Lynch syndrome: five unanswered questions
Despite the great advances that have occurred in the century following the first description of a Lynch syndrome (LS) family and more especially, in more than 20 years since the discovery of causal mutations in the mismatch repair genes (MMR), long-standing clinical questions remain unanswered. Moreover, as a result of novel technologies, new questions have arisen. With this commentary, we aim to briefly cover some of these aspects of LS. We will focus on current challenges regarding the definition of LS-related tumors, their prevention and early detection, the role of next-generation sequencing (NGS) in the molecular diagnostics of LS and advances in the treatment of LS tumors.
Tumor spectrum of LS: is there a risk for breast cancer?
Although LS was initially thought of as a colorectal cancer (CRC) syndrome, the established spectrum of LS-associated tumors includes endometrial, stomach, ovarian, pancreas, ureter and renal pelvis, biliary tract, and brain (Turcot syndrome) tumors, sebaceous gland adenomas and keratoacanthomas (Muir–Torre syndrome), and carcinoma of the small bowel 1. Rarely, MMR deficiency has been found in other tumors 2, including breast cancer (BC). As BC is a common diagnosis, its inclusion or exclusion as a LS-related tumor is perhaps the most important ‘LS tumor spectrum’ question.
Recently, in an effort to resolve this matter, Win et al. put together the results of all published studies analyzing the role of BC in LS 2, including 15 molecular studies reporting microsatellite instability (MSI) and/or immunohistochemistry (IHC) in BC tumors of MMR gene mutation carriers, and 21 studies that investigated the risk of BC in a LS context. Fifty-one percent of breast tumors showed MMR deficiency, suggesting its involvement in the breast tumorigenesis of some LS individuals 2. Regarding the risk assessment, they concluded that an increased risk for BC was shown in 8 of the 21 epidemiological studies. In the only prospective study, an increased risk of BC was observed for MMR gene mutation carriers without prior diagnosis of cancer compared to non-carrier unaffected family members 3. Further risk studies need to be done in order to elucidate if, for example, periodic breast magnetic resonance imaging should be indicated in MMR mutation-carrying women 2. Studies of BC in LS families that limit analysis to breast tumors that are deficient in MMR might be helpful in answering this question. Until such time as more definitive data are available, immunohistochemical evidence of MMR deficiency in a breast tumor in a LS family may suggest that MMR deficiency is driving tumorigenesis.
Can LS-associated cancers be diagnosed early or prevented?
The initial colorectal surveillance protocol consisting of colonoscopic examination every 3–4 years has been revised. Three prospective and one retrospective studies that analyzed the effectiveness of colonoscopic surveillance observed advanced CRC detected between 2 and 3 years after colonoscopy, which led a group of European experts to recommend to reduce the interval to 1–2 years 4. Although this pattern was recently supported by a meta-analysis pooling 1114 cases, the authors suggested that this regimen might not be justifiable for young individuals in their 20s, due to the low 5-year CRC risk of these patients 5. The small number of studies that have been conducted to prevent gynecological cancers in LS patients do not permit evidence-based decisions 4. However, it is accepted that gynecological examination, transvaginal ultrasound and aspiration biopsy from 30 to 35 years should be offered to mutation carriers every year to diagnose early endometrial and ovarian cancers 4, 6, 7. Importantly, prophylactic hysterectomy and salpingo-oophorectomy should be strongly considered when childbearing is completed or at the age of 40 4, 6, 7.
A large proportion of deaths in LS are associated with extra-colonic and extra-endometrial cancers, and the benefit of surveillance for these cancers, however, is still unknown and should only be performed in a research setting and in the context of family history 4, 6. Despite this, for gastric cancer, some experts recommend the search for the presence of Helicobacter pylori as well as upper gastrointestinal endoscopy every 1–3 years 4, 6. Additionally, the risk of developing a specific cancer and the age of onset might change between the different MMR defects; therefore, specific preventive measures considering the MMR gene that is mutated have been discussed 7, 8. Growing but not conclusive evidence exists that the use of relatively high-dose of aspirin is beneficial in preventing cancer in LS patients 4, 7.
Should all colorectal and endometrial carcinomas be tested for MMR proteins?
Traditionally, clinical criteria (Amsterdam and Bethesda guidelines) and/or, more rarely, computer modeling assessed risks (MMRpredict, MMRpro, PREMM1,2,6) have helped to identify those patients who should undergo tumor testing by MSI and/or IHC – the gold standard techniques to identify a possible underlying inherited defect in an MMR gene 7. However, these guided approaches to detect LS patients have been criticized for non-optimal sensitivities and specificities as well as for the difficulty and the costs of obtaining reliable family history 7, 9. Universal testing of MMR deficiency in all newly diagnosed CRCs, or in CRCs diagnosed at ≤70 years, and in individuals older than 70 years who have a family history concerning for LS has been proposed as a cost-effective approach 4, 6, 7. Similarly, a universal approach to identify LS has been proposed for endometrial carcinoma (EC) through MSI and/or IHC 10.
Although universal testing is presently recommended by most experts worldwide, it has not been broadly implemented 11. Its development requires cooperation and effective communication across multiple disciplines, ensuring identification, notification and proper referral of affected patients to genetic counseling and genetic testing units 10, 11. Moreover, the current revolution of NGS may result in germline testing of all CRC and EC patients becoming the most cost-effective approach to identify LS patients 12 (Fig. 1).
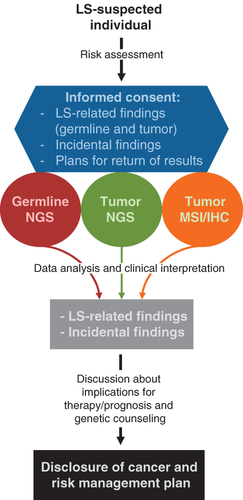
Are we ready to properly implement NGS into the diagnostics of LS?
NGS technologies now allow simultaneous analysis of multiple susceptibility genes at a cost that is modestly greater than single-gene testing representing an alternative strategy to the standard approach of serial testing, which is time consuming and expensive 13-15. NGS strategies that are currently used in cancer predisposition genetic screening go from predefined gene panels to whole-exome sequencing, the analysis of which can also be restricted to preselected genes. High-penetrance and/or unknown significant variants will appear in genes that might or might not be linked with the disease or in genes that confer only a moderate risk. Established cancer genetic testing and counseling models were not designed in this high-throughput context, thus, a putative increased distress due to receiving unsolicited results is a concern 14, 16. To address this, Sie et al. evaluated patient experiences with gene panels based on exome sequencing and concluded that most adult patients report high satisfaction and low distress after results acquirement 17. Whether or not incidental findings of pathogenic mutations should be transmitted to patients has been widely debated 18 but discussing the possibility of identifying variants of unknown significance, particularly in the less well-studied genes, will probably pose even greater challenges 15.
LS is associated with mutation in at least five genes, so could benefit from a panel-based NGS approach. In this multiplex testing context, LS predisposition is studied in the more general framework of inherited CRC, thus not limiting the analysis to LS-causing genes, but extending it to other CRC syndromes and their high-penetrant causing genes, as well as to other genes with moderate penetrance 13, 19, 20. Given the increased perception that the broad spectrum of LS cancers can overlap with other hereditary cancer syndromes 21-23, the use of multiple gene panels can help to elucidate the genetic cause in patients that would have not been diagnosed by using a gene-by-gene limited screening. Somatic NGS panels 15 as well as MSI/IHC testing should also be taken into account in a NGS-based LS-predisposition model (Fig. 1). Implementing such a comprehensive model, however, might be difficult in a publicly funded health system.
Should treatment of cancer be influenced by MMR status?
LS tumors are MMR deficient due to biallelic inactivation of MMR genes. Therefore, secondary unrepaired mutations become scattered throughout the genome enhancing the likelihood of oncogenes and tumor suppressor genes to be targeted. This particular mutator phenotype, appearing also in sporadic tumors due to somatic biallelic inactivation of MMR genes through mutation or hypermethylation 24, 25, has the potential to be exploited to develop targeted therapies and can easily be recognized in tumors through the presence of MSI 26. MSI has been confirmed as an independent good prognostic marker and although it has been proposed that it should guide stage-adjusted treatment decisions, its correlation with the response to 5-fluorouracil (5-FU) treatment remains puzzling 26, 27. Specific response to other commonly used chemotherapeutic drugs has been seen in MMR-deficient tumors and has been discussed by Hewish et al. 26. One of the most promising ways to exploit sensitivity to chemotherapy for MMR-deficient tumors is probably through synthetic lethal strategies 26. While MMR deficiency is compatible with cellular viability, the additional loss of other genes, proteins or pathways may lead to cell death. Synthetic lethal relationships have been shown between MMR-deficient cells and inhibition of PINK1 28, treatment with methotrexate 29, and cytarabine-based chemotherapy 30. Last but not least, a recent study has shown why MSI CRCs are not naturally eliminated despite their particularly active Th1/CTL immune microenvironment: high expression of checkpoint molecules PD-1, PD-L1, CTLA-4, LAG-3, and IDO occurs and creates an immunosuppressive microenvironment that may help MSI tumors evade immune destruction by the infiltrating immune cells 31, 32. Some of these immune checkpoints are currently being targeted with inhibitors 33. Importantly, these findings clearly point the MSI subset of CRCs as good candidate tumors to benefit from checkpoint immunotherapy 31, 32.
Concluding remarks
The next steps in LS management will focus on our capability to correctly interpret and responsibly transmit the huge amount of data generated by NGS approaches which will accelerate the diagnostic aspect of patient management. Prevention via established and novel agents can then be effectively and widely introduced. The recent exciting findings on MMR-deficient tumor microenvironment will translate into novel and original approaches to specifically treat MMR-deficient tumors.
Acknowledgements
E. C. is a recipient of a Marie Curie International Outgoing Fellowship (PIOF-GA-2012-327193), co-sponsored with the Catalan Institute of Oncology-Bellvitge Institute for Biomedical Research, Barcelona, Spain, and is a recipient of a travel grant by the Ministère des Relations internationales et de la Francophonie-Comité mixte Québec-Catalogne (07.307). W. D. F. is partially funded by the Ministère de l'enseignement supérieur de la recherche de la science et de la technologie du Quebec (PSR-SIIRI-846).




