Recognizing the tenascin-X deficient type of Ehlers–Danlos syndrome: a cross-sectional study in 17 patients
Abstract
The tenascin-X (TNX) deficient type Ehlers–Danlos syndrome (EDS) is similar to the classical type of EDS. Because of the limited awareness among geneticists and the challenge of the molecular analysis of the TNXB gene, the TNX-deficient type EDS is probably to be under diagnosed. We therefore performed an observational, cross-sectional study. History and physical examination were performed. Results of serum TNX measurements were collected and mutation analysis was performed by a combination of next-generation sequencing (NGS), Sanger sequencing and multiplex ligation-dependent probe amplification (MLPA). Included were 17 patients of 11 families with autosomal recessive inheritance and childhood onset. All patients had hyperextensible skin without atrophic scarring. Hypermobility of the joints was observed in 16 of 17 patients. Deformities of the hands and feet were observed frequently. TNX serum level was tested and absent in 11 patients (seven families). Genetic testing was performed in all families; 12 different mutations were detected, most of which are suspected to lead to non-sense mRNA mediated decay. In short, patients with the TNX-deficient type EDS typically have generalized joint hypermobility, skin hyperextensibility and easy bruising. In contrast to the classical type, the inheritance pattern is autosomal recessive and atrophic scarring is absent. Molecular analysis of TNXB in a diagnostic setting is challenging.
Ehlers–Danlos syndrome (EDS) is characterized by joint hypermobility, skin hyperextensibility, and tissue fragility; latter leading to spontaneous ecchymoses, easy bruising and atrophic scarring. EDS is a clinically and genetically heterogeneous group of connective tissue disorders 1. Many new types have been discovered over the past years and the nosology is in the process of being updated.
The tenascin-X (TNX)-deficient type (OMIM 606408) is a non-collagenous EDS type caused by homozygous or compound heterozygous mutations in the TNXB gene (MIM 600985), that leads to non-sense mediated mRNA decay of TNXB and result in a complete absence of TNX or, occasionally, in misfolding of the protein 2, 3. The clinical diagnosis can be confirmed by the absence of TNX in the serum and by mutation analysis of the TNXB gene 3. The clinical phenotype resembles the classical type of EDS with two major differences: an autosomal recessive inheritance and the absence of atrophic scarring 3.
In 1997, the TNX-deficient type was first reported in a 26-year-old male patient with congenital adrenal hyperplasia due to 21-hydroxylase deficiency as well as hyperextensible skin, hypermobile joints, easy bruising, and poor wound healing 2. Skin biopsy of this patient showed small collagen fibres and a complete absence of TNX. Cultured dermal fibroblasts also lacked TNX. Genetic analysis revealed a deletion on chromosome 6, including both the CYP21A2 gene and the TNXB gene. Subsequently, Schalkwijk et al. measured TNX in the serum of 151 EDS patients (classical, hypermobile and vascular type), 168 disease controls (psoriasis or rheumatoid arthritis), and 21 healthy controls. A complete TNX deficiency was observed in four additional EDS patients and three affected siblings (one of which with the contiguous gene syndrome similarly as initially reported by Burch et al.) 3. Mutation analyses revealed homozygous or compound heterozygous mutations in the TNXB gene.
After the initial publication, additional cases have been reported, with a focus on specific clinical features, including neuromuscular, genito-urethral, gastrointestinal, and cardiovascular findings 4-13. So far, of 14 families only 18 patients with TNX-deficiency and two 2 with the contiguous gene syndrome have been reported, 14 of whom from the Netherlands. Only in six families, four of which from the Netherlands, three different TNXB mutations have been reported.
In order to improve the clinical recognition of this EDS type it is important to increase physicians' and patients' awareness of its clinical features. Therefore, it is essential to have a well-defined description of the phenotype. This will improve genetic counselling and management of the disease. Therefore, we performed a cross-sectional clinical study in 17 patients, and aimed to provide a detailed report of the TNX-deficient EDS phenotype. We also summarize and discuss the methods of molecular analysis of TNXB and present the results of DNA testing in this cohort. This will enable geneticists worldwide to recognize this phenotype and enable the setting up of diagnostic facilities.
Materials and methods
Patients
All known Dutch patients with either a homozygous or compound heterozygous mutation in the TNXB gene or a complete deficiency of TNX in serum in patients with a homozygous or compound heterozygous TNXB mutation in a sibling were invited to participate in this study. These patients were identified among the cohort known at the Radboud university medical centre (Radboudumc) (including the initial cohort of Schalkwijk et al.) 3, and among the patients identified at the DNA diagnostic laboratory of the VU University Medical Centre (VUMC), which offers next-generation sequencing (NGS) of a panel of EDS genes including TNXB since 2013. In addition, an invitation was posted on the patient organization website (www.ehlers-danlos.nl). Patients with the contiguous-gene syndrome involving both the TNXB gene and the CYP21A2 gene were excluded, as this genotype may lead to a more severe phenotype 14, 15. We also excluded patients in whom a heterozygous mutation was detected and who were not tested for complete absence of TNX on serum measurements, as they may represent patients with hypermobility type EDS and TNXB haploinsufficiency 16.
The study was approved by the medical ethical committee of the Radboudumc and informed consent forms were collected prior to enrolment.
Clinical features
A history and physical examination were performed by five independent clinical geneticists using a standardized self-designed form. This form was based on the overall features of EDS included in the Villefranche criteria, a systematic literature review to define the TNX-deficient EDS phenotype, and the clinical experience of all geneticists involved (Table 1) 1.
| Patient no | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | Number of patients (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Family no. | I | II | III | IV | V | VI | VII | VIII | IX | X | XI | |||||||
| Family 1 in Schalkwijk et al. 3 | Family 2 in Schalkwijk et al. 3 | Not reported | Voermans et al. 7 | Voermans et al. 7 | Voermans et al. 7 | Patiënt 2 in Hendriks et al. 10 | Not reported | Not reported | Patiënt 1 in Hendriks et al. 10 | Not reported | ||||||||
| Patient characteristics | ||||||||||||||||||
| Age (years) | 69 | 59 | 60 | 55 | 59 | 48 | 36 | 32 | 31 | 29 | 27 | 23 | 16 | 14 | 12 | 12 | 6 | n/a |
| Sex | F | F | F | F | M | F | M | F | F | M | F | F | M | M | F | F | M | 6 M (35) |
| Parental consanguinity | – | – | – | – | – | – | – | – | – | + | – | – | – | – | – | – | – | 1 (6) |
| TNXB mutations detected | +/+ | +/+ | +/+ | +/n/a | +/n/a | +/+ | +/+ | +/+ | n/a | +/+ | +/+ | +/+ | +/+ | +/+ | +/+ | +/+ | +/+ | |
| Serum TNX (ng/ml) | 0 | 0 | 0 | 0 | 0 | n/a | 0 | 0 | 0 | 0 | 0 | n/a | n/a | n/a | 0 | n/a | n/a | n/a |
| Dysmorphic changes | ||||||||||||||||||
| Dysmorphic traits | – | + | – | – | – | – | – | – | – | + | + | – | – | – | – | – | – | 2 (12) |
| High arched palate | – | + | – | – | – | – | – | – | + | + | – | – | – | – | – | – | – | 2 (12) |
| Narrow palate with dental crowding | – | – | – | + | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 (6) |
| Dermatological features | ||||||||||||||||||
| Velvety/smooth skin | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | 17 (100) |
| Hyperextensible skin | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | 17 (100) |
| Easy bruising | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | 17 (100) |
| Spontaneous ecchymosis | + | + | + | – | – | + | + | + | + | + | + | + | + | – | + | + | – | 13 (77) |
| Piezogenic papules feet | + | – | – | + | – | – | + | + | – | + | + | + | + | + | + | + | + | 12 (56) |
| Oedema of ankles and/or feet | – | – | – | – | + | – | + | – | + | – | + | – | – | – | – | – | – | 4 (24) |
| Raynaud/acrocyanosis | – | + | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 (6) |
| Varicose veins | – | – | – | + | – | + | – | + | – | – | – | – | – | – | – | – | 3 (18) | |
| Complaints of delayed wound healing | – | – | + | – | – | – | – | – | – | + | + | + | – | + | – | + | + | 7 (41) |
| Umbilical hernia | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | + | 1 (6) |
| Inguinal hernia | + | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 (6) |
| Musculoskeletal features | ||||||||||||||||||
| Delayed motor development | – | – | – | – | + | – | – | – | – | – | + | – | – | – | – | + | – | 3 (18) |
| Infancy/childhood joint hypermobility | + | – | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | 16 (94) |
| Infancy/childhood articular dislocations | – | + | + | + | – | – | + | + | + | + | + | + | + | + | + | + | – | 13 (77) |
| Congenital dislocations | – | – | – | + | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 (6) |
| Residual joint hypermobility | + | – | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | 16 (94) |
| Beighton score | 5 | 3 | 7 | 8 | 7 | 9 | 9 | 9 | 6 | 8 | 8 | 8 | 8 | 8 | 8 | 6 | 8 | n/a |
| Articular (sub)luxation of the | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | – | 16 (94) |
| shoulder | + | + | + | + | – | + | + | + | – | + | + | + | + | + | + | + | n/a | 14 (82) |
| ankle | – | – | – | – | – | + | – | – | – | – | – | + | – | – | – | – | n/a | 2 (12) |
| hip | – | + | + | + | – | + | – | – | – | – | – | + | – | – | – | + | n/a | 6 (36) |
| knee | – | + | + | – | – | – | – | + | – | – | – | + | – | – | + | + | n/a | 6 (36) |
| patella | – | + | – | – | – | – | – | – | – | – | – | – | – | – | – | – | n/a | 1 (6) |
| finger(s) | + | – | + | + | + | + | – | + | + | – | – | – | – | + | – | + | n/a | 9 (53) |
| toe(s) | – | – | – | – | – | + | – | – | – | – | – | – | – | – | – | – | n/a | 1 (6) |
| thumbs | – | + | – | – | – | – | – | – | – | – | – | – | – | – | – | – | n/a | 1 (6) |
| temporomandibular | – | + | – | – | – | – | – | – | + | – | – | – | – | – | – | + | n/a | 3 (18) |
| elbow | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | n/a | 0 (0) |
| clavicula | – | + | – | – | – | – | – | – | – | – | – | – | – | – | – | – | n/a | 1 (6) |
| wrist | – | + | + | + | – | – | – | – | – | – | – | – | – | – | – | – | n/a | 3 (18) |
| Bursitis | – | + | + | – | + | – | – | – | – | – | – | – | – | – | – | – | n/a | 3 (18) |
| Frequent ankle sprains | – | + | – | + | + | + | + | – | + | – | – | – | + | + | + | – | + | 10 (59) |
| Rec/chronic back pain | – | – | – | + | + | – | + | – | + | – | – | + | – | – | – | + | + | 7 (11) |
| Rec/chronic myalgias | – | – | + | + | + | – | + | – | + | + | + | + | – | + | – | + | – | 10 (59) |
| Rec/chronic arthralgias | – | – | + | + | + | + | – | – | + | – | + | + | + | + | – | + | + | 11 (65) |
| Hallux valgus | – | + | + | + | – | – | – | + | + | – | – | – | – | – | – | – | – | 5 (29) |
| Pes planus | + | + | + | + | – | – | – | + | – | + | + | + | – | + | + | + | + | 12 (71) |
| Painful foot soles | – | – | + | – | – | – | – | – | + | – | – | – | – | + | – | – | – | 3 (18) |
| Deformed feet: brachydactyly (short and broad) | – | + | – | + | – | – | – | – | – | – | – | + | – | – | + | – | – | 4 (24) |
| Deformed hands (brachydactyly and short broad palms) | – | – | – | – | – | – | – | – | – | – | + | + | – | – | – | – | – | 2 (12) |
| Deformed fingers (campto/clinodactyly or swan neck) | – | + | – | + | – | – | – | – | – | – | – | – | – | – | – | – | – | 2 (12) |
| Deformed toes (clawing) | – | + | – | – | – | – | + | – | – | – | – | – | – | – | + | – | – | 3 (18) |
| Fatigue | ||||||||||||||||||
| Complaints of fatigue | + | + | + | – | + | + | + | + | – | + | + | + | + | + | – | + | + | 14 |
| CIS fatigability score; in right column number of patients with severe fatigue | 36 | 43 | n/a | 44 | 53 | 49 | 21 | 32 | 44 | 43 | 33 | 51 | n/a | n/a | n/a | n/a | n/a | 7/11 (64) |
| Neurological features | ||||||||||||||||||
| Paraesthesia of the | ||||||||||||||||||
| and/or hand | – | + | – | – | + | + | + | – | – | + | – | – | – | – | – | – | – | 5 (29) |
| legs and/or feet | – | – | – | – | + | + | + | – | – | – | – | – | – | – | – | – | – | 3 (18) |
| Hypotonia of | ||||||||||||||||||
| hands and/or feet | – | + | + | – | + | – | + | + | + | – | – | – | – | + | – | – | – | 7 (35) |
| legs and/or arms | – | + | + | – | + | – | + | – | + | – | – | – | – | + | – | – | – | 6 (35) |
| Subjective muscle weakness | – | + | + | – | + | – | + | + | + | – | – | – | – | + | – | – | – | 6 (35) |
| Distal muscle atrophy | – | + | + | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 2 (12) |
| Polyneuropathy | + | + | – | – | + | + | + | – | + | – | – | – | – | – | – | – | – | 6 (35) |
| Cardiological features | ||||||||||||||||||
| Congenital heart defects | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 0 (0) |
| Hypertension | – | + | – | – | + | + | + | – | – | – | – | – | – | – | – | – | – | 4 (24) |
| Valve abnormalities | + | + | – | + | – | + | – | – | – | – | – | – | – | – | – | – | – | 4 (24) |
| Gastrointestinal features | ||||||||||||||||||
| Bowel perforation | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 0 (0) |
| Gastric ulcer | – | – | – | – | – | + | – | – | – | – | – | – | – | – | – | – | – | 1 (6) |
| Gastrointestinal bleed | – | – | – | – | – | + | – | – | – | – | – | – | – | – | – | – | – | 1 (6) |
| Rectal prolapse | – | + | – | + | – | + | – | – | – | – | – | – | – | – | – | – | – | 3 (18) |
| Diverticulosis/itis | + | – | + | – | – | + | – | – | – | – | – | – | – | – | – | – | – | 3 (18) |
| Haemorrhoids | – | – | – | – | + | + | – | – | – | – | – | – | – | – | – | – | + | 3 (18) |
| Obstetric/gynaecological features | ||||||||||||||||||
| Number of pregnancies | 2 | 1 | 3 | 0 | n/a | 3 | n/a | 2 | 3 | n/a | 0 | 1 | n/a | n/a | 0 | 0 | n/a | 15 pregnancies |
| Number of intra-uterine death/miscarriage(s) | 0 | 0 | 0 | 0 | n/a | 1 | n/a | 0 | 1 | n/a | 0 | 0 | n/a | n/a | 0 | 0 | n/a | 2/15 (13) pregnancies |
| Number of premature membrane rupture | 0 | 0 | 0 | n/a | n/a | 1 | n/a | 0 | 0 | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | 1/15 (7) pregnancies |
| Perineal rupture | 1 | 0 | 0 | n/a | n/a | 0 | n/a | 1 | 1 | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | 3/15 (20) pregnancies |
| Number of post- or peri-partum haemorrhage | 0 | 0 | 0 | n/a | n/a | 2 | n/a | 1 | 1 | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | 4/15 (27) pregnancies |
| Uterus prolapse | + | – | – | – | n/a | – | n/a | – | – | n/a | – | – | n/a | n/a | n/a | n/a | n/a | 1/9 (11) |
| Vaginal prolapse | – | – | + | – | n/a | – | n/a | – | – | n/a | – | – | n/a | n/a | n/a | n/a | n/a | 1/9 (11) |
| Pelvic instability | – | – | – | – | n/a | – | n/a | – | – | n/a | + | – | n/a | n/a | n/a | n/a | n/a | 1/9 (11) |
| Ophthalmological features | ||||||||||||||||||
| Ptosis | + | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 (6) |
| Refractive error | + | + | + | – | + | + | + | – | – | – | – | + | – | + | – | – | – | 8 (47) |
| Frequent subconjunctival haemorrhage | – | – | + | + | + | – | – | – | + | – | + | – | – | – | – | – | – | 5 (29) |
| Dental features | ||||||||||||||||||
| Caries | – | – | – | – | – | – | – | – | – | – | – | – | – | + | – | + | – | 2 (12) |
| Enamel defects | – | – | – | – | – | – | – | – | – | – | – | – | – | + | – | + | + | 3 (18) |
| Recurrent periodontitis | – | – | – | – | – | – | – | – | – | – | – | – | – | + | – | + | – | 2 (12) |
| Other | ||||||||||||||||||
| Sleep apnoea with overnight CPAP | – | + | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 (6) |
| Spina bifida occulta | – | – | – | + | – | – | – | – | – | – | – | – | – | – | – | – | – | 1 (6) |
- CIS, checklist individual strength; CPAP, continuous positive airway pressure; F, female; M, male; n/a, not applicable/not available or not assessed.
To assess fatigue and related behaviour in the adult patients we used the subset ‘subjective fatigue’ from the ‘checklist individual strength’ (CIS) 17. It consists of eight questions each with a score of 1–7; a sum score of ≥27 indicates abnormal fatigue and a sum score of ≥37 indicates severe fatigue.
Statistical analysis
All obtained data were transferred to ibm spss Statistics 21 in which analyses were performed. Results are presented in a descriptive manner.
Laboratory analyses
Results from serum TNX measurements were collected retrospectively from patient records. TNX (a large 450 kDa extracellular matrix glycoprotein) can be detected with antibodies directed against its carboxy-terminal domain. Details concerning the serum measurement of TNX have been described previously by Schalkwijk et al. 3 and are summarized in Appendix S1, Supporting Information.
DNA diagnostics of the index patient of each family (except for family I) was offered using a NGS platform targeted for Ehlers–Danlos genes (see Appendix S1). In family I, only Sanger sequencing for a mutation previously identified in a research setting was performed 3. The pseudogene-homolog region of TNXB was analysed by Sanger sequencing in a few large multi-exon amplicons. Deletion/duplication analysis of the TNXB gene was performed using a Multiplex ligation-dependent probe amplification assay (MLPA, MRC-Holland, kit P155, Amsterdam, the Netherlands) and/or a bioinformatic tool for copy number variants (CNVs) detection on the NGS sequencing data (eXome-Hidden Markov Model (XHMM) analysis). Segregation analysis in family members was performed by Sanger sequence or MLPA analysis. Detailed information on the applied methodologies is reported in Appendix S1.
Results
Patients
A total of 20 patients was invited to participate, of whom only 17 were able to participate. This included the five living patients from the original cohort of Schalkwijk et al. 3 Patient 3 from this series was excluded because she had the contiguous-gene syndrome [a homozygous deletion encompassing the CYP21A gene and the TNXB gene 3], and two patients had died (patient 4, male, at the age of 57 due to septic shock following an oesophageal rupture after trans-oesophageal ultrasound; and patient 5, female, at the age of 49 due to deep venous thrombosis and cardiomyopathy). Furthermore, six patients of the subsequent reports from the Radboudumc participated (four reported by Voermans et al., and patient 1 and 2 reported by Hendriks et al.) 7, 10. Finally, nine patients diagnosed with TNX-deficient type EDS based on DNA testing at the DNA diagnostics laboratory of the VUMC were invited to participate through the geneticist who requested the test. Of them, six were able to participate. One patient declined participation because he was undergoing surgery at the time of the study, and two did not respond to the invitation by their geneticist. The invitation posted on the website of the patient organization resulted in three responses of patients in whom only one mutation was detected; they were not included.
In total 17 patients [mean age 34.6 years (range: 6–69 years)] from 11 families were clinically evaluated. All patients had Caucasian ancestry. The results of each patient are presented in Table 1, and Table 2 displays the mutations detected in each patient or family. This cohort consisted of five families (four families with two affected sibs, one with three affected sibs) and six isolated cases.
| Family | Serum TNX | Performed DNA testing | Mutation allele 1 | Predicted effect | Mutation allele 2 | Predicted effect |
|---|---|---|---|---|---|---|
| I 3 | 0 | Sanger sequencing (mutation previously detected in research) | c.3290_3291del p.(Lys1097Argfs*48)a , b | Non-sense mediated mRNA decay | c.3290_3291del p.(Lys1097Argfs*48)a , b | Non-sense mediated mRNA decay |
| II 3 | 0 | NGS analysis (CTD02 platform) + XHMM and MLPA | c.11435_11524 + 30del (TNXA-derived variation, probably due to gene conversion)b , c | Unknown | c.12174C > G p.(Cys4058Trp) (TNXA-derived variation, probably due to gene conversion)b , d , e |
Unknown Variant affects a cysteine residu which is predicted to form a disulfide bond, stabilizing tertiary protein structured |
| III | n/a | NGS analysis (CTD01 platform) + MLPA | c.12553C > T p.(Arg4185*)c , e | Non-sense mediated mRNA decay | c.2590C > T p.(Gln864*)b , c | Non-sense-mediated mRNA decay |
| IV 7 | 0 | NGS analysis (CTD02 platform) + XHMM and MLPA |
TNXB/TNXA fusion gene, probably due to unequal crossover causing a 30 kb deletion encompassing CYP21A2. This fusion gene is characterized by the TNXA-derived variations: c.11435_11524 + 30del and c.12174C > G p.(Cys4058Trp)a , b , f |
Unknown | c.11435_11524 + 30del (TNXA-derived variation, probably due to gene conversion)b , c | Unknown |
| V 7 | 0 | NGS analysis (CTD02 platform) + XHMM | c.903del p.(Tyr301*)c | Non-sense mediated mRNA decay | c.12464-1G > A p.(?)c |
Unknown Use of a cryptic acceptor splice site 1 bp downstream will possibly lead to a frameshift and to non-sense mediated mRNA decay |
| VI 7 | 0 | NGS analysis (CTD02 platform) + XHMM | c.3290_3291del p.(Lys1097Argfs*48)a , b | Non-sense mediated mRNA decay | c.3290_3291del p.(Lys1097Argfs*48)a , b | Non-sense mediated mRNA decay |
| VII 10 | 0 | NGS analysis (CTD02 platform) + XHMM and MLPA | c.3290_3291del p.(Lys1097Argfs*48)a , b | Non-sense mediated mRNA decay |
TNXB/TNXA fusion gene, probably due to unequal crossover causing a 30 kb deletion encompassing CYP21A2. This fusion gene is characterized by the TNXA-derived variations: c.11435_11524 + 30del and c.12174C > G p.(Cys4058Trp)a ,b ,f |
Unknown |
| VIII | n/a | NGS analysis (CTD01 platform) + MLPA | c.2590C > T p.(Gln864*)b , c | Non-sense mediated mRNA decay |
TNXB/TNXA fusion gene, probably due to unequal crossover causing a 30 kb deletion encompassing CYP21A2. This fusion gene is characterized by the TNXA-derived variations: c.11435_11524 + 30del and c.12174C > G p.(Cys4058Trp)a ,b ,f |
Unknown |
| IX | n/a | NGS analysis (CTD01 platform) + MLPA | c.2461C > T p.(Arg821*)b , c , e | Non-sense mediated mRNA decay | c.11435_11524 + 30del (TNXA-derived variation, probably due to gene conversion)b ,c | Unknown |
| X 10 | 0 | NGS analysis (CTD02 platform) + XHMM |
TNXB/TNXA fusion gene, probably due to unequal crossover causing a 30 kb deletion encompassing CYP21A2. This fusion gene is characterized by the TNXA-derived variation: c.12174C > G p.(Cys4058Trp)g |
Unknown Variant affects a cysteine residu which is predicted to form a disulfide bond, stabilizing tertiary protein structureII |
c.12174C > G p.(Cys4058Trp) (TNXA-derived variation, probably due to gene conversion)b ,d ,e |
Unknown Variant affects a cysteine residu which is predicted to form a disulfide bond, stabilizing tertiary protein structured |
| XI | n/a | NGS analysis (CTD01 platform) + MLPA | c.107_108delinsA p.(Ala36Aspfs*68)b , c | Non-sense mediated mRNA decay | c.7826-1G > C p.(?)c , e |
Unknown Use of a cryptic acceptor splice site 2 bps downstream will possibly lead to a frameshift and to non-sense mediated mRNA decay |
- MLPA, multiplex ligation-dependent probe amplification; n/a, not applicable/not available or not assessed.
- a Mutation previously described by Schalkwijk et al. 3.
- b Recurrently identified (either in heterozygous or compound heterozygous/homozygous state) in this study and/or in a cohort of patients with a suspicious diagnosis of EDS (A.M., unpublished data).
- c Not previously described in patients.
- d Variant previously described by Morrissette et al. 15. According to Morrissette et al, and in contrast with our serum measurements, this variant would disrupt TNX function but not affect expression of TNX, suggesting a dominant-negative effect.
- e Low allelic frequency (<0.0005) in ExAc (European, not Finnish population).
- f TNXA/TNXB (CAHX-CH1) in Morrissette et al. 15.
- g TNXA/TNXB (CAHX-CH2) in Morrissette et al. 15.
Medical history
Initial symptoms
Medical history from all patients revealed that clinical onset was in childhood, ranging from the neonatal age to puberty. Initial symptoms consisted of (sub)luxations (most frequently of the shoulder, 10/17 patients), hypermobility (16/17 patients) and velvety and hyperextensible skin (all patients).
Cardiological features
No congenital heart defects were reported. Four patients had hypertension starting in adult life, all treated with medication. Four patients had mitral valve abnormalities: one with prolapse, one thickened mitral valve with mild insufficiency and two patients with mild mitral valve insufficiency. One patient developed a cardiomyopathy post-partum, of which the evaluation is ongoing.
Gastrointestinal features
Severe gastrointestinal problems were not apparent in the patients. Only one patient reported a gastric ulcer at the age of 16 and a bowel perforation due to diverticulitis at the age of 48. Three patients reported rectal prolapse. In addition to our standardized assessment, two patients spontaneously mentioned swallowing difficulties. Evaluation by a speech therapist, including a swallowing video, confirmed reduced swallowing speed in one of them, but a cause could not be identified.
Surgical history
A total of 10 patients had a history of surgery; 4 patients had surgeries involving the hands (fixation of a thumb that spontaneously dislocated and tendon cleavage of fingers). One patient had the fourth digit of the left foot removed due to progressive clawing. Non-EDS related surgeries were ovarian cyst removal and adeno-tonsillectomy.
Gynaecologic and obstetric history
Of the women, who had been pregnant (n = 7; 15 pregnancies), three of seven had post-partum haemorrhage (after eight deliveries; one of them twice) and three of seven had a perineal tear during labour (of seven deliveries). Two other women reported uterine or vaginal prolapse.
Family history
All parents and children of patients are obligate heterozygote carriers. We found that 4 of 11 mothers complained of symptoms possibly related to TNX-deficient type EDS; hypermobility without (sub)luxations (all four), pes planus (n = 1), easy bruising (n = 1), joint pain (n = 1). Of the fathers 5 of 11 had complaints; arthralgia and hyperextensible skin (n = 3), hypermobility (n = 1), pes planus and inguinal hernia (n = 1). Of the patient's siblings 11 of 26 were tested and proven heterozygous carriers. Three of these 11 carrier siblings reported hypermobile joints.
Reported symptoms
Musculoskeletal symptoms
Of the 17 investigated patients, a total of 16 patients complained of hypermobility in one or more joints. (Sub)luxations manifested in 16 patients: the shoulder was most frequently affected whereas the fingers, hip and knee were also commonly affected joints. Interestingly, one patient (age 6 years old) had never suffered any (sub)luxations. Frequent ankle sprains were reported in 10 patients. Bursitis was reported by five patients; four in the elbows and one in the metacarpal/metatarsal joints. Furthermore, 10 patients reported myalgia and 11 complained of joint pain. Fatigability was reported by 14 patients: they complained of an overall fatigued feeling and heavy legs after walking for at least 300 m. Eleven of these 14 patients filled in the CIS questionnaire. The questionnaire revealed that 10 of them experienced an abnormal severity of fatigue, and 7 of these 10 patients scored as severely fatigued.
Mucocutaneous symptoms
All patients mentioned hyperextensible, velvety and soft skin that bruises easily with mild trauma. Spontaneous ecchymoses were reported by 13 patients. Seven of the patients in this cohort reported subjective delayed wound healing. Two patients reported an inguinal hernia (requiring surgical correction) and an umbilical hernia, respectively.
Neurological symptoms
The gross motor development was normal; three patients started walking at the age of 18 months, and all others before that. Of the assessed patients, 5 of 14 complained of paraesthesias in the extremities and 7 patients complained of muscle weakness (mostly a lowered grip strength).
Ophthalmological and audiological symptoms
Only minor ophthalmological problems were encountered. Eight patients had refractive errors for which they used (reading) glasses. Recurrent subconjunctival haemorrhage was explicitly reported by 5 of 17 patients. Hearing was not impaired in any of our patients.
Dental symptoms
Three patients reported frequent caries and enamel defects.
Physical examination
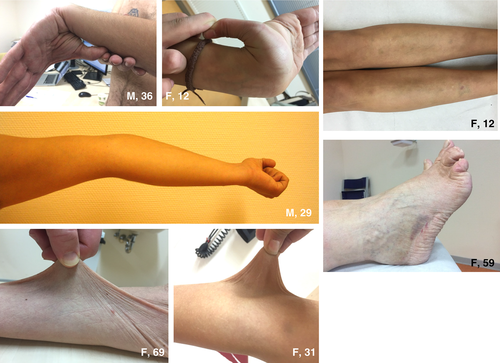
The most frequently observed symptoms during physical examination were hypermobile joints (16/17 patients) (Fig. 1), hyperextensible and velvety skin (17/17 patients) (Fig. 1) and piezogenic papules of the feet (12/17 patients) (Fig. 2). The Beighton score was elevated (a score of ≥5/9) in 16 of 17 patients. Hematomas (Fig. 1) were seen mostly on the legs and gluteal area; atrophic scarring was encountered in none of the patients.
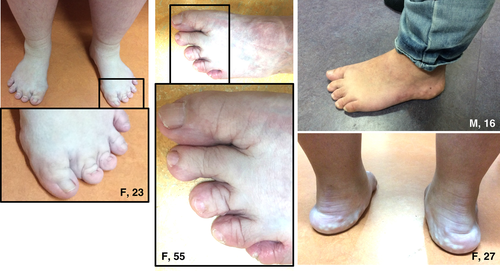
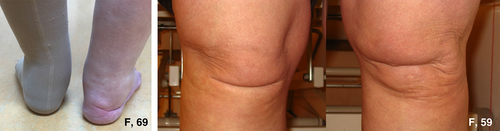
Deformities of the hands and feet were observed frequently (Figs. 2 and 3). A total of 12 patients had pes planus and four patients had short/broad feet with brachydactyly of the toes. One patient had swan neck fingers and clawing of all toes (one removed for this reason), furthermore, she had acrogeric hands with very poor muscle strength. Three more patients had finger deformities, but these were less pronounced: camptodactyly of digits III (n = 1) and broad hands with short fingers (n = 2) (Fig. 3). Interestingly, in 4 of 17 patients mild to severe oedema was seen in the ankles and/or feet and patients reported to experience an increase when standing long or feeling tired (Fig. 4).
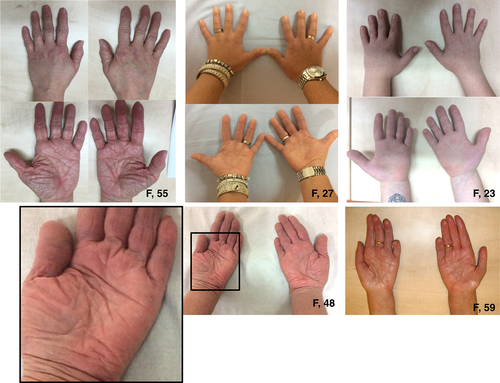
Other interesting features found in some of our patients, although not systematically examined, were the occurrence of axonal neuropathy (n = 4), atrophy of the hand muscles (n = 1), and increased wrinkling of the skin of the hands causing an acrogeric appearance (n = 3).
Laboratory analysis
Serum TNX concentrations were available for 11 of 17 patients from 7 families. All of them showed a complete absence of TNX.
Genetic analysis
Mutation analysis was completed in all 11 families participating in this study, showing homozygous or compound heterozygous TNXB mutations in all families (Table 2). In total, DNA analysis led to the identification of 12 different TNXB mutations, 8 of which had not been described before (Table 2). A 2 bps deletion (c.3290_3291del), two different 30 kb deletions both generating a TNXB/TNXA fusion gene, and a pseudogene-derived missense variant [c.12174C > G p.(Cys4058Trp)] were previously reported 3, 15. The novel sequence variants included four mutations introducing a premature stopcodon [c.903del p.(Tyr301*), c.12553C > T p.(Arg4185*), c.2461C > T p.(Arg821*) and c.2590C > T p.(Gln864*)], two splice site mutations [c.7826-1G > C p.(?) and c.12464-1G > A p.(?)] and a small deletion/insertion [c.107_108delinsA p.(Ala36Aspfs*68)]. Moreover, a novel pseudogene-derived 120 bps deletion (c.11435_11524 + 30del) was found.
Discussion
Clinical features
We performed a cross-sectional study in 17 patients with the TNX-deficient type of EDS to improve clinical recognition of this EDS type. Typical features are generalized joint hypermobility (with or without recurrent dislocations), skin hyperextensibility (with a velvety aspect) and easy bruising. Furthermore, piezogenic papules and brachydactyly of the feet were frequently observed. The TNX-deficient type can be distinguished from the classical type of EDS by the absence of atrophic scarring and an autosomal recessive inheritance pattern. Other frequent recurrent features were foot deformities (pes planus, hallux valgus), oedema in the legs in absence of cardiac failure, mild proximal/distal muscle weakness and complaints of chronic musculoskeletal pain and chronic fatigue. In addition, less common features were deformities of the hands and fingers and increased wrinkling of the skin of the hands and vaginal/uterus/rectal prolapse. Both axonal neuropathy and atrophy of the muscles in hands and feet were observed in this study, although not objectified. More detailed information on these features in Dutch patients with TNX-deficient type EDS can be found in earlier work by Voermans et al. 7.
Most clinical manifestations can be linked to the lack of TNX in connective tissue. The foot and hand deformities (broad feet and hands, and brachydactyly), which are probably to be congenital, point to a role for TNX during development. Interestingly, a previous study showed a high expression of TNX in the interdigital space of the distal forelimb of rat embryos at E15 18. Few craniofacial features were observed (high arched palate and narrow palate with dental crowding), but most patients had no abnormalities. Oedema in EDS patients has rarely been described so far 19. We found peripheral oedema in 24% of our patients. The differential diagnosis of oedema is very broad and further investigations are needed to identify the exact aetiology of peripheral oedema in patients with (TNX-deficient type) EDS. Peripheral blood pooling as part of postural tachycardia syndrome or venous insufficiency related to subcutaneous tissue laxity might play a role 20. Cardiovascular abnormalities included valve abnormalities (24%) and hypertension (24%). These findings are in line with a systematic study on cardiovascular features in this type of EDS, which resulted in the recommendation to perform echocardiography at the first evaluation and when a cardiac murmur appears 4. Furthermore, one patient developed a post-partum cardiomyopathy, and one patient initially reported by Schalkwijk et al. (not included in this study) suffered of cardiomyopathy when she died at the age of 49. It is unclear whether this is related to the TNX deficiency.
Gastrointestinal manifestations consisted of gastrointestinal bleeding (6%), rectal prolapse (18%), diverticulosis or diverticulitis (18%) and haemorrhoids (18%). A recent case report on a TNX-deficient type EDS patient with a gastrointestinal bleeding and a literature review on gastrointestinal symptoms (3 diaphragmatic hernias, 3 diverticuloses, 2 bowel perforations, 1 recurrent rectal prolapse and 1 gastrointestinal bleeding in a total cohort of 19 patients) suggests that gastrointestinal involvement is probably to be more prevalent in this EDS type 13. Furthermore, even rare gastrointestinal complications may occur, as in one of the patients reported by Schalkwijk et al., who experienced an oesophageal rupture after trans-oesophageal ultrasound 10. Recently, we have identified two additional patients with compound heterozygote TNXB mutations (unpublished data). The first patient was diagnosed with classical EDS before he died in his mid-fifties due to an infection following a bowel perforation. Interestingly, post-mortem studies showed aneurysmatic abdominal arteries. In the second patient, TNXB sequence analysis was performed after accidental finding of a heterozygote TNXB deletion during analysis of the COL3A1 gene (probes for both genes are present in the MLPA kit P155 of MRC-Holland). This 58-year-old man presented with an aneurysm of the thoraco-abdominal aorta and aneurysmata of both common iliac artery and the superior mesenteric artery.
Further study is also needed for fatigue in patients with TNX-deficiency as it presents an important clinical problem. The majority of TNX-deficient patients are scoring very high on the CIS fatigue questionnaire. This is in-line with our previous questionnaire study among 273 patients with various types of EDS, which showed severe fatigue in more than three quarters of the patients. The five possible determinants of fatigue were sleep disturbances, concentration problems, social functioning, self-efficacy concerning fatigue, and pain severity. Cognitive behavioural intervention for fatigue in EDS should focus on these aspects 21. Pain was also a frequent symptom (myalgia in 10 and joint pain in 11 patients), in line with our previous study 22. We did not measure Creatine Kinase (CK) levels in this study, because no hyperCKemia was encountered in our previous study on neuromuscular features in 10 patients with this type (median 107; range: 50–287 U/l) 7.
First degree family members may show signs of hypermobility on examination. A formal clinical evaluation was not performed in the first degree relatives of patients in this study, but the family history revealed that in 8 of 33 (24%) carrier individuals joint hypermobility was present (36% of the mothers, 9% of the fathers, 27% of the tested siblings), which is probably to be slightly higher than in the general population. Symptoms of joint hypermobility (dislocations, functional limitations or joint pain) were not reported and relatives were not reported to have paid any medical visits for their joint hypermobility. This was similar as the findings in the 10 haploinsufficient TNXB subjects investigated in this study on neuromuscular features: asymptomatic hypermobility (Beighton score ≥ 5) was detected in four female patients [Beighton scores not published; patients included in study by Voermans et al 7]. In a previous study, haploinsufficiency of TNX was also shown to associate with hypermobility symptoms in relatives of patients with TNX-deficiency 16. Furthermore, patients with congenital adrenal hyperplasia and a contiguous deletion of CYP21A2 and TNXB resulting in TNX haploinsufficiency are reported to have a more severe phenotype 14, 15. This suggests that the endocrine milieu of Congenital Adrenal Hyperplasia (CAH) influences the severity of EDS 14, 15.
These results underlie the need of a further evaluation of the association of TNX haploinsufficiency and the hypermobility type of EDS.
Molecular analysis
In this study, we report the identification of homozygous or compound heterozygous TNXB mutations in all 11 families with TNX-deficient type EDS. This represents the largest group of patients with this type of EDS which has been molecularly characterized so far. In all these patients, homozygous or compound heterozygous mutations in TNXB were detected. In total, 12 different mutations were identified, 8 of which had not been described before. Four of the mutations had been detected at least once in a heterozygous state in a large European population cohort (ExAC). Seven of them were recurrently found (either in heterozygous or compound heterozygous/homozygous state) in this study and/or in a large cohort of patients with a suspicious diagnosis of EDS (unpublished data). The genetic heterogeneity encountered in this cohort confirms the problem of underdiagnosis of this EDS type, and stresses the need of extending clinical and genetic testing outside the Netherlands.
Six of the identified mutations introduce a premature stop-codon and are predicted to result in non-sense mediated decay of the mutant RNA. The splice site mutations identified in this cohort of patients, c.7826-1G>C and c.12464-1G>A, both abolish the consensus sequence of an acceptor splice site. The effect of these mutations has not been studied at RNA level, but splice prediction software suggests the activation of a new cryptic acceptor splice site, respectively two and one base downstream, which is predicted to shift in the reading frame and as such will also result in non-sense mediated mRNA decay. This is in line with the results of serum TNX measurements, that showed a complete loss-of-function of TNX in all tested patients.
More complex is the interpretation of the effect of the TNXA pseudogene-derived mutations in these patients. It concerns here a 120 bp deletion, c.11435_11524 + 30del, which abolishes part of exon 35 en intron 35 of TNXB. This deleted region represents the only large TNXB-specific sequence in the TNXA-homolog region (exons 32–44) of TNXB. The second “TNXA-derived” mutation is a single nucleotide substitution (c.12174C>G), that substitutes a highly conserved cysteine residue with a tryptophan, p.(Cys4058Trp). Cysteine at position 4058 is predicted to be engaged in a disulfide bond with Cys4028, which stabilizes the structure of the C-terminal fibrinogen domain of TNX 15. Both of these TNXA-mutations can be introduced in the TNXB gene by gene conversion. Gene conversion (i.e. the exchange of DNA segments between duplicated genes) is a known phenomenon which often takes place between a functional gene and a pseudogene. This mechanism is probably to be responsible for introducing a TNXA-derived mutation in 5 of 22 mutant alleles in this study (Table 2). Moreover, these TNXA-derived mutations characterize the TNXB/TNXA fusion genes identified in 4 of 22 mutated alleles in this study. These fusion genes have presumably arisen by an unequal crossover, causing in both cases a 30 kb deletion encompassing the CYP21A2 gene. These TNXB/TNXA fusion genes have been previously described by Morissette et al. 15, respectively as TNXA/TNXB (CAHX-CH1) en TNXA/TNXB (CAHX-CH2). TNXA/TNXB (CAHX-CH1), characterized by both the 120 deletion c.11435_11524 + 30del and the c.12174C > G p.(Cys4058Trp) mutation, is the fusion gene originally described by Schalkwijk et al. 3 and has been identified in 3 of 22 alleles in this study. TNXA/TNXB (CAHX-CH2) is characterized only by the c.12174C > G p.(Cys4058Trp) mutation and has been identified in family X in this study, in combination with a c.12174C > G p.(Cys4058Trp) mutation due to gene conversion.
The effect of the 120 bp deletion c.11435_11524 + 30del is not known. However, complete deficiency of TNX in the serum of five patients carrying this deletion in at least one of the mutated alleles strongly suggests that this mutation also results in a null-allele. The same line of reasoning should apply to the c.12174C>G p.(Cys4058Trp) mutation, which is probably to be the causal defect in two patients in this study: patient 3 (family II), who is compound heterozygote for this missense mutation and the 120 bp deletion, both probably arisen by gene conversion events in both alleles; patient 15 (family X) is homozygote for the c.12174C > G p.(Cys4058Trp) mutation, due to a gene conversion event in one allele and a TNXB/TNXA fusion gene in the other allele. In both of these patients, TNX measurement showed previously a complete deficiency of TNX in serum. This is in striking contrast with the results of TNX expression studies in fibroblasts of patients with the contiguous gene deletion syndrome CAH-X and the c.12174C > G p.(Cys4058Trp) mutation. In these patients, western blot analysis of whole-cell lysate showed no alteration of TNX expression 15. Based on these results, Morissette et al. suggest a dominant-negative effect for the c.12174C > G p.(Cys4058Trp) mutation 15. A possible explanation of this discrepancy is that mutant p.(Cys4058Trp) TNX is produced in fibroblast cells but is not secreted from the cells. Also, conformational change of the C-terminal fibrinogen domain, due to the p.(Cys4058Trp) mutation, may affect antibody recognition of the carboxy-terminal fragment of TNX, leading to false-negative TNX serum measurements. Additional studies, e.g. TNX expression analysis in fibroblasts of patient 3 or 15, are necessary to clarify the pathogenetic mechanism of the c.12174C > G p.(Cys4058Trp) mutation.
Diagnostic testing of TNX
Molecular testing of the TNXB gene represents a challenging task for a DNA diagnostic laboratory, due to the presence of a pseudogene (TNXA) which is more than 97% identical to the 3′ end of TNXB (exons 32–44) 3. With the only exception of exon 35, which partially shows a TNXB-specific sequence (due to the presence of a 120 bp deletion in the pseudogene), exon and intron sequences in this region are identical or almost identical in both the gene and the pseudogene. This has implications both for sequencing and deletion/duplication analysis.
- Sequence analysis of TNXB. Two approaches can be chosen: (i) Sanger sequencing of the entire TNXB gene; (ii) NGS analysis of the entire TNXB gene + Sanger sequencing of the pseudogene-homolog region. The NGS approach has the advantage of allowing simultaneous analysis of all known EDS-related genes, allowing rapid achievement of a molecular diagnosis when recognition of the type of EDS based on clinical examination is uncertain.
- TNXB deletion/duplication analysis. The importance of deletion/duplication analysis in TNX-deficient patients is underlined by this study, as large deletions in TNXB were identified in 7 of 22 alleles. Deletion/duplication analysis should be performed using an exon specific CNV detection tool. Suitable for this purpose is the MLPA.
TNX concentration measurements in serum of patients with suspected TNX-deficient type EDS can be a valuable diagnostic tool when DNA testing is not available. Furthermore, because of the difficulties encountered in DNAtesting it can also be favourable to first (or in parallel) assess serum TNX concentrations in order to make the diagnosis, as this is a much faster technique.
In fact, due to the difficulties in DNA testing, TNXB analysis is probably to detect none or one of the TNXB mutations in some of the patients. The complete absence of TNX in serum confirms the diagnosis in these cases. Moreover, this test can be useful for the interpretation of variants of unknown significance. Therefore, this test should be available in a number of specialized laboratories.
Schalkwijk et al. used a sandwich enzyme-linked immunosorbent assa (ELISA) test and detected TNX also in serum. The level of TNX in serum probably reflects the level of synthesis and/or breakdown at the tissue level. For a more detailed description of the mutation analysis and of TNX measurement in serum we refer to Appendix S1.
Conclusions
Patients with TNX-deficient type EDS typically have generalized joint hypermobility [with or without (sub)luxations], skin hyperextensibility and easy bruising. Other common features are foot and hand deformities (piezogenic papules, pes planus, hallux valgus, broad forefeet, brachydactyly, and acrogeric skin of hands), neurological symptoms including muscle weakness and severe fatigue. In contrast to the classical type of EDS, the inheritance pattern is autosomal recessive and atrophic scarring is absent. Asymptomatic generalized joint hypermobility was reported in approximately a quarter of the first degree family members (who are obligate heterozygous TNXB mutation carriers). The differential diagnosis includes collagen six myopathies (Bethlem myopathy), the vascular type of EDS (in patients with gastrointestinal bleedings), and the classical type EDS (in case of an uninformative family history).
Homozygous or compound heterozygous point-mutations and deletions, triggering non-sense mediated decay of mutant TNXB-RNA are usually found in these patients. Nowadays, NGS offers a valuable tool allowing simultaneous analysis of all known EDS-related genes and rapid achievement of a molecular diagnosis when recognition of the type of EDS based on clinical examination is uncertain. However, DNA testing of TNXB remains challenging and TNX-serum concentration measurement can offer an advantageous alternative, allowing to achieve a diagnosis when DNA analysis is not available. Due to the limited awareness for this type among clinical geneticists and other clinicians and the challenge of the molecular analysis of TNXB, the TNX-deficient type EDS is probably to be underdiagnosed in most parts of the world.
Acknowledgements
The authors would like to thank the patients for their kind cooperation. We thank M. Smit and L. van Sornses de Koste for expert technical contribution, Y. Cornelissen for her logistical support in organizing the study, and Dr A. Plomp and Dr M. Baars for their communication on the additional patients mentioned in the discussion. Travel expenses were covered by the Department of Neurology of the Radboudumc, Nijmegen, the Netherlands.
Author contributions: S. D. contributed in planning, conducting and reporting. E. D. contributed in conducting and reporting. L. R. contributed in conducting and reporting. M. K. contributed in conducting and reporting. D. B. contributed in conducting and reporting. D. M. contributed in conducting and reporting. B. G. E. contributed in planning and reporting. B. H. contributed in planning and reporting. J. S. contributed in reporting. B. L. contributed in conducting and reporting. A. M. contributed in conducting and reporting. N. C. V. contributed in planning, conducting and reporting.




