Patients With Hereditary Hemorrhagic Telangiectasia Diagnosed by Nanopore Long Read Sequencer
Funding: This work was supported by Japan Agency for Medical Research and Development (JP24ek0709672), and JSPS KAKENHI, Grant-in-Aid for Scientific Research (C) (23K09446).
ABSTRACT
Hereditary hemorrhagic telangiectasia (HHT) is an autosomal dominant disorder characterized by excessive and frequent epistaxis, telangiectasia, and visceral involvement, with a strong positive family history. The genes responsible for HHT are endoglin (ENG), activin A receptor-like kinase 1 (ACVRL1 or ALK-1), and SMAD4 are known. As opposed to epistaxis and telangiectasia, whether or not the disease is prone to any visceral lesions depends on the causative gene. Arteriovenous malformations (AVMs) of the brain and lung are common in ENG, hepatic AVMs and cutaneous telangiectasia are common in ACVRL1, and juvenile polyposis (JP-HHT) is characteristic in SMAD4. Therefore, the diagnosis of HHT and its subclasses in patients with recurrent epistaxis is clinically important for predicting cerebral and visceral complications and for prophylactic medical or surgical treatment. Genomic techniques for the molecular diagnosis of HHT have advanced remarkably in recent years. Long-read sequencing using nanopore technology is now recognized as a new and effective approach for the detection of structural aberrations, such as large deletions, that cannot be detected by conventional short-read analysis. In particular, a new technique called adaptive long-read sequencing can selectively sequence only pre-defined target regions. Here we report two patients with HHT in whom no variants were found by short-read targeted sequencing, but structural variants were detected by adaptive nanopore sequencing. These molecular diagnostic results were used in their clinical management.
1 Introduction
Hereditary hemorrhagic telangiectasia (HHT) is an autosomal dominant disorder characterized by excessive and frequent epistaxis, telangiectasis, visceral lesions, and a strong positive family history [1]. The known disease-causing genes of HHT include endoglin (ENG), activin A receptor type like kinase 1 (ACVRL1 or ALK-1), and SMAD4 [2-4]. Patients with variants in ENG, ACVRL1, and SMAD4 are classified as HHT1, HHT2, and JP-HHT, respectively [5].
The common shared symptoms among the three types are epistaxis and telangiectasias, but the susceptibility to accompanying visceral lesions varies depending on the causative gene: ENG variants are frequently associated with arteriovenous malformations (AVMs) in the brain and lungs and ACVRL1 variants are frequently associated with liver AVMs and skin telangiectasis [6]. SMAD4 variants are characterized by juvenile polyposis (JP-HHT) [4]. Hence, making diagnosis of HHT and its subclass in patients with history of recurrent epistaxis is clinically relevant in predicting cerebral and visceral complications and preventive medical or surgical management.
Molecular diagnosis of HHT has traditionally been carried out by the Japanese insurance-covered gene panel testing based on short-read targeted sequencing. Short-read targeted or exome sequencing is a method in which synthesized nucleic acid capture probes are used to enrich targeted exonic regions through hybridization, followed by short-read next-generation sequencing to analyze these regions. This method can detect single nucleotide variants, small insertions and deletions, and large copy number variations involving several exons. However, as previously reported, structural variants containing only a short single exon are difficult to detect using tools that analyze exonic data [7].
In contrast, adaptive sampling using nanopore long-read sequencing enables selective enrichment of specific genomic regions by electronically defined targeted regions for sequencing. The most notable difference from short-read sequencing is the ability to sequence long DNA fragments, which facilitates effective detection of structural variants [8, 9]. A recently developed modification called adaptive long read sequencing allows sequencing of predefined targeted regions, rather than the entire genome, and lowers analytic costs to a clinically accessible level [10].
Here, we report on two HHT patients in whom no variants were found by targeted sequencing, but structural variants were detected using the adaptive nanopore sequencing method. These molecular diagnostic results were then utilized in their clinical management.
2 Case Presentation
2.1 Patient 1
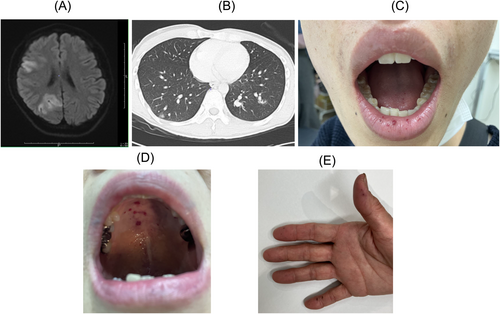
A 28-year-old male, with a history of daily episode of epistaxis since childhood, developed sudden consciousness disturbance, left hemiplegia, and was diagnosed as having right middle and posterior cerebral artery territory infarction (Figure 1A). The infarction was attributed to paradoxical embolism caused by pulmonary arteriovenous fistula (Figure 1B). On physical examination, telangiectasis on the lips, tongue, and oral mucosa were observed (Figure 1C). Imaging studies showed multiple pulmonary arteriovenous fistulas were observed as visceral lesions (Figure 1B), but no liver AVMs were noted. His father and paternal grandmother had a history of frequent epistaxis as well. He met the Curaçao Diagnostic Criteria for HHT. However, the Japanese insurance-covered gene panel testing specific for HHT (ENG, ACVRL1, SMAD4, and BMPR2) using short-read sequencing was negative. In this gene panel testing, copy number analysis was not performed due to the difficulty of achieving high-accuracy detection.
The molecular research protocol was approved by The World Medical Association Declaration of Helsinki and a local institutional board review (No. 20110262 Ethics Committee of Keio University School of Medicine). After obtaining informed consent from the patient, genomic DNA was extracted from peripheral blood leukocytes using the standard phenol extraction protocol. Genomic DNA from patient 1 was subjected to targeted sequencing by means of nanopore adaptive sampling technology [11]. The full length and ±10 kb of 15 genes, including ACVRL1 and ENG of Hereditary Haemorrhagic Telangiectasia (Version 3.6) in the PanelApp of Genomics England (Table S1) were targeted for adaptive sampling. Sequencing libraries were prepared using the Ligation Sequencing Kit SQK-LSK114 (Oxford Nanopore Technologies, Oxford, UK). Each library was loaded onto an R10.4.1 flow cell FLO-PRO114M for sequencing on the PromethION 2 solo instrument (ONT, Oxford UK). Sequence data was generated by Dorado basecaller (v0.4.3) with the super accurate basecalling model. The base-called reads were then aligned to the human reference genome (GRCh38) using Minimap2 (v2.24) [12]. The target contained 1 081 059 bases. The coverage depth for patient 1 was a mean of 24.7 (median of 25). Haplotype-aware small variant calling was performed by PEPPER-Margin-DeepVariant (v0.8.5) [13]. Structural variants were called by Sniffles (v2.22) [14].
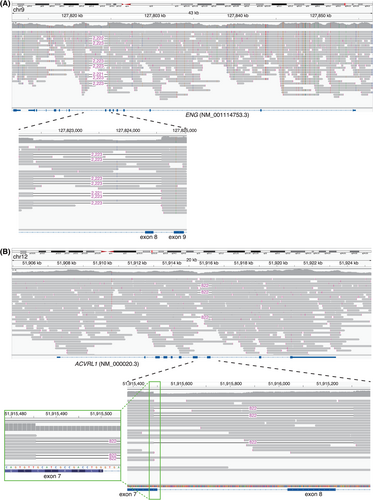
A structural variant, chr9(GRCh38):127822364_127824588delinsT spanning exon 8 of the ENG gene was identified by nanopore long-read sequencing (Figure 2A). This variant was subsequently validated by Sanger sequencing.
2.2 Patient 2
A 62-year-old female, with a history of daily epistaxis lasting about 10 min since her 30s, developed hematemesis. Upper gastrointestinal endoscopy revealed gastric telangiectasis. On physical examination, telangiectasis were observed in the oral cavity and fingers (Figure 1D,E). Imaging studies showed visceral lesions including AV shunts and PV shunts in the liver, but no lesions were found in the lungs or brain. Several family members, including her sister and deceased father, had a history of epistaxis and liver AVMs. She met the Curaçao Diagnostic Criteria for HHT. However, after obtaining informed consent from the patient, the Japanese insurance-covered gene panel testing specific for HHT (ENG, ACVRL1, SMAD4, and BMPR2) using short-read sequencing was negative. As well as patient 1, the insurance-covered gene panel testing did not conduct copy number analysis. Long read sequencing with adaptive sampling technique was performed as described above. For patient 2, the mean coverage depth was 31.6 (median = 32).
A structural variant, chr12(GRCh38):51915486_51916340delinsTGCAAACCTGGGAGGTTTGCAGTCAGACCTCCTGGCACC spanning exon 7 and exon 8 of the ACVRL1 gene was identified by nanopore long-read sequencing (Figure 2B). This variant was subsequently validated by Sanger sequencing.
3 Discussion
We here report two patients with recurrent epistaxis who were molecularly diagnosed as having HHT finally by application of nanopore long-read sequencing with adaptive technology but not by short-read targeted sequencing. The documentation of pathogenic variants in ENG and ACVRL1 in these two families had several clinical implications.
First, the identification of variants of the HHT causative genes provided the patients with strong motivation toward a focused preventive screening. For example, personalized regular screening for visceral lesions can be planned with a focus on cerebral and pulmonary AVMs in patient 1 and on liver AVMs in patient 2. Indeed, both patients had some doubts about their clinical diagnosis and need in preventive screening.
For family members with recurrent epistaxis, molecular diagnosis provided an opportunity to offer screening for possible visceral lesions associated with HHT or cascade genetic testing. In family testing, it is possible to screen for only the pathogenic variants rather than the entire regions of the four causative genes of HHT, allowing for more rapid evaluation.
The lack of SMAD4 variants in patient 1 or patient 2 obsoletes the need for preventive endoscopic screening for gastrointestinal polyposis is unnecessary. Given that endoscopic screening of the gastrointestinal tract is relatively costly and invasive, the ability to rule out SMAD4 variants (JP-HHT) is highly significant.
4 Conclusion
We encountered two patients in whom HHT was diagnosed after repeated episodes of epistaxis leading to otolaryngologic consultation. Both patients had severe visceral complications. Initially, the molecular genetic diagnosis was negative, making it difficult to explain the condition to the patients and recommend screening to their families. Once the diagnosis was confirmed, we were able to proceed with team-based medical care involving relevant departments beyond otolaryngology, depending on the causative genes (i.e., ENG vs. ACVRL1).
Acknowledgments
We thank Ms. Hiroko Nakamura, Ms. Yumi Obayashi, and Ms. Keiko Tsukue for the technical assistance in the preparation of this article.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.




