Mimickers of hydroxychloroquine retinal toxicity
Gagan Kalra and Peter Jones share equal authorship.
Abstract
Hydroxychloroquine (HCQ) retinal toxicity is an important entity that can be challenging to differentiate from its mimickers. Bull's eye maculopathy is the classical presentation of HCQ retinopathy; however, its differential includes several drug-related retinal toxicities, inherited retinal disorders, and systemic conditions with associated retinopathies. Given the similarities in clinical presentation, imaging findings, and other diagnostic data, it can be quite challenging for all but the retina specialists to successfully identify retinal toxicity from HCQ. This review summarises HCQ retinopathy and its mimickers of with a special emphasis on key clinical and ancillary distinctions that can help comprehensive ophthalmologists and primary care offices in clenching this diagnosis.
Abbreviations
-
- AAO
-
- American Academy of Ophthalmology
-
- AMD
-
- age-related macular degeneration
-
- AOFVD
-
- adult-onset foveomacular vitelliform dystrophy
-
- BBS
-
- Bardet-Biedl Syndrome
-
- BCAMD
-
- benign concentric annular macular dystrophy
-
- BCNU
-
- carmustine
-
- BPD
-
- butterfly pattern dystrophy
-
- CACD
-
- central areolar choroidal dystrophy
-
- COD
-
- cone dystrophy
-
- CORD
-
- cone-rod dystrophy
-
- DME
-
- diabetic macular edema
-
- ELM
-
- external limiting membrane
-
- EZ
-
- ellipsoid zone
-
- FAF
-
- fundus autofluorescence
-
- FFA
-
- fluorescein angiogram
-
- FSMD
-
- fenestrated sheen macular dystrophy
-
- GA
-
- geographic atrophy
-
- HCQ
-
- hydroxychloroquine
-
- IZ
-
- interdigitation zone
-
- JNCL
-
- juvenile neuronal ceroid lipofuscinosis
-
- LCA
-
- Leber Congenital Amaurosis
-
- MacTel
-
- macular telangectasia
-
- mfERG
-
- multifocal electroretinogram
-
- NCL
-
- neuronal ceroid lipofuscinosis
-
- OCT
-
- ocular coherence tomography
-
- ONL
-
- outer nuclear layer
-
- PDSFF
-
- multi-focal pattern dystrophy simulating fundus flavimaculatus
-
- PKAN
-
- pantothenate kinase-associated neurodegeneration
-
- PRPH2
-
- peripherin 2
-
- RA
-
- rheumatoid arthritis
-
- RP
-
- retinitis pigmentosa
-
- RPE
-
- retinal pigment epithelium
-
- SD-OCT
-
- spectral-domain optical coherence tomography
-
- SLE
-
- Systemic lupus erythematosus
1 INTRODUCTION
Hydroxychloroquine (HCQ) is a commonly used medication worldwide for various rheumatological conditions including systemic lupus erythematosus and rheumatoid arthritis. It has long been known that these medications cause retinal toxicity, most commonly with prolonged usage. Both general ophthalmologists and retina specialists are commonly asked to screen for retinal toxicity in patients treated with these medications. Guidelines advise that patients receive an ophthalmological exam and testing prior to starting treatment with these medications as these are often continued for many years. Thus, when a patient develops retinal abnormalities while on HCQ, there should be some thought given to whether the abnormalities are from HCQ toxicity or another cause. In this article, we will review the findings associated with HCQ toxicity and summarise a variety of alternative conditions which can present with similar findings, with an emphasis on how to differentiate these entities.
2 METHOD OF LITERATURE SEARCH
The following databases were utilised for literature search—Google scholar, Pubmed (MEDLINE), and Scopus. The key search phrases that were used to perform the search were ‘hydroxychloroquine retinopathy differentials’, ‘mimickers of hydroxychloroquine retinopathy’, ‘Bull's eye maculopathy’. The search was performed on 12th December 2022. Search results in English language published prior to the date of search were included in the study. Case reports and case series were only included if they contributed new information to the characteristics, diagnosis, or treatment of the disease. The search results were then reviewed by two graders (G.K. and P.J.) to identify, group, and summarise the distinct clinical and imaging features of mimickers of HCQ retinopathy.
3 PATHOPHYSIOLOGY OF HCQ RETINOPATHY
The definitive mechanism of retinal toxicity due to HCQ and derivatives is unclear. Presence of multilamellar structures in the histological assessment of both human and animal retinas has been documented with long-term HCQ therapy. This is associated with subsequent loss of outer neurosensory retina comprising ganglion cells, photoreceptor cells, and retinal pigment epithelium.1 The accumulation of these abnormal structures have been hypothesized to result from inhibition of lysosomal phospholipases or erroneous inhibition of protein synthesis in outer retinal layers.2, 3 It has additionally been postulated that some genetic mutations, such as mutations in ABCA4 (in which homozygous mutations are well known to cause Stargardt disease) may play a role in predisposing individuals to toxicity.4
4 OVERVIEW OF RETINAL FINDINGS ASSOCIATED WITH HYDROXYCHLOROQUINE TOXICITY AND SCREENING RECOMMENDATIONS
The main risk factors for retinal toxicity from HCQ are dosage (both cumulative and daily) and duration of therapy. At lower dosages, the risk of retinal toxicity is very low. Even with its daily dosage approaching the maximum dosages (4–5 mg/kg/day) recommended by the American Academy of Ophthalmology (AAO), the chance of developing retinal toxicity is <2% within 10 years, but rises by approximately 1% per year afterwards.5 Therefore, current AAO guidelines6 recommend screening initially at therapy initiation and again at 5 years of therapy if daily dosage remains <5.0 mg/kg/day and the patient does not have additional risk factors such as kidney disease or concurrent tamoxifen use. Subsequently, yearly screening with visual fields (10-2 in non-Asian individuals, 24-2 in Asian individuals) and spectral domain optical coherence tomography (SD-OCT) of the macula, with autofluorescence (AF), and multifocal electroretinogram (mfERG) to corroborate deficits.
Early toxicity, although non-reversible, is likely to be asymptomatic for the patient and have subtle or no gross clinical findings. Thus testing is key for early detection. Formal visual fields can illustrate paracentral deficits and it is recommended that visual fields be interpreted with high suspicion of toxicity for at risk individuals.6 The classic OCT finding is parafoveal ellipsoid zone (EZ) disruption. Other early OCT signs are outer nuclear layer thinning, disruption of the parafoveal interdigitation zone (IZ), and reduced reflectivity of the parafoveal EZ band in the absence of definitive disruption.7 A potential very early sign is accelerated macular thinning, as identified by a recent study showing a higher HCQ retinopathy prevalence in patients showing macular thinning in a retrospective study.8 Fundus autofluorescence (FAF) images often show a smooth parafoveal, possibly bullseye pattern of hyperfluorescence in early toxicity, which correlates with areas of IZ/EZ disruption in the OCT. The classic bullseye maculopathy on clinical fundoscopy is a late finding, at which time the other findings described should be clearly present. Additionally, toxicity may not be localised perifoveally in patients of Asian descent and outer macula findings may be seen closer to the vascular arcades, thus necessitating wider visual field testing in these individuals. Central visual acuity is relatively preserved until development of advanced toxicity. Wide-field ERG recordings are variable and can be normal in early toxicity. When affected these show delayed flicker responses and evenly affected rod and cone responses.9 Figure 1 shows an example of a case on long-term HCQ therapy and classical clinical signs of HCQ retinopathy with bulls eye pattern.
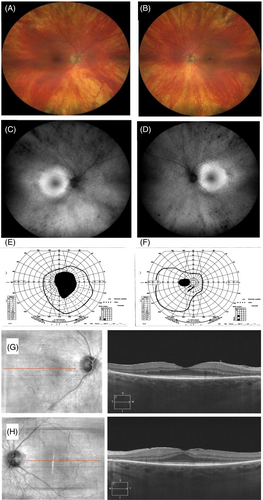
There are several other clinical entities that could mimic HCQ retinal toxicity. The differential diagnoses for these entities are summarised in Table 1.
| Drug toxicities affecting the RPE |
|
|
|
|
|
|
| Retinal dystrophies/degenerations |
|
|
|
|
|
|
|
|
| Systemic Diseases w/associated retinopathies |
|
|
|
|
|
- Abbreviations: BCNU, Carmustine; RPE, retinal pigment epithelium.
Figure 2 shows an example of bulls eye maculopathy that was attributable due to ABCA4 gene mutation in the setting of no HCQ use. This review will provide a summary of each mimicker entity and distinguish it from retinal toxicity from HCQ using typical clinical and imaging findings.
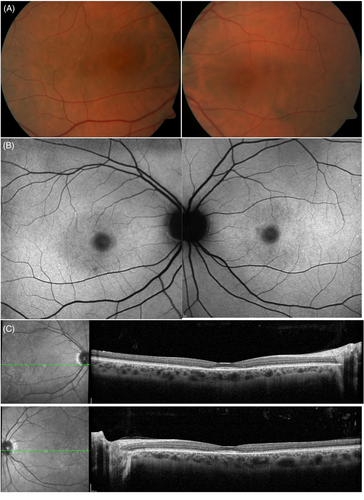
5 MEDICATION TOXICITIES
There are a number of other retinal drug toxicities that have been reported to be associated with a bull's eye maculopathy. If the use of a drug is associated with a parafoveal RPE or photoreceptor disruption, it could present as a bull's eye retinopathy. Drugs that have been reported to cause such retinopathies include clofazimine, ritonavir, pentosan polysulfate, desferoxamine/defasirox, phenothiazines, and alkylating chemotherapeutic agents.
Clofazimine is a drug used to treat mycobacterial infections and some inflammatory conditions including vitiligo and has been reportedly associated with a bullseye maculopathy.10-12 Ritonavir, a protease inhibitor used to treat HIV, has been reported to produce a parafoveal RPE degradation with potential bull's eye presentation.13 Pentosan polysulfate, a drug used to treat interstitial cystitis, has been more recently reported to cause a pigmentary maculopathy that can be parafoveal, especially early on,14, 15 but can also progress to patchy geographic atrophy in severe cases. Desferoxamine and defasirox are iron chelation medications used to treat iron overload that can produce parafoveal pigmentary retinopathies.16 These effects can be produced after as little as a single dose, and stopping the medication can improve the resulting visual field defects.16, 17 Phenothiazines (e.g., Thioridazine, Chlorpromazine) are current infrequently used antipsychotics that are known to cause maculopathy, which can have its onset either soon after initiation if at high dose, or with chronic use.18
Chemotherapeutic alkylating agents (cisplatin, carmustine/BCNU) have also been reported to cause a pigmentary retinopathy, though also have been reported to cause a retinal vascular occlusive events.19
6 MACULAR DYSTROPHIES AND DEGENERATIONS
6.1 Age-related macular degeneration
Age-related macular degeneration (AMD) is the most common maculopathy to mimic HCQ toxicity. Distinguishing between early AMD changes and early HCQ toxicity is challenging since the variability and non-specific nature of early AMD clinical signs could belong to either pathology. Figure 3 shows an example of a patient on long-term HCQ therapy and concurrent AMD but no HCQ retinopathy as confirmed with additional testing.
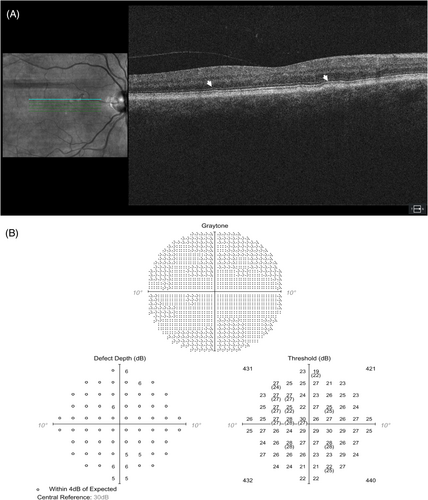
Both HCQ retinopathy and AMD can have outer retinal changes including EZ/IZ disruption or RPE changes on OCT, and given the long-term nature of HCQ therapy, new changes could thus represent one or both pathologies. Importantly, current AAO guidelines recommend a baseline exam and testing at the time of HCQ therapy initiation for detection of pre-existing retinopathy for future comparisons. Even with an established baseline, new changes on subsequent follow-up may be consistent with either pathology.
OCT and FAF are the most useful differentiators between these entities. OCT features indicative of either exclusive or concurrent AMD diagnosis include drusen, pigment epithelial detachments, and intraretinal fluid, subretinal fluid, or other signs of exudative disease. The geographic distribution of OCT findings most consistent with HCQ toxicity (IZ/EZ disruption, RPE changes) would be expected to be perifoveal or more peripheral in eastern Asians, but could be more stochastic in AMD. Some patterns of GA with a central island could potentially mimic a bulls-eye maculopathy. Early subfoveal involvement would be more indicative of AMD as this region is typically spared in early HCQ retinopathy. The FAF pattern in HCQ tends to be smoothly hyperfluorescent early, in contrast to AMD in which the FAF pattern is quite variable and much more irregular.20
If there is suspicion of concurrent HCQ toxicity and AMD, then HCQ cessation or dose reduction should be recommended to the prescribing physician. The management is less clear in a patient with AMD and unclear diagnosis of HCQ toxicity (without definite or likely toxicity). The literature is sparse on examples of these cases since most reports of HCQ retinopathy in the literature tend to exclude patients with other macular co-morbidities (e.g., AMD, DME, rod-cone dystrophy).21-27 Although it seems likely that concurrent maculopathy from AMD or other source may increase the risk or rate of HCQ toxicity, this has not been definitively shown. Therefore, the risks of continuing the medication versus stopping should be clearly discussed with the patient and prescribing physician in cases where toxicity is possible but cannot be definitively confirmed. A referral to a retinal specialist should be considered.
6.2 Stargardt's disease
Stargardt's disease is the most common inherited retinal dystrophy occurring in about 1 in 10 000 individuals in the United States.28 The clinical findings can be quite variable between individuals and disease stage, ranging from foveal arophy, to bulls eye maculopathy and a much more wide-spread dystrophy that resembles retinitis pigmentosa.29, 30
ABCA4 mutations, both homozygous and heterozygous, have been shown to cause Stargardt disease.4, 31 The occasional phenotypic similarity between HCQ retinopathy and Stargardt disease, sparked interest in exploration of prevalence of ABCA4 mutation in HCQ retinopathy patients.4 In a report discussing nine cases with ABCA4 mutations, authors had described two cases of Stargardt disease with a ring maculopathy resembling the bulls eye maculopathy seen in HCQ retinopathy.32 A report assessing eight patients with HCQ retinopathy, two patients showed heterozygous mutations of the ABCA4 gene that was thought to have resulted in severe reduction of ABCA4 activity and thereby predisposing the development of HCQ retinopathy.4 Based on the genotypic expression, the authors argued that the patient would typically be thought to develop Stargardt disease. However, due to absence of classical findings on clinical examination and fluorescein angiography, the diagnosis was ruled out.
Features that differentiate Stargardt's disease from HCQ retinopathy include initial presentation with decreased vision in the first to third decade of life, yellow, pisciform RPE flecks extending from posterior pole to mid-periphary on fundoscopy with characteristic peripapillary sparing, geographic atrophy in the macula commonly without pigmentary changes, and rarely choroidal neovascularization may be noted.29 On fluorescein angiography, characteristic darkening or hypofluorescence of the choroid is noted. This finding is called as ‘silent’ choroid and is not seen in HCQ retinopathy. OCTA can show thinning of the superficial vasculature.
6.3 Pattern dystrophy
Pattern dystrophies are a heterogenous category of inherited retinal dystrophies that normally present in middle age with macular pigmentary changes and deposits in a variable distribution They are typically classified into adult-onset foveomacular vitelliform dystrophy (AOFVD), butterfly pattern dystrophy (BPD), multi-focal pattern dystrophy simulating fundus flavimaculatus (PDSFF), reticular dystrophy of the retinal pigment epithelium, and fundus pulverulentus based on the clinical features.33 They are most associated with mutations in the PRPH2 gene (encoding Peripherin-2/RDS) but have also been associated with mutations in other genes, including VDM2, ABCA4, BEST1, IMPG1/2, and CTNNA1.34, 35
Typically, visual acuity is moderately affected, clinical findings are quite variable based on stage and type, and though involvement is bilateral, it can be asymmetric. Typical clinical findings include pigmentary changes in the macula; yellow, white, or greyish deposits which can be in a reticular or flecked pattern, vitelliform foveal lesions (which tend to be smaller than those in Best disease), and at times drusen can be present.36 RPE atrophy can be present in later stages, and CNVM is a possible complication. OCT imaging typically shows hyperreflectivity between the RPE/Bruch's membrane which can extend to the ONL, and disruption of the EZ/IZ.37
The spatial pattern of RPE changes in the macula is quite variable and may not fit cleanly into one of the defined patterns, thus is possible to present with a bullseye pattern of RPE changes similarly to HCQ toxicity. Clinical features to differentiate pattern dystrophy from HCQ toxicity include family members with macular dystrophy, markedly asymmetric presentations, patterns of RPE disruption other than bullseye/perifoveal distribution, and the presence of drusen or flecked deposits. The main OCT feature more suggestive of a pattern dystrophy is the hyperreflective material in the EZ/IZ to ONL, rather than primarily disruption of the EZ/IZ which is seen in both conditions. If a pattern dystrophy is suspected, genetic testing with a retinal dystrophy panel could be considered.
6.4 Macular telangectasia type 2
Macular telangectasia type 2 (MacTel type 2) is a bilateral disease of unknown cause which results in capillary changes and neurosensory retinal atrophy, usually becoming symptomatic in the fifth or sixth decade of life.38-40 The most characteristic findings are ectatic capillaries and parafoveal venules that do not show normal narrowing and which dive at right angles into the retina, retinal greying, intraretinal cysts, and sometimes lamellar or full-thickness macular holes.41 The vascular and retinal changes tend to be more prominent temporal to the foveola, but can extend concentrically, giving the changes a bull's-eye appearance.42 Neovascularization can occur, but in a minority of patients. The visual acuity is often very good (often >20/50) until later in the disease progression, with temporally biased paracentral scotomas being reliably observed as the disease progresses. In advanced stages, the disease where there is photoreceptor atrophy or in the setting of neovascularization, visual acuity can be much worse, around 20/200 with eccentric fixation. Common OCT findings include inner retinal hyperreflectivity and hyporeflective inner retinal cystic spaces in early stages, and in later stages outer retinal cystic cavities, outer retinal atrophy, and outer retinal pigment migration with hyperreflectivity. All of these changes are commonly foveal in location or in the temporal slope of the fovea. The intraretinal cystic changes are not associated with leakage on angiography. The FFA does show the abnormal venules and capillaries in the early phase and staining of the macula in the late phase.43 FAF findings depend on the wavelength used because there is a known decrease in macular pigment density, which attenuates 488 nm AF signals more than 514 nm AF signals, thus at 488 nm the typical foveal FAF attenuation is missing.44 One can otherwise see patchy areas of hyper-FAF corresponding to photoreceptor atrophy or intraretinal cavitations and sometimes hypo-FAF corresponding to areas of RPE atrophy.41
Since retinal changes do sometimes manifest in a bullseye configuration, and late stages do result in outer retinal atrophy, one should take into the other features of MacTel in order to distinguish between it and HCQ toxicity, including the small vessel findings, temporal bias, OCT features uncommon in HCQ toxicity including intraretinal cysts and pigmentary migration, and FFA leakage/staining. If the presentation is suggestive of MacTel on the initial HCQ toxicity testing, an FFA should reliably distinguish between the two entities. There is no literature that we can find to guide management of HCQ usage for patients with coexisting MacTel.
6.5 Central areolar choroidal dystrophy
This is an inherited macular dystrophy most commonly arising from autosomal dominant mutations in the peripherin/RDS gene (PHPR2) that most often has symptomatic onset at 30–60 years of age.45, 46 Stages of severity have been described with stage 1 showing parafoveal RPE changes with corresponding hyperautofluorescence in the affected areas, stage 2 showing diffuse macular RPE changes in a round or oval area 1.5 to several disc diameters in area, which can have relative foveal sparing. Stage 3 and greater have areas of geographic atrophy which expand over time. On OCT imaging during stages 1 and 2, affected areas can show either disruption of both RPE and EZ, with hyperreflectivity possibly extending into the ONL, or thickening of the photoreceptor outer segments with irregular increase in reflectance. This progresses to loss of the EZ and RPE in later stages. Thus, in early stages, the thickening of photoreceptor outer segments or hyperreflectivity extending above the EZ and ELM would distinguish it from HCQ toxicity, along with the patchy distribution of outer retinal changes rather than a more even distribution. The GA of later stages of CACD are more likely to be mistaken for atrophic AMD than HCQ toxicity.45 The FAF shows focal changes in stage 1, speckled FAF throughout a sharply demarcated macular area in stage 2 and later, with areas of geographic atrophy that develop lacking FAF. Additionally, because CACD is generally inherited in an AD fashion, a family history of central vision loss will be common although not universal. Genetic testing with a retinal dystrophy panel is recommended if CACD is suspected.
6.6 Benign concentric annular macular dystrophy
This is a rare autosomal dominant dystrophy first described by Deutman in 197447 which produces an annular parafoveal hypopigmention early in the disease course, but later in the disease patients have an RP-like phenotype with peripheral bone-spicule like pigmentation, waxy pallor of the optic nerve and attenuated arterioles.48 Reports describing this dystrophy focus on several identified Dutch families, and mutations in the IMPG1 gene have been implicated in its development.49 Patients sometimes had nyctalopia even when young, but generally started to have mildly reduced vision in the 20/40 range in the 4th or 5th decades. Bulls-eye pigmentary changes can occur prior to symptom onset. Generally, ERG showed rod greater than cone dysfunction even prior to symptom onset, and could be non-recordable for patients in their 40's. Visual fields varied from normal to paracentral defects, to diffuse constriction. Fluorescein angiograms showed mid-peripheral punctate hyperfluorescence even in patients without otherwise evident peripheral changes on exam. In the earlier stages of the disease course, this dystrophy has significant phenotypic overlap with HCQ toxicity in both mild and severe disease phenotypes. The potential differentiators to indicate BCAMD are symptoms of nyctalopia prior to HCQ usage, family history of retinal disease, very severely attenuated ERG with modest clinical retinopathy, bone spicule pigmentary changes. Lack of these, in the context of HCQ exposure of proper dosage and duration would suggest that HCQ toxicity is the far more likely diagnosis. Genetic testing would be necessary for definitive diagnosis of BCAMD.
6.7 Fenestrated sheen macular dystrophy
Fenestrated sheen macular dystrophy (FSMD) is another rare, slowly progressive macular dystrophy, first described in 1979 by O'Donnell and Welch50, 51 and in a second family by Slagsvold a year later.52 It is characterised by the macula appearing to have a whitish to yellowish-green diffuse sheen. There is central hyperpigmentation at the fovea with a parafoveal annulus of hypopigmentation, with the macula showing reddish tinged spots in the macula that appear to be windows or breaks in the sheen. These features have been seen in patients as young as 4 years old. FA is either normal or shows a punctate choroidal hyperfluorescence in the peri-foveal region in younger patients. In later life, one patient was observed to have complete geographic RPE depigmentation in an annular pattern consistent with geographic atrophy, but not definitely shown as the studies predate OCT imaging.50 The patient with the observed atrophy was asymptomatic until age 55 when he noticed a scotoma. No genetic mutation has been identified to be the main cause of FSMD, however recent case report described two patients with the same biallelic mutations in CRB1 (mutations of which are associated with RP, LCA, and various maculopathies) one of whom had clinical features of FSMD. This patient was initially seen at an age of 11 years old, who presented at 26 with small areas of foveal RPE and outer retinal atrophy in both eyes. The sister of that patient, who carried the same two alleles, had a much more mild phenotype, only showing mild petaloid RPE changes at 21 years of age.53 All patients examined with FSMD in the original papers had normal visual acuity with normal to moderate red-green deficient colour vision, and normal or nearly normal ERG testing.
6.8 Cone and cone-rod dystrophies
Cone dystrophy (COD) and cone-rod dystrophy (CORD) are inherited retinal disorders arising from a large number of identified gene mutations that affects the function and survival of cone and rod photoreceptor cells (cones more severely than rods), leading to progressive central vision loss.54-57 These diseases and HCQ retinal toxicity can present with similar symptoms such as vision loss and defects in colour vision.58, 59 Although in many patients COD and CODR present as children, there is significant variability in symptom onset and severity, which could lead to diagnostic uncertainty in some patients. The differentiation between these two conditions is crucial since HCQ retinal toxicity can be arrested if caught early, while cone-rod dystrophy is a progressive and irreversible condition with no known cure.6
In addition to the clinical history of long-term HCQ use, visual field testing, electroretinography (ERG), and spectral-domain optical coherence tomography (SD-OCT), and genetic testing all play roles in differentiating these entities. Patients with COD/CORD are quite symptomatic with decreased visual acuity and colour perception, while HCQ toxicity is often asymptomatic in the early stages. On visual field testing, early cone-rod dystrophy may reveal a central scotoma that can be associated with a bulls-eye maculopathy or central macular pigmentary changes or atrophy on fundus exam with corresponding hypo and hyperautofluorescence on FAF imaging. In the case of bull's eye maculopathy in COD/CORD there is often still lesser involvement of the subfoveal outer retina.58 In contrast, HCQ toxicity spares the central fovea until advanced stages, showing paracentral relative scotomas on visual field testing with corresponding outer retinal changes on OCT.54 Superonasal paracentral scotoma is the most common presentation with the inferotemporal parafoveal region most commonly the first involved.59 FAF imaging in HCQ toxicity tends to show a parafoveal hyperautofluorescence in early toxicity, with hypoautofluorescent and mottling in later stages as RPE degeneration progresses.60 COD/CORD can have quite variable FAF changes, but can show FAF mottling even atrophy quite quickly after symptom onset. A thin hyper-autofluorescent ring at the transition between affected and more normal retina/RPE can also be seen in COD/CORD, whereas the hyperautofluorescent ring of HCQ toxicity is frequently more diffuse. SD-OCT may reveal thinning and disorganisation of the ONL, EZ, and IZ in both conditions.22, 56, 58, 61, 62 Full field and mfERG can be very useful in differentiating these pathologies. In cone-rod dystrophy, both cone and rod responses are reduced, with cone responses being more severely affected than rod responses. In contrast, HCQ toxicity generally shows an isolated reduction in the amplitude of the cone response with little to no effect on rod response.21 Responses in COD/CORD tend to be centrally depressed also, thus would not be expected to show the same changes to the ring ratios.63 Ultimately, if there is suspicion for COD/CORD, then genetic testing should be persued, though a genetic mutation is identified in only approximately 60% of cases of COD/CORD, the majority of which are inherited in an autosomal recessive pattern.58
7 RETINOPATHIES ASSOCIATED WITH SYSTEMIC DISEASES
There are several retinopathies associated with systemic conditions that do result in a bulls-eye maculopathy that we will review. For most of these conditions, either the age or prominent systemic symptoms would likely keep these conditions from being commonly considered in most patients at risk for HCQ toxicity.
7.1 Bardet-Biedl syndrome
Bardet-Biedl syndrome (BBS) is one of a class of conditions with affect proteins involved structure and function of the cilia, and the photoreceptor outer segment is a modified cilium, thus many ciliopathies display an associated retinal dystrophy.64-66 Other well-known syndromes in this class include Leber Congenital Amaurosis, Usher syndrome, and Alstom syndrome, which all mainly produce retinal dystrophies of types or early enough in life to be unlikely to be considered in patients take HCQ.66
BBS is an autosomal recessive ciliopathy caused by mutations in one or more of 21 different genes, causing a constellation of polydactyly (most commonly postaxial), variable cognitive impairment, hypogonadism, renal anomalies, and retinopathy.64-66 The retinopathy can be an be either rod-cone, cone-rod, or global phenotype, depending on the mutation. Similarly, there can be patients that present with central involvement first, but given the young age and rapid progression, tends to become global quickly. Visual symptoms are similar to other primary retinal dystrophies (e.g., nyctolopia, peripheral vision issues), and tend to start in within the first decade, progressing to legal blindness by the second decade of life, though some may be later presenting. Many will have significant ERG abnormalities even in early childhood.
Though there is overlap of features of HCQ toxicity and the retinopathy in BBS, the other syndromic features of BBS and generally early age of presentation make for it to not be commonly considered on the differential with HCQ toxicity. Look for other syndromic features, such as polydactyly, obesity, and cognitive impairment in a patient with BBS.65, 66 If the patient is an older child or young adult, BBS patients are likely to have severe visual impairment and global photoreceptor dysfunction on ERG, rather than more focal deficits on mfERG seen with HCQ toxicity.
7.2 Juvenile neuronal ceroid lipofuscinosis (Juvenile Batten's disease)
This disorder is part of a family of disorders neuronal ceroid lipofuscinoses (NCL's) that cause progressive neurological decline and death. The classical juvenile NCL, or juvenile Batten's disease, is associated with mutations in CNL3, which encodes a transmembrane protein of unclear function. Children generally present with visual changes as the first manifestation at 3–8 years old, with fairly rapid progression of severe vision loss, with functional blindness within 1–2 years.67 On exam, the vision on presentation can be poor bilaterally and nystagmus may be present. Optic disc pallor, arteriolar attenuation, and subtle granularity of the RPE in the macula are consistently observed. A bull's eye maculopathy is common though not always present. Systemically, some do have concurrent changes in behaviour, mood, cognition, or memory on careful history, but will likely develop further neurological symptoms within several years of vision loss.
The young age of presentation and severe vision loss with features similar to cone or cone-rod dystrophy lead to non-syndromic retinal dystrophies to be the most likely items on the differential with JNCL, but since it can present in the less commonly teenage years, HCQ toxicity could potentially be considered in appropriately exposed patients. Concomitate neurocognitive or neurological symptoms should raise suspicion for systemic disease. Imaging findings for JNCL include marked central foveal hypofluorescence on FAF with possible ring of hyper-FAF, and diffuse profound EZ and general outer greater than inner retinal atrophy on OCT that does not respect the immediate parafoveal area in most cases. Electrophysiological testing shows severe rod and cone dysfunction, with complete lack of ERG either photopic or scotopic flash ERG in many cases.67, 68 Diagnostic tests for JNCL include characteristic vacuoles observed in the lysosomes of lymphocytes on a peripheral blood smear, with confirmation by genetic testing or CNL3 mutations.
7.3 Pantothenate kinase-associated neurodegeneration (formerly Hallervorden-Spatz syndrome)
This is a rare (incidence in 1–2 per million) autosomal recessive progressive neurodegenerative condition causing progressive dystonia, dysarthria, and rigidity, along with iron accumulation in the brain and in some cases a retinopathy.69, 70 It is caused by mutations in the PANK2 gene and presents classically in the first decade of life but can present atypically in the second decade. A retinopathy is present about 70% of classical cases, less frequently in atypical cases.71 The first symptom is often nyctalopia, followed by progressive loss of visual fields and central acuity. The retinopathy is most often is a pigmentary retinopathy similar in appearance to RP, but can also present as a bullseye maculopathy.69 ERGs in patients without apparent retinopathy often show mild to moderate cone abnormalities and show more severe generalised photoreceptor dysfunction in those with pigmentary retinopathy.72 All patients who have retinopathy (which can present early in the disease process) also have neurological symptoms, thus it poses less of a diagnostic challenge as long as the clinician has PKAN on the differential if the patient does not already have the diagnosis. Diagnosis of PKAN is made by characteristic findings on brain MRI of iron deposition, including the ‘eye of the tiger’ sign and genetic testing.71 Referral to neurology is recommended if suspected.
7.4 Alport syndrome
Alport syndrome is an inherited clinical syndrome caused by defective type IV collagen synthesis, causing some combination of ocular abnormalities, progressive renal failure, and sensorineural hearing loss. It is caused by mutations in COL4A5 (inherited in X-linked fashion), or COL4A3 and COL4A4 (autosomal recessive) genes. Ocular findings associated with this syndrome are varied, including corneal opacities, anterior lenticonus, retinopathy (fleck retinopathy is most common), temporal retinal thinning, lamellar or giant macular holes.73 There has been a case report showing bulls eye maculopathy resulting from Alport syndrome.74 The patient reported, who also had anterior lenticonus in both eyes, had a bulls-eye pattern of parafoveal depigmentation in only one eye, with BCVA of 20/40 in the affected eye, and 20/50 in the other. The fundus had perifoveal hypopigmentation, which had corresponding flecked hyperautofluorescence on FAF, hyperfluorescence with subtle leakage on FA, and subfoveal (but not perifoveal) RPE thickening on OCT. There was subtle thinning of the photoreceptor outer segments overlying the RPE changes, and more pronounced temporal thinning of the outer plexiform and inner retinal layers. The EZ/IZ were intact in this patient. No comment was made regarding the visual fields for this patient. These subfoveal RPE changes are distinct from HCQ toxicity, which will affect the parafoveal EZ/IZ. Therefore, the combination of OCT findings and flecked hyperautofluorescence easily distinguish reported findings of Alport syndrome from HCQ toxicity, with the array of features more closely matching those typically found in MacTel type 2.
7.5 Conclusion
HCQ retinal toxicity is an important and distinct clinical entity that can be differentiated from its mimickers by thorough clinical and ancillary testing as summarised in this review. This review highlights the clinical stages of the disease that can resemble various other disorders, along with the key differentiating features that can nudge a comprehensive ophthalmologist or a primary care physician in the right direction. With newer diagnostic methods and informatics techniques on the horizon, early differentiation and identification of this disease continues to evolve and merits ongoing investigation.
FUNDING INFORMATION
None.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.




