A case series study on the safety of cefditoren pivoxil use during the first trimester of pregnancy in Japan
Abstract
We evaluated the teratogenic risk associated with exposure to cefditoren pivoxil during the first trimester of pregnancy using the integrated databases of the Toranomon Hospital and the National Center for Child Health and Development. Among 13 599 registered individuals, the analysis included 285 subjects who had taken cefditoren pivoxil during the first trimester of pregnancy. The rates of stillbirth, miscarriage, and elective terminations were 0.4%, 5.6%, and 2.1%, respectively. Among 262 live births, the rates of preterm birth, low birth weight, and major congenital malformations were 4.6%, 5.7%, and 1.2%, respectively. Our results suggest that exposure to cefditoren pivoxil during the first trimester of pregnancy does not significantly increase the risk of adverse pregnancy outcomes and infant outcomes.
1 INTRODUCTION
Cephalosporin antibiotics, including cephalosporin-based and cefamycin-based drugs, have been commonly used as therapeutic agents for infections during pregnancy. Czeizel et al. reported in a case–control study evaluating the use of cephalosporin antibiotics in pregnancy, including the entire group of cephalosporins, that there appeared to be no teratogenic risk to the fetus.1 However, their study did not include cefditoren pivoxyl, because cefditoren pivoxil was sold in a limited number of countries.
Cefditoren pivoxil is a cephalosporin antibiotic developed in Japan, and it has been widely used since 1994. However, information regarding its use during pregnancy is limited to the results of post-marketing surveillance by the pharmaceutical industry.2 Therefore, in this study, we report the evaluation of the teratogenic risk associated with exposure to cefditoren pivoxil during the first trimester of pregnancy in Japan.
2 MATERIALS AND METHODS
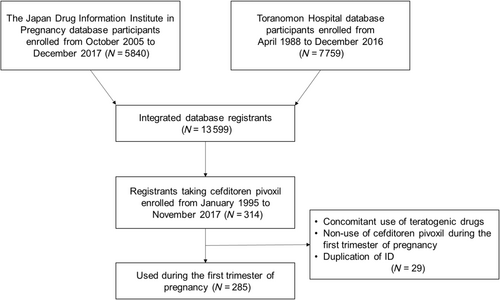
2.1 Study settings and data collection
Counseling on the safety of medication during pregnancy and the collection of pregnancy outcomes and infant outcomes were conducted at the Toranomon Hospital from April 1998 to December 2016 and the Japan Drug Information Institute in Pregnancy (JDIIP) from October 2005 to December 2017.3-7 This study was based on an integrated database that combines information from these two facilities. The database consists of pre-counseling questionnaires, interviews with pregnant women during counseling, and postal surveys conducted approximately 1 month after delivery. The database includes maternal survey items such as age, pregnancy history, delivery history, smoking status, alcohol consumption, names of medications taken, timing of medication use, duration of medication use, dosage, and concomitant medications. Investigation items related to pregnancy outcomes and infant outcomes include miscarriage, stillbirth, preterm birth, gestational age at delivery, birth weight, and the presence of congenital malformations. The assessed pregnancy outcomes included miscarriages before 22 weeks of gestation and the occurrence of stillbirths. The assessed infant outcomes included major and minor congenital malformations, low birth weight (<2500 g at birth), and premature delivery (<37 weeks of gestation). Reported malformations were defined following the European Surveillance of Congenital Anomalies (EUROCAT).8 In cases of malformations not included in EUROCAT, an experienced dysmorphologist from the National Center for Child Health and Development provided diagnoses, referring to the necessity of surgical treatment, and a further two specialists approved the diagnoses.
We extracted women who took cefditoren pivoxil tablets during the first trimester of pregnancy. Cases with unknown pregnancy outcomes or cases involving the concomitant use of teratogenic drugs were excluded from the analysis. The determination of teratogenicity for concomitant medications considered the list of teratogenic substances/drugs by Koren9 and the report by Ishikawa et al.10
2.2 Statistical analysis
Categorical data were presented as numbers (n) and percentages (%). Continuous variable data were presented as means and standard deviations (SD). Frequencies for congenital malformations, preterm birth, and low birth weight were calculated with 95% confidence intervals (CIs).
2.3 Ethical considerations
The research protocol was approved by the ethics review committee of Niigata University Medical & Dental Hospital (Approval number: 2017-0355). The principles of the Helsinki Declaration were followed, and informed consent was obtained from all participants.
3 RESULTS
Among the 13 599 registered women in the integrated database of the two facilities, there were 285 women who took cefditoren pivoxil tablets during the first trimester of pregnancy (Figure 1). Table 1 shows the characteristics of these women, with the majority having no underlying medical conditions. The pregnancy outcomes for these 285 women were as follows: one stillbirth (0.4%), 16 miscarriages (5.6%), and six elective terminations (2.1%) (Table 2). The rate of miscarriages among pregnant women whose exposure for cefditoren pivoxil was only 0–3 weeks gestation, only 0–14 weeks gestation, and 0–3 weeks and 4–13 weeks gestation were 6.7%, 6.1%, and 2.0%, respectively. The rate of preterm birth was 4.6% (12/262, 95% CI: 2.4–7.9%), and the rate of low birth weight (<2500 g) was 5.7% (15/262, 95% CI: 3.2–9.3%).
| Variables | Mean (SD)/n (%) |
|---|---|
| Age (years), mean (SD) | 31.0 (4.8) |
| Classification of age, n (%) | |
| <30 years | 106 (37.2) |
| 30–34 years | 113 (39.7) |
| ≥35 years | 66 (23.2) |
| Body mass index (kg/m2), mean (SD)a | 21.1 (2.7) |
| Nulliparous, n (%) | 125 (43.9) |
| Alcohol consumption, n (%)a | 2 (0.7) |
| Smoking, n (%)a | 7 (2.5) |
| Underlying disease, n (%) | |
| Mental disorders | 1 (0.4) |
| Diabetes mellitus | 0 (0.0) |
| Epilepsy | 1 (0.4) |
| Hypertensive disorders | 1 (0.4) |
| Thyroid disease | 6 (2.1) |
| Gestational weeks of consultation, n (%) | |
| 3–8 | 61 (21.4) |
| 8–15 | 189 (66.3) |
| 16–27 | 23 (8.1) |
| ≥28 | 1 (0.3) |
| Missing | 11 (3.9) |
| Year of consultation, n (%) | |
| 1995–1999 | 43 (15.1) |
| 2000–2004 | 56 (19.6) |
| 2005–2009 | 68 (23.9) |
| 2010–2014 | 81 (28.4) |
| 2015–2017 | 37 (13.0) |
| Gestational weeks of exposure for cefditoren pivoxil, n (%) | |
| 0–3 weeks only | 104 (36.5) |
| 4–13 weeks only | 131 (46.0) |
| 0–3 and 4–13 weeks | 50 (17.5) |
- a Data on alcohol consumption (n = 1), smoking (n = 1), and body mass index (n = 151) were missing.
| Pregnancy outcomes (N = 285) | n (%) |
|---|---|
| Live births, n (%) | 262 (91.9) |
| Miscarriages, n (%) | 16 (5.6) |
| Artificial abortions, n (%) | 6 (2.1) |
| Stillbirths, n (%) | 1 (0.4) |
| Multiple births, n (%)a | 0 (0.0) |
| Infant outcomes for live birth infants (N = 262) | Mean (SD)/n (%) |
|---|---|
| Male, n (%)a | 142 (54.2) |
| Gestational period (weeks), mean (SD)a | 38.9 (1.6) |
| Preterm births, n (%)b | 12 (4.6) |
| Birth weight (g), mean (SD) | 3045.3 (406.3) |
| Low birth weight, n (%)c | 15 (5.7) |
| Major congenital malformations, n (%) | 3 (1.2) |
| Minor congenital malformations n (%) | 3 (1.2) |
- a Data on the number of infants (n = 5), gestational period (n = 1), and birth weight (n = 1) were missing.
- b Defined as <37 weeks.
- c Defined as <2500 g.
Table 3 provides details of cases with major and minor congenital malformations in six infants. Major congenital malformations included complete transposition of great vessels, polydactyly, and cleft lip and palate, each observed in one infant, with a rate of 1.2% (3/262, 95% CI: 0.24–3.3%). Even in the infants whose mothers took cefditrene pivoxyl during 4–13 weeks, the rate of major congenital malformations was 1.8%. Minor congenital malformations included patent foramen ovale, umbilical hernia, congenital wheezing, and undescended testes, each observed in one infant, with a rate of 1.2% (3/262, 95% CI: 0.24–3.3%).
| Participant number | Year of consultation | Sex of infant | Major congenital malformations | Minor congenital malformations | Maternal age at consultations (years) | Timing of cefditoren pivoxil exposure (gestational weeks) | Underlying disease | Additional drug exposures of interest during 0–3 gestational weeks | Additional drug exposures of interest during 4–13 gestational weeks |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2010 | Male | Transposition of great vessels | N/A | 27 | 4–13 | Loxoprofen, Rebamipide, Lysozyme, Tranexamic acid | ||
| 2 | 2015 | Female | N/A | Patent foramen ovale | 33 | 0–3 | Mental disorder, Thyroid disease | Clomifene, Levothyroxine, Dydrogesterone, Paroxetine | Levothyroxine, Paroxetine |
| 3 | 2017 | Female | Polydactyly | N/A | 24 | 0–3, 4–13 | Loxoprofen, Betamethasone butyrate propionate | Betamethasone butyrate propionate | |
| 4 | 2000 | Female | N/A | Umbilical hernia | 39 | 4–13 | Azithromycin, Tulobuterol, Mefenamic acid, Serrapeptase | Bacitracin, Loxoprofen, Mefenamic acid, Serrapeptase | |
| 5 | 2002 | Male | Cleft lip and palate | N/A | 30 | 4–13 | Salicylamide/Acetaminophen/Anhydrous caffeine/Promethazine, CefcapenePivoxi, Serrapeptase, Pseudoephedrine/Chlorpheniramine/Belladonna total alkaloids/Anhydrous caffeine, Trazodone, Flunitrazepam, Triazolam | Serrapeptase, Gualenate/l-Glutamine, Loxoprofen | |
| 6 | 2006 | Male | N/A | Congenital wheezing, undescended testes | 39 | 4–13 | Fluticasone, Bakumondoto, Codeine, Tipepidine, Carbocisteine, OTC stomachic |
- Abbreviations: N/A, not applicable; OTC, over-the-counter.
4 DISCUSSION
In this study, we examined the safety of cefditoren pivoxil exposure during the first trimester of pregnancy in both pregnant women and infants. No adverse effects were observed on pregnancy outcomes or the infants.
The incidence rate of major congenital malformations in our study was low (1.2%, 3/262, 95% CI: 0.24–3.3%), and there was no overlap in the types of congenital malformations. Therefore, there might be no congenital malformations that were attributable to the drug. The incidence rate of minor congenital malformations was 1.2% (3/262, 95% CI: 0.24–3.3%). Because minor congenital malformations are not a problem in many cases, physicians often do not inform mothers of minor congenital malformations when they find them in routine practice, such as health check-up at the 1 month after birth. Therefore, there is a possibility that the reporting of minor congenital malformations by mothers is underreported. Additionally, reproductive and developmental toxicity tests of cefditoren pivoxil in rats and rabbits did not show any teratogenic effects (Meiact MS® Tablet 100 mg Interview Form, 14th edition, 2022). In a post-marketing surveillance of Meiact MS® Tablet 100 mg, among 18 cases where the drug was administered during the early pregnancy period (including absolute and relative sensitive periods), one case had abnormal findings in the infant.2 In this case, the drug was administered at 15 weeks of pregnancy, and the infant had hyperbilirubinemia at birth, which was treated with phototherapy. However, hypervirrubinemia is not related to the administration of Meiact MS® Tablet 100 mg, as it is suspected to be causally related to the underlying Rh incompatible pregnancy. Considering the absence of teratogenic effects in reproductive and developmental toxicity studies and the lack of causal relationship between Meiact MS® Tablet 100 mg administration and infant malformations in post-marketing surveillance, the malformations observed in this study may be part of the naturally occurring malformations, and there were no congenital malformations that were clearly attributable to the drug.
Furthermore, the rate of miscarriage in this study (5.6%) was obviously lower than the reported frequency of approximately 15%.11, 12 Because most consultations occurred after the favorable period for miscarriage has passed, the rate might be low. The rate of preterm birth has remained constant at around 5.7% based on population statistics in Japan over the past 15 years, and the rate of low birth weight (<2500 g) has remained stable during the same period, with reported rates of 8.3% for males and 10.6% for females in 2016.13 The rates of preterm birth and low birth weight in this study were lower than the frequencies reported in Japanese population statistics. Therefore, cefditoren pivoxil as an antimicrobial agent during pregnancy may not be considered as having additional risks on the baseline risks.
There are several limitations to this study. The purpose for the use of cefditoren pivoxil could not be collected. Pregnancy outcomes and infant outcomes were based on reports from mothers approximately 1 month after delivery. The follow-up rate was not necessarily 100%, which may have introduced reporting bias. Additionally, the evaluation of infant outcomes was limited to approximately 1 month after delivery, and long-term effects could not be assessed. Because postal surveys were conducted approximately 1 month after delivery, information on miscarriage rates may not be provided sufficiently. Furthermore, the post-counseling use of medication was not confirmed, and the possibility of its impact on the results cannot be ruled out. Therefore, it is hoped that the results of this study will be validated by other data sources and study designs.14
In conclusion, the results of this study suggest that exposure to cefditoren pivoxil during the first trimester of pregnancy does not increase the risks of adverse pregnancy outcomes and infant outcomes. This finding can help reduce anxiety in pregnant women who are taking cefditoren pivoxil during the first trimester of pregnancy.
ACKNOWLEDGMENTS
This study was supported by the members of the JDIIP, Toranomon Hospital, and support medical institutions (Hokkaido University Hospital, Hirosaki University Hospital, Iwate Medical University Hospital, Akita Red Cross Hospital, Yamagata University Hospital, Tohoku University Hospital, Fukushima Medical University Hospital, Maebashi Red Cross Hospital, Tsukuba University Hospital, Saiseikai Utsunomiya Hospital, Jichi Medical University Hospital, Chiba University Hospital, Saitama Medical University Hospital, Saitama Medical Center, Jichi Medical University Hospital, Yokohama City University Hospital, Yamanashi Prefectural Central Hospital, Shinshu University Hospital, Niigata University Medical and Dental Hospital, Toyama University Hospital, Kanazawa Medical Center, Fukui University Hospital, Hamamatsu Medical University Hospital, Japanese Red Cross Aichi Medical Center Nagoya Daiichi Hospital, Nagoya City University Hospital, Nagara Medical Center, Gifu University Hospital, Mie University Hospital, Shiga University Hospital, Kyoto Prefectural University of Medicine Hospital, Osaka Maternal and Child Health Center, Osaka University Hospital, Osaka Medical and Pharmaceutical University Hospital, Kobe University Hospital, Nara Medical University Hospital, Japanese Red Cross Wakayama Medical Center, Tottori University Hospital, Okayama Medical Center, Okayama University Hospital, Shimane University Hospital, Hiroshima University Hospital, Yamaguchi University Hospital, Shikoku Children and Adult Medical Center, Tokushima University Hospital, Ehime University Hospital, Kochi University Hospital, Kyushu University Hospital, Saga University Hospital, Oita University Hospital, Kumamoto Red Cross Hospital, Kumamoto University Hospital, Nagasaki University Hospital, Miyazaki University Hospital, Kagoshima City Hospital, Kagoshima University Hospital, and Okinawa Prefectural Chubu Hospital). We appreciate Dr. Rika Kosaki for her work in defining the malformations recorded in this database. We would also like to thank the participants who donated their clinical information. We also appreciate Dr. Mami Ishikuro for her work in advising the analyses.
CONFLICT OF INTEREST STATEMENT
Taku Obara is an Editorial Board member of CGA and a co-author of this article. The authors declare no conflict of interest.




