Focal dysplasia of the cerebral cortex and infantile spasms associated with somatic 1q21.1-q44 duplication including the AKT3 gene
Abstract
Somatic and germline duplications or activating mutations of AKT3 have been reported in patients with hemimegalencephaly and megalencephaly. We performed array comparative genomic hybridization on brain tissue and blood in 16 consecutive patients with symptomatic epilepsy due to focal or multilobar malformations of cortical development who underwent surgical treatment of epilepsy. One patient with infantile spasms and a dysplastic left frontal lobe harboured a somatic trisomy of the 1q21.1-q44 chromosomal region, encompassing the AKT3 gene, in the dysplastic brain tissue but not in blood and saliva. Histopathology revealed severe cortical dyslamination, a thin cortex in the premotor area with microgyri and microsulci, immature neurons with disoriented dendrites and areas of cortical heterotopia in the sub-cortical white matter. These cytoarchitectural changes are close to those defining type Ib focal cortical dysplasia. Immunohistochemistry in brain specimens showed hyperactivation of the PI3K/AKT/mTOR pathway. These findings indicate that AKT3 upregulation may cause focal malformations of cortical development. There appears to be an etiologic continuum between hemimegalencephaly and focal cortical dysplastic lesions. The extent of brain malformations due to AKT3 upregulation may be related to the embryonic stage when the post-zygotic gene alteration occurs.
Focal cortical dysplasia (FCD) is the most frequent histological finding in paediatric patients undergoing epilepsy surgery and is classified in different clinico-pathological subtypes 1. In spite of the overwhelming literature on FCD, current knowledge of the molecular pathology for distinct entities is still limited 2.
Recent observations suggest that hyperactivation of the PI3K/AKT/mTOR pathway plays an important role in brain overgrowth. Trisomy of chromosome 1q, encompassing many genes, including AKT3, and activating mutations in AKT3, PIK3CA and mTOR genes have been identified as somatic mosaicism in dysplastic brain tissue surgically removed from patients with hemimegalencephaly 3, 4. A germline, de novo duplication of 3 Mb in 1q43q44 containing 15 genes, including AKT3, was identified in a patient with megalencephaly 5. De novo, germline or post-zygotic mutations in AKT3, PI3KR2 and PIK3CA have been identified in patients with megalencephaly–capillary malformation syndrome (MCAP) and megalencephaly–polymicrogyria–polydactyly–hydrocephalus syndrome (MPPH) 6, 7.
A possible involvement of the PI3K/AKT/mTOR pathway has also been hypothesized in type II FCD, in which an altered expression or subcellular localization of proteins belonging to this pathway has been demonstrated 8.
Using an array-comparative genomic hybridisation (array-CGH) approach on 16 patients who underwent surgical treatment of epilepsy, we identified a patient with cortical dysplasia in the left frontal lobe and intractable infantile spasms harbouring a trisomy of the 1q21.1-q44 chromosomal region in the removed brain tissue, but not in blood or saliva.
Immunohistochemistry analysis of the dysplastic brain specimens revealed hyperactivation of the PI3K/AKT/mTOR pathway in neurons. This observation suggests that mosaic duplications of AKT3 may have a causative role for focal as well as major hemispheric malformations of cortical development.
Subjects and methods
Subjects
We performed array-CGH on 16 consecutive patients with symptomatic epilepsy due to focal or hemispheric malformations of cortical development (Table S1, Supporting Information) who underwent surgical treatment of epilepsy. Blood, brain and saliva samples were obtained from patients after informed consent, according to the guidelines of the Human Research Ethics Committee of the A. Meyer Children's Hospital. Genomic DNA was extracted from brain specimens using the QIAamp DNA Mini Kit (Qiagen, Hilden, Germany), from blood leukocytes using an automated DNA isolation robot (QIASymphony; Qiagen), and from saliva using the Oragene Kit (DNA Genotek; Kanata, Ontario, Canada), according to the manufacturer's protocol.
Array CGH analysis
Array-CGH was performed using the Agilent Human Genome CGH Microarray Kit 4x180K (Agilent Technologies, Santa Clara, CA), following the manufacturer's protocols. Briefly, 500 ng of purified DNA of a patient and a control (Agilent) were double digested with RsaI and AluI enzymes (Agilent) for 2 h at 37°C. Each digested sample was labelled for 2 h with the Agilent Genomic DNA Labelling Kit, using Cy5-dUTP for the patient DNA and Cy3-dUTP for the control DNA. Labelled products were purified and prepared according to the Agilent protocol. After probe denaturation and pre-annealing with 50 µg of Human Cot-1 DNA (Invitrogen, Carlsbad, CA), hybridization was performed at 65°C for 24 h in a rotating oven. After two washing steps the array slide was scanned with the Agilent C Scanner (Agilent). The spot intensities were measured and the image files quantified using the Agilent Feature Extraction 10.5 software. Text outputs from the quantitative analyses were imported into Genomic Workbench Standard Edition 5.0 software (Agilent). Copy number variation (CNV) calls were filtered to eliminate events comprising <5 probes and events with >70% overlap with a CNV detected in healthy subjects from the database of genomic variants 9. Breakpoint positions were reported according to Hg 19, build 37.
Microsatellite analysis
Five commercial, highly polymorphic fluorescent-labelled markers (ABI PRISM Linkage mapping sets v 2.5; Life Technologies, Foster City, CA) were polymerase chain reaction (PCR) amplified and analysed on an ABI Prism 3130XL sequencer (Life Technologies), according to the manufacturer's protocol. We selected two markers (D1S450 and D1S2726), mapping on the short arm of chromosome 1 and three markers (D1S213, D1S2800 and D1S2836), mapping on the long arm. Peak intensity was evaluated using the Peak Scanner software (Life Technologies).
Quantitative PCR (qPCR) analysis
qPCR was performed on DNA extracted from dysplastic brain tissue and from blood using oligonucleotides mapping on chromosome 1p31.3 and 1q44. Amplification was carried out using SYBR Green chemistry (Roche, Mannheim, Germany) on a Light Cycler 480 (Roche). DNA from a control individual (Agilent) was used for comparison. All experiments were performed in quintuplicate. Primers and qPCR conditions are available upon request.
Neuropathology
Formalin-fixed, paraffin-embedded sections from clinical resection specimens were cut at 5 µm thickness and stained with haematoxylin and eosin for anatomopathological evaluation. Additional sections were used for immunohistochemical analysis using commercially available antibodies against neurofilaments (1/50, Invitrogen), Neu-N (1:100, Millipore, Billerica, MA), phosphorylated ribosomal protein S6 (Ser240/244) (1:1200), mTOR (1/100), AKT (1:100), phospho-AKT (Thr308) (1:100), phospho-PDK1 (Ser241) (1:100), PI3 Kinase p110α (1:100) and PTEN (138G6) (1:100) (Cell Signalling Technology, Danvers, MA). Images were acquired using an Eclipse 80i microscope (Nikon Corporation, Tokio, Japan) with Digital Sight DS-U1 camera (Nikon) or an IX51 microscope (Olympus America, Center Valley, PA) with Retiga 2000R camera (QImaging, Surrey, BC, Canada).
Results
Genetic study
Array-CGH analysis, performed in paired blood/brain samples, revealed a mosaic duplication of about 105 Mb (hg19, Chr1:144009707-249212809) in 1q21.1-q44 in the brain of 1/16 (≈6%) screened patients (Fig. 1a). The duplication was confirmed by analysing a series of highly polymorphic microsatellite markers distributed along the short and the long arm of chromosome 1 in DNA extracted from brain, blood and saliva. Microsatellite analysis revealed the duplication in DNA extracted from brain specimens, but not in DNA extracted from blood or saliva (Fig. 1b). Using qPCR, we estimated a copy number of 2.69 in the dysplastic brain (Fig. 1c). This value is consistent with mosaic duplication in which about 70% of cells carry the trisomy.
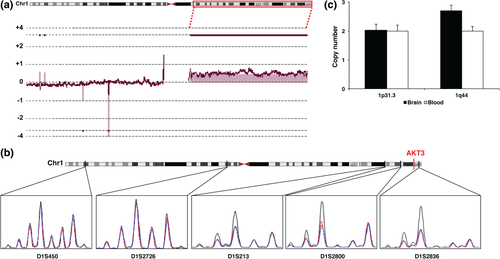
Array-CGH analysis revealed additional mosaic CNVs on chromosome 5 and chromosome 17 in the brain of 5/16 patients (≈31%), although neither qPCR analysis, nor Sanger sequencing of high heterozygosity rate SNPs mapping in these regions could confirm these findings (data not shown).
Clinical findings
The patient harbouring the duplication (patient 7, Table S1) was an 8 years old girl with an uneventful family history. The patient did not exhibit any dysmorphic feature or extra-neurological developmental abnormality. Since age 7 months, she manifested clusters of asymmetric epileptic spasms. Brain magnetic resonance imaging (MRI) revealed an area of increased cortical thickening and abnormal sulcation with increased signal intensity on T1-weighted and fast inversion recovery, extending from the left frontal pole to the ipsilateral rolandic fissure (Fig. 2a–c). Neurological examination revealed slowness and awkward distal movements of the right hand. Neuropsychological assessment revealed mild–moderate intellectual disability with fluent aphasia and constructive apraxia. Because spasms were drug-resistant, the child underwent pre-surgical evaluation. Video-electroencephalography (EEG) monitoring captured several clusters of spasms.
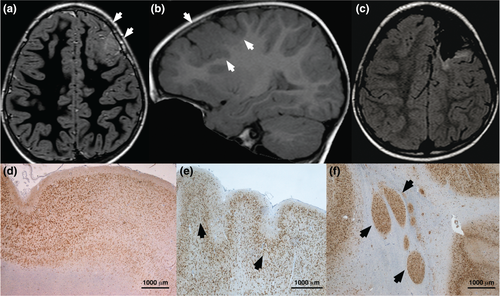
To better define the area of resection and possibly spare the sensori-motor cortex, we explored the left fronto-temporal area using 14 stereotactically implanted depth electrodes. Surgery, tailored according to anatomical and neurophysiological data, included the left prefrontal and premotor areas (Fig. 2c). Three years after the operation, the child has been seizure free on medication, except for a cluster of focal motor seizures involving the right arm, appearing upon attempted drug withdrawal.
Neuropathological analysis
Neuropathology revealed severe disorganization of cortical layering, a thin cortex in the premotor area, with microgyri and microsulci and small areas of lentiform cortical heterotopia in the sub-cortical white matter (Fig. 2d–f). Under the molecular layer, there were areas containing medium and small sized neurons, distributed in a single layer organization, intermingled with disorganized areas featuring crowded neurons and areas of sparse neuronal arrangement in a thin layer (Fig. 2d,e). Immature neurons with disoriented dendrites were spread across the specimen (Fig. 3a). We could detect no microcolumnar neuronal arrangement, dysmorphic neurons, balloon cells or abnormal findings in glial or vascular epithelial cells. Cytoarchitectural abnormalities were overall consistent with FCD type Ib, associated with subcortical heterotopia.
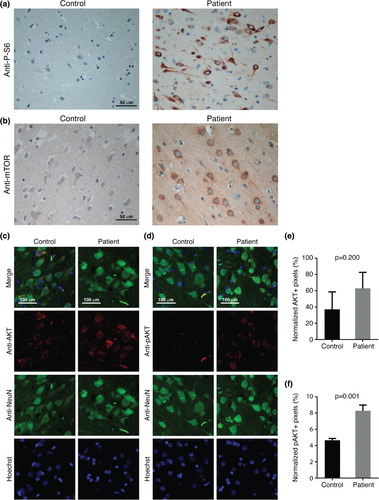
Immunohistochemistry for antibodies against phosphorylated ribosomal protein S6 (P-S6), AKT, its activated, phosphorylated form (pAKT), mTOR, PI3K (p110α), PTEN and phospho-PDK1 revealed an increased immunosignal for all antibodies (Fig. 3, Fig. S1). These findings confirm a possible hyperactivation of the PI3K/AKT/mTOR pathway and are in line with hyperexpression of PS-6 observed in hemimegalencephaly 3 as well as hyperactivation of phospho-AKT and phospho-PDK1 observed in balloon cells of type IIb FCD 10.
Discussion
We investigated the possible role of somatic CNVs in causing focal or hemispheric malformations of cortical development in 16 patients who had been surgically treated for their epilepsy and identified a duplication of chromosome 1q21.1-q44 in the dysplastic frontal lobe of a child with intractable infantile spasms. The observation that the 1q21.1-q44 duplication could not be demonstrated in DNA extracted from blood leukocytes and from saliva show somatic mosaicism in the dysplastic brain tissue. Chromosome 1q21.1-q44 contains more than 450 genes (http://genome.ucsc.edu/cgi-bin/hgTables). Although more than a single gene included in this large genomic interval might exert a dosage sensitive effect on the brain, AKT3, mapping to 1q34-q44, is a strong candidate for causing the observed phenotype. AKT3 is predominantly expressed in the brain and encodes a serine/threonine kinase that mediates the activation of mTOR 11. De novo, somatic, activating point mutations in AKT3 or trisomy of the long arm of chromosome 1 encompassing this gene have been identified in hemimegalencephaly 3, 4, a malformation involving one entire cerebral hemisphere but sharing several histopathological features with FCD. The mosaic duplication we identified appears to be overlapping, in terms of breakpoints, to those described by Poduri et al. 4, albeit it cannot be firmly established whether these duplications involve the centromere and thus the entire 1q arm.
Neuropathological analysis of dysplastic brain specimens in our patient revealed severe cortical dyslamination with immature neurons and neurons with disoriented dendrites, a thin cortex in the premotor area with microgyri and microsulci and subcortical neuronal heterotopia. All of these features have also been reported in hemimegalencephaly 3, 12, while cortical dyslamination with immature neurons and neurons with disoriented dendrites are also observed in type Ib FCD 1.
Immunohistochemistry demonstrated an aberrant activation of the PI3K/AKT/mTOR pathway, as also reported in dysplastic brain specimens in hemimegalencephaly and in balloon cells of type IIb FCD 3, 10. Therefore, the malformation spectrum associated with AKT3 upregulation appears to be broad, ranging from megalencephaly, observed in association with chromosome 1q gains detected in blood cells 5 to hemimegalencephaly down to focal dysplasia as a consequence of post-zygotic alterations in the malformed brain tissue.
Studies on human embryonic pluripotent stem cells and human neural stem cells have showed that gains of chromosome 1q are recurrent chromosomal aberrations in these cell lines 13, 14. It has been suggested that recurrence of this genomic instability might imply that gains of chromosome 1q endow a clonal advantage to cultured cells 13, 14. This observation might explain why in both hemimegalencephaly and cortical dysplasia there appears to be increased cell proliferation, with increased volume of the involved brain tissue 2.
Both histopathological and genetic findings support the hypothesis of an etiologic continuum between hemimegalencephaly and FCD in which phenotype variability may be related to the embryonic stage in which the post-zygotic gene alteration occurs and consequently to the number of brain cells harbouring it. Focusing on duplications involving AKT3, those that are present in one of the two gametes or that occur early in post-zygotic life are transmitted to all daughter cells or to a large number of them and might result in megalencephaly or hemimegalencephaly 4, 5, 15. Mutations or duplications occurring later during development are transmitted to a smaller number of daughter cells and might result in focal anomalies, as observed in our patient.
False positive array CGH results identified on chromosomes 5 and 17 in the brain of 5/16 patients may have different explanations. First, array CGH analysis has technical limitations and certain regions of the genome carry a higher likelihood of generating false positive or false negative results 16. Single-cell sequencing of endogenous human frontal cortex neurons has showed that 13–41% of neurons have at least one megabase-scale de novo CNV 17. This high level of mosaicism in neurons might introduce biases in the hybridization of array CGH probes in DNA extracted from brain samples with respect to blood leukocytes, resulting in false positive results.
The finding that only 1 of 16 screened patients harboured the 1q21.1-q44 somatic duplication suggests that focal malformations of cortical development are aetiologically heterogeneous. Nevertheless, removed dysplastic brain tissues are inhomogeneous samples, as they contain a mixture of wild type and mutated cells and a low percentage of mutated cells may result in normal array-CGH findings. In addition, the 4x180k Microarray Kit, which contains 180,880 probes with a median probe spacing of 17,627 bp, has a relatively low sensitivity to detect CNVs. Further studies with microarray kits with higher sensitivity (i.e. 1 × 1 M array kit, which contains 974,016 probes with a median probe spacing of 2,092 bp) might identify more CNVs.
Combined genome and exome sequencing studies of dysplastic tissues at single cell level will be the next fundamental step, as a complement to array-CGH studies, to quantify somatic mutation rates in different brain malformations. Single cell studies will be also fundamental to identify specific progenitor cells in which somatic genetic defects occur, providing the opportunity to unravel which cell lineages are involved in the development of focal malformations.
Acknowledgements
We gratefully acknowledge the patients and their families for participating in the research. This work was supported by funding from the European Research Projects on Rare Diseases (E-Rare-2, TUB-GENCODEV, 11-027) to R. G. and from the European Union Seventh Framework Programme FP7/2007-2013 under the project DESIRE (grant agreement n°602531) to R. G.




