Analysis of triptan use during pregnancy in Japan: A case series
Abstract
To evaluate the safety of triptan use during pregnancy in a Japanese population, we descriptively analyzed the data on pregnancy and fetal outcomes from 128 pregnant women using triptans for migraine treatment at two Japanese facilities that provided counseling on drug exposure in pregnancy between 2001 and 2017. The risks of miscarriage, low birth weight, and preterm birth were similar to those reported in the demographic statistics in Japan. The incidence proportion of malformation was also within the baseline risk range. Accumulated data suggest that exposure to triptans during pregnancy does not clearly increase the risk of negative pregnancy and fetal outcomes. This finding can help reduce anxiety in pregnant women with migraines who are taking triptans.
1 INTRODUCTION
Migraine is a primary paroxysmal and chronic headache disorder.1 The negative impacts of migraines are not limited to the pain itself, known as migraine attacks, but they also have substantial effects on both work-related and daily life activities.2 The prevalence of migraines in women of childbearing age is estimated to be between 11% and 20% in East Asia.3 In Japan, stratified (severity-based) migraine treatment is recommended and triptans are used to treat moderate to severe migraine, or mild migraine when therapy by non-steroidal anti-inflammatory drugs has previously failed.4 In pregnant patients with migraines, however, acetaminophen is known for its relatively high safety profile and is, therefore, more commonly used during pregnancy; it is the mainstay of treatment according to some guidelines.4, 5 Although no evidence of teratogenicity or prematurity was reported in a previous meta-analysis investigating triptan exposure during pregnancy,6 most of the reports included in the analysis were from Western countries. This requires further attention to determine whether the physical differences in Japanese people may affect these results. Therefore, we aimed to analyze the relationship between triptan use during pregnancy and any potential adverse outcomes in a Japanese population. To our knowledge, this is the first time such a study has been undertaken in the Japanese population.
2 MATERIALS AND METHODS
2.1 Study design, settings, and data collection
This is a descriptive study using the integrated database of the Toranomon Hospital and the Japan Drug Information Institute in Pregnancy (JDIIP); both institutes provide counseling and collect data regarding the safety of drug use in pregnancy.
Pregnant women who consulted with the Counseling Clinic for Pregnancy and Medicine of the Toranomon Hospital between April 1988 and December 2016, and with the JDIIP between October 2005 (when it was established under a program of the Ministry of Labour and Welfare) and December 2017, were registered in this study. Items in the database were collected mainly from questionnaire surveys that were filled out before counseling or from maternal interviews during counseling. They included maternal data such as age, pregnancy and reproductive history, smoking status, alcohol intake, medical history, and medication use (drug name, date of initiation and discontinuation, and daily dose). Information on pregnancy and neonatal outcomes was obtained from maternal reports approximately 1 month after delivery. The assessed pregnancy outcomes included miscarriages before 22 weeks of gestation and the occurrence of stillbirths. The assessed infant outcomes included major and minor congenital anomalies, low birth weight (LBW, <2500 g at birth), and premature delivery (<37 weeks of gestation). Reported malformations were defined following the European Surveillance of Congenital Anomalies (EUROCAT).7 In cases of malformations not included in EUROCAT, an experienced dysmorphologist from the National Center for Child Health and Development provided diagnoses, referring to the necessity of surgical treatment, and a further two specialists approved the diagnoses.
2.2 Statistical analysis
Categorical data were described as numbers (n) and percentages (%). Continuous variable data were described as mean and standard deviation (SD). A 95% confidence interval (CI) was calculated for the prevalence of malformation, LBW, and preterm birth. Statistical analysis processing was performed using JMP Pro 14.0 (SAS Institute Inc., Cary, NC, USA).
2.3 Ethical considerations
The study protocol was approved by the ethics committee of Kyoto University (No. R2671). The principles of the Declaration of Helsinki were adhered to in the conduct of this study. Informed consent concerning the construction of these databases was obtained from all participants.
3 RESULTS
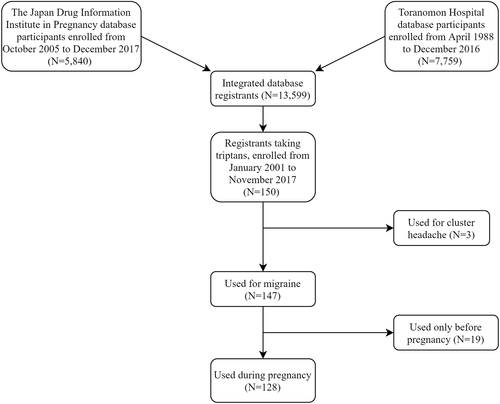
Of the 13 599 registrants possessing complete data profiles on medication use and pregnancy and neonatal outcomes in the integrated database, our study included 128 women who were taking triptans to treat migraines during pregnancy, between January 2001 and November 2017 (Figure 1). The characteristics of these patients are presented in Table 1. Mental and behavioral disorders (18.8%) were the most common underlying diseases.
| Age (y), mean (SD) | 33.0 (4.8) |
| Classification of age, n (%) | |
| 10s | 2 (1.6) |
| 20s | 26 (20.3) |
| 30s | 92 (71.9) |
| 40s | 8 (6.3) |
| Body mass index (kg/m2), mean (SD)a | 20.4 (2.7) |
| Nulliparous, n (%) | 74 (57.8) |
| Alcohol consumption, n (%)a | 2 (1.6) |
| Smoking, n (%)a | 6 (4.8) |
| Underlying disease, n (%) | |
| Mental disorders | 24 (18.8) |
| Diabetes mellitus | 1 (0.8) |
| Epilepsy | 1 (0.8) |
| Hypertensive disorders | 1 (0.8) |
| Thyroid disease | 2 (1.6) |
- a Data on alcohol consumption (n = 3), smoking (n = 4) and body mass index for all cases at the Toranomon Hospital (n = 33) were missing.
Of these 128 cases, 12 were cases of miscarriage (9.4%) and three of artificial abortions (2.3%) (Table 2). The three women who chose artificial abortions were exposed to triptans in the first trimester.
| Live births, n (%) | 113 (88.2) |
|---|---|
| Miscarriages, n (%) | 12 (9.4) |
| Artificial abortions, n (%) | 3 (2.3) |
| Stillbirths, n (%) | 0 (0.0) |
| Multiple births, n (%) | 0 (0.0) |
As shown in Table 3, there were seven preterm births (6.2%, 95% CI: 3.0–12.2) and eight LBW infants (7.1%, 95% CI: 3.6–13.4). All cases of preterm birth were classified as late preterm (ranging from 34 to 36 weeks of gestational age at delivery).
| Gestational period (weeks), mean (SD) | 38.9 (1.4) |
|---|---|
| Birth weight (g), mean (SD) | 3007.5 (389.6) |
| Preterm births, n (%) | 7 (6.2) |
| Low birth weight,a n (%) | 8 (7.1) |
| Congenital malformations, n (%) | 4 (3.5) |
- a Defined as <2500 g.
Table 4 outlines the malformations identified in four infants. The incidence of all malformations was 3.5% (4/113, 95% CI: 1.4–8.7). Of the four, one case with a bicuspid pulmonary valve was classified as a major defect; the mother of this infant also took paroxetine during the first trimester. The incidence of major malformation was 0.9% (1/113, 95% CI: 0.02–4.8).
| Participant number | Major malformations | Minor malformations | Triptan exposure in the first trimester | Maternal age at consultation | Additional drug exposures of interest in the first trimester |
|---|---|---|---|---|---|
| 1 | Bicuspid pulmonary valve | N/A | Yes | 37 | Alprazolam, paroxetine, zolpidem, sertraline |
| 2 | N/A | Cryptorchidism | Yes | 27 | Clonazepam, domperidone, lomerizine, indomethacin |
| 3 | N/A | Hemangioma | Yes | 29 | Risperidone, clonazepam, estazolam, levomepromazine, valaciclovir |
| 4 | N/A | Patent foramen ovale | Yes | 32 | Tizanidine, metoclopramide, etizolam |
- Abbreviation: N/A, not applicable.
4 DISCUSSION
In this case series, we detected no evidence of an excess of adverse pregnancy or infant outcomes due to triptan use. The proportion of miscarriages reported in this series was within previously reported rates (12%–15%).8 All three women who chose artificial abortions were exposed to triptans in the first trimester. The reasons for choosing an artificial abortion in these cases were unknown.
In Japan, the prevalence of LBW has increased from 7.5% in 2001 to 8.2% in 2017 for single births, while that of preterm birth has stayed constant at approximately 5.7% in the last 15 years.9 The prevalence of LBW and preterm birth in the present analysis was similar to that of the general population.
The incidence proportion of malformations was within the baseline risk (3%–5%)10 and that of major malformation was similar to that of control cases in this database, as previously reported (1.7%, 95% CI: 1.1–2.5).11 Therefore, in cases where typical migraine attacks persist and decrease quality of life and acetaminophen is insufficient, triptans may be a viable alternative, following an informative consultation with an appropriate physician.
This study had several limitations. First, the sample size was too small to estimate low-frequency data such as congenital malformations. Second, reporting bias could have occurred because participants experiencing poor outcomes, such as miscarriage, tend to refuse to report them. Additionally, information regarding infant outcomes was only collected at health checkups for 1-month-old infants; and no additional information regarding follow-up checkups was obtained. The use of triptans after the initial consultation was also not recorded. According to a previous report, however, more than 80% of pregnant women reported that both the frequency and severity of migraines decreased during their second and third trimesters,12 therefore we can presume in our study that the necessity of triptan use also decreased as gestation progressed.
In conclusion, the accumulated data suggest that exposure to triptans during pregnancy does not significantly increase the risk of adverse pregnancy and fetal outcomes. This finding can help reduce anxiety in pregnant women with migraines who are taking triptans.
ACKNOWLEDGMENTS
We appreciate Dr. Rika Kosaki for her work in defining the malformations recorded in this database. We would also like to thank participants who donated their clinical information.
CONFLICT OF INTEREST
Takeo Nakayama received honorariums from Pfizer Japan Inc. and MSD K.K. The other authors have no competing interests to declare.