Pitfalls and challenges in genetic test interpretation: An exploration of genetic professionals experience with interpretation of results
Katherine E. Donohue and Catherine Gooch are co-first authors.
Funding information: National Human Genome Research Institute (NHGRI) with co-funding from the National Institute on Minority Health and Health Disparities (NIMHD) and the National Cancer Institute (NCI), Grant/Award Numbers: U01HG007301, U01HG009599, U01HG009610, U24HG007307
Abstract
The interpretation of genetic testing results is subject to error. This observational study illustrates examples of pitfalls and challenges in interpretation of genetic testing results as reported by genetics professionals. We surveyed genetics professionals to describe interpretation challenges, the types of variants that were involved, and the reported clinical impact of misconception of a test result. Case studies were then collected from a select group to further explore potential causes of misunderstanding. A total of 83% of survey respondents were aware of at least one instance of genetic test misinterpretation. Both professionals with and without formal training in genetics were challenged by test reports, and variants of unknown significance were most frequently involved. Case submissions revealed that interpretation pitfalls extend beyond variant classification analyses. Inferred challenges in case submissions include lack of genetic counseling, unclear wording of reports, and suboptimal communication among providers. Respondents and case submitters noted that incorrect interpretation can trigger unnecessary follow-up tests and improperly alter clinical management. Further research is needed to validate and quantify large-scale data regarding challenges of genetic results interpretation.
1 INTRODUCTION
Genetic testing is increasingly employed in the evaluation of medical conditions, ranging from neurocognitive disorders with childhood onset to the development of cancer in adults.1 The involvement of all age groups and expansive clinical indications for genetic testing have led to an increased demand for genetic tests and ordering of these tests by health professionals without formal training in genetics.2 Coupled with this increase demand for testing is the rapid advancement of genomic technology, resulting in an increased complexity of genetic testing options to clinicians who may need to order and interpret genetic tests as a part of patient care.3 These advances in the genomic era have been swift, and medical schools may not be able to cover the nuances of genomic test interpretation in a traditional course schedule.4-8 Nevertheless, there is a recognized shortage of trained medical geneticists and genetic counselors, so health professionals without formal training in genetics have increasingly been involved in ordering and interpreting genetic testing especially as the enthusiasm for genomic medicine continues to rise.9-12
Despite the spread of genomic medicine throughout the US healthcare system, its benefits cannot be fully realized—and perhaps, may be negated—if genetic testing results are misinterpreted. The potential for pitfalls in the analysis of genetic testing results has long been appreciated. Testing that is misdirected can reveal unwarranted information, such as disease status, carrier status, and family relationships. Uncertainty in variant interpretation can lead to misdiagnosis, and prognostication is often problematic due to incomplete or age-dependent penetrance and variable expressivity.13, 14 In addition, communication of genetic testing results to patients and providers can be complicated not only by the complexity of the results, but also by the technical language used in writing reports.15 Misinterpretation of genetic results can ultimately have significant consequences, including incorrect diagnoses, unnecessary treatments and interventions, increased psychosocial stress on patients and their families, and sometimes missed diagnoses, which may even lead to death.16-24 Compounding this, there may be legal ramifications to providers and/or hospital systems that could present as malpractice. The genomics era has brought with it liabilities that are unique and it is likely that some misinterpretation lawsuits will set legal precedent.25-27 The ramifications of genetic test result misinterpretations can also lead to the misuse of healthcare dollars, costing families, payers, and the entire healthcare system.21, 28
While there have been case studies providing examples of genetic test misinterpretation and its consequences, little is known regarding the frequency of challenges in interpreting genetic results, the contributing factors to these challenges, and the impact that such errors can have on clinical care. Determining the frequency of misinterpretation of genetic test results across multiple specialties, test types, and geographic regions is difficult to objectively measure. Without access to patient records and test reports, collecting instances of misinterpretation can be subjective and influenced by ascertainment bias. This study is an initial exploration of perceived instances of misinterpretation described by genetics professionals, including geneticists and genetic counselors, using self-reported data. The goal of this study was primarily descriptive: to identify and highlight instances in which these genetic professionals have encountered situations where they consider that medical professionals have misunderstood a genetic test result. We polled providers with training in genetics (MDs, PhDs, and genetic counselors), asking how often they had experienced a perceived misinterpretation of a genetic test result and for information about the genetic tests involved, the type of misinterpretation, and the clinical setting. Case-based reports from genetic professionals were also collected to further explore the circumstances surrounding perceived examples of genetic test misinterpretation. Our ultimate goal was to better understand the situations that can challenge interpretation of a genetic test result. The observational data may therefore help future development of strategies to ensure safe and accurate interpretation of genetic testing results while maximizing access to such testing.
2 MATERIALS AND METHODS
This study was approved by the Institutional Review Board (IRB) of the University of Alabama at Birmingham. We designed a survey in which we asked respondents about examples of experienced genetic test misinterpretation (Appendix S1). The Qualtrics platform was used to send the survey to three groups of genetics professionals: (1) medical geneticists certified by the American Board of Medical Genetics and Genomics, (2) genetic counselors who are members of the National Society of Genetic Counselors (NSGC), and (3) members of the Clinical Sequencing Evidence-Generating Research (CSER) Consortium, which includes medical geneticists, laboratory geneticists, and genetic counselors. Participants completed the survey in January and February in 2019 and did not receive compensation. Due to the sensitive nature of the topic and to ensure complete anonymity, no participant demographics were obtained. Participants were aware that their responses were being used as part of a study by investigators at the University of Alabama at Birmingham. Responses were compiled in graphical and tabular forms and the results were generated using Qualtrics and Microsoft Excel. Respondents were also given the option to share anecdotes about cases with misinterpretations before ending the survey.
The goal of the survey was to identify instances of genetic result misinterpretation and to assess the clinicians' experience in how those cases affected patient care. The survey asked a series of questions regarding the experience of clinicians with results misconception. These questions asked clinicians to focus on instances when a provider was challenged by a genetic testing report, as opposed to instances when a provider disagreed with the variant assessment from a clinical laboratory. These questions also did not include examples of amended lab reports or discordant variant calls among laboratories. The goal of the survey was to elicit qualitative responses among genetics professionals, rather than to generate data for a quantitative analysis. As such, the survey questions were written by study authors and not subject to review of validity or reliability of the data that were generated. Here we describe the descriptive statistics that were collected through the survey.
A qualitative case-based approach was then used to explore experiences with perceived misinterpretation of genetic testing results among genetics professionals who were members of the CSER consortiums Education and Return of Results (E-ROR) working group, which consists of about 65 members.29 This approach allowed for a more nuanced exploration of instances of perceived misinterpretation, as our survey only allowed participants to respond with “yes” or “no,” with an optional open-ended response to share anecdotes. Members of this working group were invited to submit examples concerning challenges to genetic test interpretation from their respective clinical research studies and to describe the perceived impact of the misinterpretation. A standardized case summary form was provided (Supplementary Table 1) that asked for information about the clinical indication for testing, the type of test performed, the testing result, the misinterpretation of the result according to the respondent, their explanation as to the cause and consequences, and how similar misinterpretations could be prevented in the future. Respondents were also asked to classify the type of misinterpretation and to provide additional aspects of the case that they believed to be relevant. Cases were collected and perceived consequences of the reported misinterpretations and contributing factors to the misinterpretation cases were identified by two authors (KED and AS) using qualitative inductive analysis to condense and summarize the textual data.29-31 Themes to describe the common pitfalls of misinterpretation exemplified in these cases were also generated using this qualitative approach. In order to ensure comprehensive analysis of the data, the perceived consequence of and contributing factors to these misinterpretation cases, as well as the observed themes in the cases, were refined through iterative processes and discussion with all study authors. All cases described by the respondents occurred in the setting of participants enrolled in IRB-approved clinical research protocols at the corresponding CSER sites. These cases, although anecdotal, allowed for an in-depth exploration of the experiences with misinterpretation of genetic testing results from a group of genetics professionals.
3 RESULTS
3.1 Survey
The survey was distributed to the three groups, totaling approximately 5000 members. One caveat to the distribution method was that survey recipients may have been members of two or even all three of the groups, making the actual number of recipients likely to be below the estimated 5000. A total of 416 individuals opened the survey and 360 individuals fully completed it, giving a response rate of 7.2%. Although this is a low response rate, the purpose of our survey was case-ascertainment, not accurate determination of the frequency of result misinterpretation. As such, the 360 responses sufficed for a rich response. Of 360 valid responses, 61 people responded that they had not been involved in a case in which a genetic test result was misinterpreted by another provider which terminated the survey, leaving 299 surveys for further study.
Demographics about the composition of the survey population were not obtained in order to preserve anonymity. A total of 160 anecdotes ranging from two to 658 words, were submitted.
A total of 299/360 (83%) respondents reported that they were aware of at least one example of misinterpretation of a genetic testing result during their career. The number of misinterpreted genetic testing cases reported by respondents varied, with the majority having experienced 2–5 cases (55%), 21% experienced 10 or more, 13% experienced 6–10 cases, and 11% experienced just one.
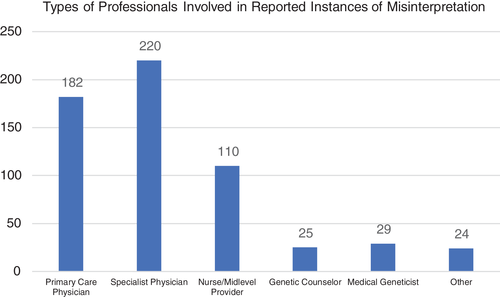
We then asked for information on the role of the healthcare professional involved in the misinterpretation of genetic results. Respondents were allowed to select more than one answer to account for the involvement of different types of healthcare professionals for respondents who had experienced multiple examples of result misconception. The breakdown of types of health professionals involved in misinterpretation events is shown in Figure 1.
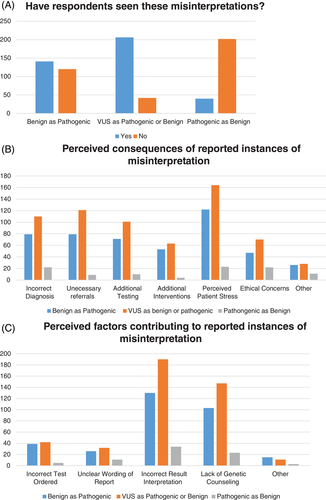
Next, survey respondents reported the types of variant classifications that were most prone to misconception. Respondents were asked if they had experienced each of the following cases: (1) a benign or likely benign variant interpreted as pathogenic, (2) a variant of unknown significance (VUS) interpreted as benign or pathogenic, and (3) a pathogenic variant interpreted as benign If respondents experienced a perceived misinterpretation in more than one scenario, they were able to respond appropriately.
3.1.1 Benign variant interpreted as pathogenic
At least one case of a benign variant being interpreted as pathogenic by a non-genetics professional was reported by 141/261 (54%) surveyed respondents who answered this question, shown in Figure 2(A). Figure 2(B) shows a breakdown of the perceived effects of misinterpretation. Perceived patient stress was often cited as an outcome, but there were multiple instances where this misconception was felt to lead to an incorrect diagnosis, referral to another specialist, additional testing, and inappropriate treatment. Many respondents shared anecdotes detailing the consequences of the misunderstanding that included unnecessary testing, organ removal, pregnancy termination and decisions to withdraw care, all based on benign results that were reportedly misinterpreted as pathogenic (see Table 1).
| Selected anecdotes |
| Benign variant interpreted as pathogenic |
| “A newborn with a congenital anomaly had a targeted genetic test panel done prenatally and found a benign parentally-inherited variant was identified. Parents told by neonatologist in the delivery room that infant had features of a specific genetic syndrome. The baby was admitted to NICU, echocardiogram and other imaging was done. I was eventually consulted and the patient was a beautiful infant, with no features of the condition. Parents were not aware, however, of other potential causes of the congenital anomaly.” |
| Uncertain variant interpreted as pathogenic |
| “A single CLN6 VUS was interpreted as diagnostic for a neurogenerative disease by a neurosurgeon who did not order the test. The family was told at a clinic visit their child had the disease. I called results out a few days later and gave the correct interpretation. The family thought their child was dying and obviously was distraught. Upon WES, a non-fatal diagnosis was found.” |
| Pathogenic variant interpreted as benign |
| “A patients breast and ovarian cancer result was interpreted as negative by her oncologist, when the result was positive. This was determined years later, after she received a second primary breast cancer diagnosis and her result was re-reviewed by a genetic counselor.” |
- Note: Select examples of the 160 anecdotes submitted by survey respondents, each representing a different type of perceived variant misinterpretation. Anecdotes are paraphrased to avoid unintentional reidentification of patients.
Respondents were asked to judge the factors that contributed to misinterpretation, shown in Figure 2(C). Most noted incorrect test interpretation by the ordering provider or lack of genetic counseling. Other causes included ordering of an incorrect test and unclear wording of the genetic testing report.
3.1.2 VUS interpreted as benign or pathogenic
A total of 206/248 (83%) of surveyed individuals had encountered at least one case of a VUS that was interpreted as benign or pathogenic. Figure 2(B) indicates the perceived consequences of this type of testing result misinterpretation; once again, perceived patient stress was cited as the most common outcome. Respondents also listed confusion, denial of life insurance, “secret” testing of minors for an adult-onset condition, and prevention of athletic participation as repercussions of miscalled VUSs. Contributing factors are listed in Figure 2(C).
3.1.3 Pathogenic variant interpreted as benign
A total of 40/242 (16%) of surveyed individuals had encountered at least one case of a pathogenic result being interpreted as benign. The consequences of this type of misinterpretation are listed in Figure 2(B). Respondents also listed delay in diagnosis and missed early screening as possible repercussions of misinterpreted pathogenic variants. Contributing factors are listed in Figure 2(C). Respondents also listed other reasons, such as lack of knowledge about VUS reclassification, as the basis for pathogenic variants being interpreted as benign.
3.1.4 Time to correction
Lastly, respondents were asked how long, on average, did patients have to wait before they were provided with the correct interpretation of their genetic testing result following prior misinterpretation of the result. Times ranged from days to years, but most often was reported to be weeks to months (45%). 34% of respondents stated that time to correction took weeks, 12% stated it took years, and 9% stated the correction was made in a matter of days (0–6).
3.1.5 Free text comments
Respondents were given the option to share anecdotes about misinterpreted cases before ending the survey. A total of 160 anecdotes were submitted. Three anecdotal examples of each misinterpretation type (benign as pathogenic, variant of uncertain significance as benign or pathogenic, and pathogenic as benign) are included in Table 1.
3.2 CASE REPORTS
Ten case reports were submitted by members of the E-ROR working group. Table 2 briefly describes each case as well as the (1) perceived consequence of the reported error and (2) perceived contributing factors to the reported error. Similar to the survey data, the case studies revealed that these perceived instances of misinterpretation can lead to perceived unnecessary stress for patients or family members, unwarranted therapeutic interventions, and incorrect diagnoses. Respondents referenced incorrect test interpretation by the provider, lack of genetic counseling, and unclear wording of the testing report as contributing factors to misinterpretation. Ordering of the incorrect genetic test was not mentioned in these cases, as testing options were predetermined for all study sites in the CSER consortium. The cases submitted provided additional, nuanced descriptions of the circumstances that can surround errors in genetic test interpretation compared to the survey data, as case submitters were not limited to specific response options. The text below describes three themes that were observed in the submitted cases and provides further detail regarding circumstances surrounding reported misinterpretations of genetic results.
| Case | Case summary | Perceived consequence of error | Perceived contributing factors to error | ||||
|---|---|---|---|---|---|---|---|
| Perceived stress for patient or family members | Incorrect diagnosis | Unwarranted therapeutic interventions | Incorrect test interpretation by provider | Lack of genetic counseling | Perceived unclear wording of report | ||
| 1 | De novo variant of uncertain significance in found in a premature infant: provider reported that the variant wasnt paternally or maternally inherited without discussing the mechanism of de novo variants. Patients mother assumed non-paternity given the variant was not inherited from either parent. | + | + | + | |||
| 2 | WGS reported 47,XXY as an incidental finding and did not identify any variants associated with the indication for testing. However, provider interpreted the test as positive and that the result explained the patients primary phenotype. | + | + | + | + | ||
| 3 | Loss of function variant in a gene reported as causative of familial hypercholesterolemia, but in fact this gene predisposes to hypercholesterolemia only with gain of function variants. | + | + | + | |||
| 4 | Two variants of unknown significance reported in cis, but provider interpreted them as in trans and assumed this caused an autosomal recessive condition in the patient. | + | + | + | + | + | |
| 5 | Provider interpreted negative genome sequencing results to mean that the test ruled out all genetic causes for patients phenotype. | + | + | + | |||
| 6 | Infant with intrauterine growth restriction (IUGR), microcephaly, brain malformations, and prenatal cardiac compromise had compound heterozygous pathogenic/likely pathogenic variants interpreted by provider as definitely causative of all of the childs symptoms, who quoted a 25% recurrence risk. The gene however is not definitively associated with IUGR, microcephaly, and brain abnormalities. |
+ | + | + | + | ||
| 7 | Heterozygous variant classified as a likely pathogenic variant by research laboratory but subsequent Sanger confirmation by outside lab called it a VUS. Reanalysis concluded variant was likely diagnostic in this case. Provider not familiar with the case called it VUS. | + | + | + | + | ||
| 8 | Two pathogenic variants were reported in an autosomal recessive disease gene, however, segregation status was not available due to proband only testing. Despite this, family was told child had diagnosis of autosomal recessive condition. Father was later tested and had neither variant, confirming variants in proband were most likely in cis. Diagnosis was overcalled. |
+ | + | + | |||
| 9 | Exome sequencing results returned with a possible homozygous exon deletion. Laboratory recommended deletion/duplication studies to confirm homozygous deletion. Results were reported to family as causative before del/dup studies completed, which were negative. |
+ | + | + | |||
| 10 | Karyotype, single gene sequencing, and del/dup studies were ordered for a patient from the same laboratory. Chromosome analysis revealed Tetrasomy X, but result of the single gene testing was labeled as “positive.” The remainder of the report included language about the condition associated with this gene, despite the fact that the results did not point towards this patient having the condition. Mother was incorrectly told that child had this condition. | + | + | + | + | + | + |
- Note: Summary of the cases submitted by the CSER Education & Return of Results Working Group, highlighting perceived pitfalls in interpretation of genomic test results among providers.
3.2.1 Misinterpretation of genetic results extends beyond errors in variant classification
Submitted cases show that pitfalls in misinterpretation of genetic results extend beyond perceived errors in variant classification. Only two cases classified the reported error as a misinterpretation in variant classification ('benign' interpreted as 'pathogenic', and 'VUS' interpreted as 'pathogenic'). All cases, however, cited incorrect application of genetic concepts by the referring provider as the perceived cause of misinterpretation. For example, case #5 discusses an infant who received whole genome sequencing (WGS). While results were negative, the ordering provider stated that the test had ruled out all genetic causes of the infant's phenotype and informed the family that there was no recurrence risk in future children. Therefore, the family was incorrectly counseled about the meaning of a negative test result and provided false reassurance regarding recurrence. Other examples of misunderstanding of genetic concepts include incorrect interpretation of de novo versus inherited variants (#1), the role of cis versus trans variants in autosomal recessive inheritance (#4), and the definition of incidental (secondary) versus primary findings (#2). Understanding of inheritance patterns, genetic terminology, and report reading are all relevant when accurately interpreting genetic test results.
3.2.2 Misinterpretation of genetic results may result from perceived miscommunication by, and to, multiple parties
While misinterpretation of genetic testing reports may often be perceived as due to provider error (particularly as it relates to the level of genetics knowledge a provider may or may not have), these case reports highlight instances where pitfalls in interpretation may be complicated by external communication factors. For example, cases #3 and #10 both cite unclear wording of reports as the perceived underlying cause of misinterpretation. In case #3, a loss of function variant in PCSK9 was listed on a laboratory report as related to familial hypercholesterolemia (FH), but it did not clearly state that only gain of function variants in this gene causes disease. Therefore, the non-genetics provider initially interpreted the test as diagnostic for FH. This is an instance where lack of communication between the laboratory and provider may not have directly caused the misinterpretation, but could have contributed to it. Other cases described perceived miscommunication between clinicians and/or research teams as the pitfall in test interpretation (cases #7 and #9). The translation of genetic testing results from provider to patient through unclear genetic counseling was also a perceived contributing factor to misinterpretation in the case reports (cases #1 and #6). In case #1, for example, the provider disclosed a VUS that was neither maternally or paternally inherited to the mother of an infant without explaining the concept of de novo inheritance. This left the mother distraught and assuming misattributed paternity, which was later included in the infant's chart. These cases demonstrated that communication between clinicians, laboratories, and/or families therefore plays a vital role in genetic testing interpretation.
3.2.3 Misinterpretation of genetic results is complex and not always easily categorizable
Finally, all cases highlight instances where interpretation of a genetic result is not straightforward, emphasizing the complexities to the interpretation process that we were unable to capture in the survey data. As seen in Table 2, there were often multiple perceived contributing factors to and consequence of the reported misinterpretation, and cases were not easily described by one factor. Furthermore, case submitters noted that there are gray areas in the interpretation of genetic results and that perceived errors may not be easily identified. Case submitter #9, for example, cited the need for caution when interpreting results and the necessity of investigating the limitations of a testing modality. Case submitter #5 discussed that disagreements may occur among providers, which itself can cloud testing interpretation. Case submissions revealed that pinpointing the exact cause of a perceived genetic test report misinterpretation may not always be possible.
3.2.4 Respondent suggestions for minimizing misinterpretation
Each case submitter was also asked to provide suggestions that may have minimized the challenges involved in the perceived misinterpretation case. These suggestions varied and included the involvement of genetic counselors or genetics professionals in the interpretation of genetic tests, increased education for health professionals without formal training in genetics, and improvement of the wording of genetic testing reports. Other suggestions included increased and clear communication between ordering providers, genetic professionals, and laboratory professionals (Supplementary Table 2).
4 DISCUSSION
The survey and case report data provide numerous examples of reported pitfalls and challenges in genetic testing interpretation by a diverse group of medical providers that include professionals with and without formal training in genetics. The data illuminate multiple ways that misconceptions regarding genetic testing results can occur. The survey data described accounts of misinterpretation of variant significance, including misconception of a benign variant as pathogenic, a pathogenic variant as benign, and a VUS as either pathogenic or benign. Of note, this is not limited to molecular genetic testing and can also occur in the provision of cytogenomic microarray results. Secondly, incorrect counseling of patients despite correct classification of the variant can also occur. This pitfall may be related to a providers familiarity with genetic concepts and can negatively impact patient care. Both the survey and case analyses revealed that the most commonly reported consequence of result misconception was perceived patient stress, though other outcomes included incorrect management or treatment decisions and increases in medical care cost through ordering of unnecessary follow-up tests. The survey did not address complications arising from amended reports or discordant variant calling among labs.
The respondents listed facets of the case that they believe contributed to the result misunderstanding. While this is helpful from an anecdotal standpoint, the data we collected are not intended to identify ways to “fix” pitfalls and challenges in providing results. Other publications have discussed ways to reduce the likelihood of misinterpretation, and improved education of medical professionals has emerged as a common theme.32-35 While the need for increased education of non-genetics professionals in clinical genetics has long been appreciated, it is clear that pitfalls in interpretation can still occur with formal genetics training. Challenges in bettering genomics education also remain, especially regarding education standardization and student knowledge retainment.36-39 Involvement of genetic counselors and medical geneticists in the training of healthcare professionals would be ideal. Further quantitative research in this area is indicated to better understand how effective healthcare provider education will be in improving result interpretation.
Many of the respondents (survey and case report) listed lack of genetic counseling as a possible cause for challenges in interpretation. Involvement of a genetic professional in pre- and/or post-test counseling may be effective in increasing the likelihood of correct interpretation of a test result, for appropriate test ordering, and for accurate patient education.26 At the same time, while the demand for clinical genetic testing continues to grow, the genetics workforce remains relatively small.40 In 2017, the NSGC supply-and-demand algorithm predicted that the genetic counseling workforce was understaffed by nearly 50%.41 A 2019 ACMG survey showed a stagnant number of medical geneticists, leading to longer wait times for patients.42 Improving access to providers trained in clinical genetics may therefore help ameliorate genetic test misinterpretation. Increasing the number and size of training programs for genetic counselors and medical geneticists is critical.43, 44 Additionally, the development of innovative service delivery models that leverage digital tools (such as chatbots and health information technology) may help increase access to genetic services, maximizing the efficiency of genetics professionals.45-50 Telegenetics has also become a popular option to reach more patients while simultaneously decreasing wait time and increasing patient volume.51-54
Respondents also listed unclear wording of a genetic test result as a potential challenge for result interpretation. There is curently is no standard in genetic test report wording and formatting. A study by Makhoon et al. showed a significant variation between cancer genetic test reports, which differed in content, length, recommendations, layout, and test description, among other parameters.55 They also reported significant difference in the reports' average reading grade level, which varied between 7th and 12th grade. Studies have evaluated what providers value in test result reporting for genome sequencing, but studies to determine what is valued in reports for other types of genetic testing, such as microarray, are lacking.56 One of the main conclusions of the CLARITY Challenge through Boston Childrens Hospital is “the need for greater attention to the development of clear, concise clinical reports, with common elements such as use of reference accession numbers and genome builds, consistent criteria for definition of pathogenicity (or degree of uncertainty)”.57 Further research is warranted to evaluate if changing laboratory reporting structure and language may help mitigate interpretation errors.
This descriptive study also adds to the valued literature which has been documenting experiences with misinterpretation of genetic results. Case series focused on errors in genetic testing, especially in the realm of hereditary cancer risk, have been key in providing awareness to the consequences of improper management of genetic testing and report interpretation.16, 21, 22, 58 Experiences of misinterpretation in pediatric, prenatal, and even direct-to-consumer genetic testing have also been reported by genetic professionals.59-62 Continued identification of pitfalls in the ordering and interpretation of genetic results—and the identification of these errors in a robust, quantitative manner—is necessary in order to realize the extent to which errors in genetic interpretation occur so that they may be adequately addressed.
5 LIMITATIONS
This was a retrospective study, so recall bias has likely been a factor in result reporting by respondents (both survey and case report). We also recognize that there may have been ascertainment bias in the responses received, wherein practitioners who had encountered an instance of results misinterpretation may have been more likely to respond to the survey or provide a case study. This is especially relevant given the low response rate. However, as we are not making conclusions about the frequency of misconception in the clinical setting, these biases are unlikely to impact our assessment of the pitfalls and challenges that do occur. As the survey was not designed to determine the frequency of misinterpretation, our results are not amenable to quantitative analyses. These qualitative approaches also limit our ability to verify or validate assertions made by the survey or case-based respondents. In addition, survey respondents were only able to select “yes” or “no” options to our three types of misinterpretations and therefore were only able to expand on examples of perceived misinterpretation in the optional, open-ended request for anecdotes. A future qualitative analysis of the 160 anecdotes submitted by survey respondents may provide another avenue to understand some of the pitfalls in genetic testing interpretation.
The survey was sent to genetic providers in three large groups based in North America and thus was not inclusive of the global genetics community; in addition, only a small subset of genetic professionals who were members of the E-ROR working group in the CSER consortium were provided with the opportunity to submit detailed cases. As this is a self-reported, observational study, the impacts of misinterpretation described by participants may be subjective. Responses to survey questions may also have varied due to differences in the interpretation of questions, as the survey was not piloted. Since providers were asked not to provide Health Insurance Portability and Accountability Act (HIPAA) classified private information, we were unable to view the actual patient reports or clinic notes and relied instead on the interpretation of respondents. As such, we could not independently verify the reports and notes, the misinterpretation described, and/or the clinical impact of any described misinterpretations. Finally, this study cannot be used to infer how often misinterpretation occurs, as respondents indicated instances of misinterpretation without specifying the total number of genetic tests with which they have been involved or the number of years that they have spent in practice. Future research regarding frequency of instances of misinterpretation among clinicians using a more structured approach with piloting and validation of survey questions is an important next step to this work.
We also recognize that there are legitimate instances in clinical genetics where a provider will interpret and disclose results to a patient in a classification that differs from the laboratory report. It is possible that this practice could be misconstrued as a misinterpretation. Clinicians have more phenotypic information about the patient that they can use to interpret a variant compared to the testing laboratory and are in a better position to assess the clinical relevance of a finding. This study supports the need for a nuanced approach to genetic test interpretation and encourages further research regarding education of healthcare providers on interpretation techniques.
6 CONCLUSION
The genetics professionals who participated in our study have encountered multiple pitfalls in the interpretation of genetic test results. These challenges can occur for both genetics and non-genetics trained professionals who are providing the results to patients and their families. The survey respondents highlighted that variant misclassification was common and interpretation of a VUS as a benign or pathogenic variant was the most frequently observed form of variant misinterpretation. Unclear wording of test reports and lack of access to genetic counseling were considered to be contributors to difficulties in test interpretation. Case report submissions demonstrated that even with correct interpretation of a variant classification, the implications of the genetic test result could still be misconstrued due to misunderstanding of or lack of familiarity with genetic concepts, misreading of the genetic report, and poor communication. Each of these pitfalls is deserving of its own quantitative research so that the genetics community can better understand how to avoid the pitfalls and challenges inherent in genetic testing report interpretation.
ACKNOWLEDGEMENTS
The authors would like to thank the following members of the CSER Consortium who provided feedback on this manuscript: Kelly East, MS, CGC (HudsonAlpha Institute for Biotechnology); Laura G Hendon, MA, MS, CGC (University of Mississippi Medical Center); Katie Gallagher, MS, CGC (Montefiore Medical Center). The Clinical Sequencing Evidence-Generating Research (CSER) consortium is funded by the National Human Genome Research Institute (NHGRI) with co-funding from the National Institute on Minority Health and Health Disparities (NIMHD) and the National Cancer Institute (NCI), supported by U01HG007301(BRK), U01HG009599 (AS), U01HG009610 (KED), and U24HG007307 (Coordinating Center). The contents of this paper are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. The CSER consortium thanks the staff and participants of all CSER studies for their important contributions. More information about CSER can be found at https://cser-consortium.org/.
CONFLICT OF INTEREST
The authors declare no potential conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




