Endoscopic cyclophotocoagulation versus second glaucoma drainage device after prior aqueous tube shunt surgery
Abstract
Background
To evaluate the efficacy in controlling intraocular pressure (IOP) with endoscopic cyclophotocoagulation (ECP) versus implantation of a second glaucoma drainage device (GDD-2) in the treatment of uncontrolled glaucoma with a prior aqueous tube shunt.
Design
A nonrandomized retrospective chart review.
Participants
Patients with refractory glaucoma following a failed initial tube shunt (Baerveldt Glaucoma Implant 350), who underwent ECP or GDD-2 with Baerveldt Glaucoma Implant as a second surgery. Twenty-five eyes underwent ECP, and 48 eyes received a GDD-2.
Methods
ECP or second tube-shunt surgery.
Main Outcome Measures
Reduction in IOP and antiglaucoma medications, and Kaplan–Meier survival with success defined as lOP ≥ 5 mmHg and ≤ 21 mmHg and ≥ 20% reduction from preoperative IOP. Secondary outcome measures were visual acuity and the presence of any postoperative complications.
Results
Both ECP and GDD-2 significantly lowered IOP (Student's t test) and number of antiglaucoma medications (Wilcoxon paired signed rank test). There were no significant differences in postoperative IOP (Student's t test) or antiglaucoma medications (Mann Whitney test) between ECP and GDD-2 at 6 and 12 months. There was also no difference in the Kaplan–Meier survival outcomes between the two groups.
Conclusion
Both ECP and GDD-2 are both effective as second surgeries for refractory glaucoma that has failed a prior aqueous shunt.
Introduction
Glaucoma treatment in patients who fail to achieve adequate intraocular pressure (IOP) control despite a patent and functional tube shunt in conjunction with maximal tolerated medical therapy continues to be a challenge. While the early results of the tube versus trabeculectomy study1-3 influenced a shift towards early implantation of a second glaucoma drainage device (GDD-2) in patients with such cases of refractory glaucoma, implantation of a second tube shunt increases the risk of strabismus, diplopia, discomfort and tube exposure.4-8
The recent advent of endoscopic cyclophotocoagulation (ECP)5, 9-13 has shown advantages of long-term safety and efficacy,9, 10, 14 particularly in its significantly lower rates of postoperative complications traditionally associated with transscleral cyclodestructive procedures, such as hypotony, phthisis, inflammation and vision loss.15 ECP provides the additional advantage of sparing vital structures of the eye, such as the sclera and conjunctiva, leaving them intact for future glaucoma surgeries, if needed.12
Currently, there is no gold standard for the treatment of refractory glaucoma that has failed a prior aqueous tube shunt. Because of its direct approach to the ciliary body, ECP may be a reasonable option that is comparable to a second tube shunt implantation in these eyes. This study was conducted to evaluate the efficacy of ECP compared to the implantation of a GDD-2 in the treatment of uncontrolled glaucoma with a prior aqueous tube shunt.
Methods
Study design
This was a retrospective, nonrandomized, interventional case series study performed at a single academic institution. By chart review, we identified pseudophakic patients with medically refractory glaucoma who underwent initial aqueous tube shunt implantation with the Baerveldt Glaucoma Implant 350 (Abott Medical Optics, Santa Ana, CA), who then required a second surgery of either ECP or GDD-2 in order to achieve adequate IOP control at the Glaucoma Service at University of Southern California Eye Institute. All patients had a minimal potential follow-up of 2 years.
Written informed consent was obtained from each patient prior to surgery. Institutional Review Board approval was obtained from the University of Southern California, and all research conformed to the tenets of the Declaration of Helsinki. Patients with all types of glaucoma refractory to other medical and surgical treatments were included except for neovascular glaucoma (Table 1). This exclusion is because of our practice of not performing ECP on patients with neovascular glaucoma, thus not having a comparison between treatment groups for this diagnosis. Inclusion criteria were a diagnosis of open angle, angle closure or secondary glaucoma, greater than 6 months after previous glaucoma aqueous tube shunt surgery, and inadequate IOP control (>21 mm Hg) on two or more topical glaucoma medications or with IOP ≤ 21 mm Hg but above a predetermined target IOP (based on baseline IOP, severity of optic nerve or visual field damage, or progression of visual field loss), or intolerant of medical therapy or on an oral carbonic anhydrase inhibitor, and visual acuity (VA) better than light perception (LP). Exclusion criteria were VA light perception or worse, prior transscleral ciliary body ablation (cyclophotocoagulation) procedures and nonfunctional (nonpatent) aqueous shunt without fluid drainage to plate. The patency of the tube shunt was assessed clinically by slit lamp biomicroscopy or by B-scan ultrasound to determine the presence of a fluid bleb over the plate.
| Types of glaucoma | ECP (n = 25) n (%) | GDD-2 (n =48) n (%) | Chi-square or Fisher exact P-value |
|---|---|---|---|
| POAG | 12 (48%) | 18 (36.7%) | 0.35 |
| CACG | 3 (12%) | 12 (24.5%) | 0.37 |
| Juvenile onset glaucoma | 1 (4%) | 2 (4.1%) | 1.00 |
| Secondary glaucoma† | 9 (36%) | 13 (26.5%) | 0.40 |
| Steroid glaucoma | 0 | 1 (2.0%) | 1.00 |
| Congenital glaucoma | 0 | 2 (4.1%) | 0.55 |
- † ECP secondary glaucoma breakdown: PK 5 (20%), ICE 2 (8%), Traumatic 2 (8%); GDD-2 secondary glaucoma breakdown: Traumatic 2 (4.1%), Inflammatory 9 (18.4%), Uveitic 2 (4.1%).
- CACG, chronic angle closure glaucoma; ECP, endoscopic cyclophotocoagulation; GDD, glaucoma drainage device; ICE, iridocorneal endothelial syndrome; PK, penetrating keratoplasty; POAG, primary open angle glaucoma.
Preoperative data collected included patient age, gender, glaucoma diagnosis, number and type of previous ocular surgeries including glaucoma surgeries and cataract extraction (CE), lens status, IOP, VA, number of antiglaucoma medications, cup-to-disc ratios, and visual field mean deviation (MD) and pattern standard deviation (PSD). IOP was measured by Goldmann applanation tonometry by a trained certified ophthalmic technician. The average of measurements taken on two consecutive visits prior surgery was used for preoperative IOP. Postoperative data collected included IOP, VA, number of antiglaucoma medications, duration of follow-up, and presence of any postoperative complications related to the study procedure, and any surgical interventions required to manage complications. Postoperative data were abstracted from follow-up evaluations at 3 months (±1 month), 6 months (±1 month), 1 year (±3 months) and 2 years (±3 months).
Main outcome measures
The main outcome measures were mean reduction in IOP and antiglaucoma medications at 12 and 24 months after ECP, and Kaplan–Meier survival with success defined as achievement of lOP ≥ 5 mmHg and ≤ 21 mmHg and ≥20% reduction from preoperative IOP. Follow up was at 1, 3, 6, 12, 18 and 24 months. Failure of treatment was defined as continued uncontrolled IOP > 21 mmHg or IOP < 5 mmHg on two consecutive follow-up visits after 1 month, IOP reduction < 20% from preoperative baseline, need for additional glaucoma surgeries or loss of LP vision. Secondary outcome measures were VA and the presence of any postoperative complications.
Surgical technique
ECP was performed by a single attending (BAF) at the Glaucoma Service of the University of Southern California, and the procedure has been previously described.15 In brief, ECP was performed with an endoscopic probe (Endo Optiks, Little Silver, NJ, USA) delivering 810-nm diode laser (Iridex OcuLight SL, Mountain View, CA, USA) at 250 mW to 350 mW through two clear corneal incisions created temporally and nasally. A continuous 330–360° span of ciliary processes was photocoagulated. The treatment endpoint was shrinkage and whitening of the entire ciliary process. The corneal incisions were closed with 10-0 nylon or vicryl, and a sub-conjunctival injection of 0.1-mL dexamethasone 1% and topical administration of tobramycin-dexamethasone ointment were administered at the conclusion of the procedure.
GDD-2 surgical interventions were performed by several attending physicians at the Glaucoma Service at the University of Southern California. GDD-2 included implantation of either a Baerveldt 350 mm2 or 250 mm2 (Abott Medical Optics, Santa Ana, CA, USA). Devices were implanted superonasally, because of the existence of a prior aqueous tube shunt superotemporally. For either implant, a 90° conjunctival and Tenon's capsule peritomy was performed. The implant plate was placed under or between the superior and medial rectus muscles, with the eyelets sutured to the sclera with 8-0 nylon suture. Both types of tubes were primed with balanced salt solution, and the tubes were tied closed with 7-0 vicryl. A 23-guage needle was used to enter the anterior chamber 1 mm posterior to the limbus and in the iris plane. The tube was cut bevel up and placed into the anterior chamber through this track. Externally, the tube was fastened to sclera using 8-0 polyglactin suture and then covered with donor pericardium or sclera patch graft. The peritomy was closed with an 8-0 polyglactin suture. Sub-conjunctival injections of 0.1-mL dexamethasone 1% and cefazolin (40 mg) were placed, and tobramycin-dexamethasone ointment was applied.
Statistical analysis
For preoperative and postoperative comparisons, the paired Student's t test was used for parametric data (IOP) and the Wilcoxon paired signed rank test was used for nonparametric data (number of medications, logarithm of the minimum angle of resolution [logMAR]) at 1, 3, 6, 12, 18 and 24 months. For comparisons between groups, Student's t test was used for parametric data (IOP, age), the Mann–Whitney test was used for nonparametric data (number of medications, logMAR) and the Fisher exact test was used for frequencies or proportions (gender, complications). The Wilcoxon's test was used to compare proportions, Sign's test for binary variables, analysis of variance (ANOVA-F) when the parametric tests basic assumptions were satisfied and Student's t test for linear variables. A Kaplan–Meier survival curve was created for both procedures, and the ECP and GDD-2 groups were compared with the log-rank test. Patients who failed were still included in the comparison of means, but censored from the survival curve from that point on. Statistical significance was defined as P < 0.05. Statistical analysis was performed with SAS V9.3 programming language (SAS Inst., Cary, N.C.)
Results
Seventy-three patients were included in the study; 25 underwent ECP and 48 had GDD-2 (26 Baerveldt 250, 22 Baerveldt 350). Only one eye of each patient was included. Glaucoma diagnoses are displayed in Table 1, and demographic data are displayed in Table 2. There were no statistical differences noted between the groups with respect to mean age at the time of surgery, gender, mean preoperative IOP, cup-to-disc ratio, MD on visual fields, PSD on visual fields and preoperative best corrected VA.
| Demographics | ECP (n = 25) | GDD-2 (n = 48) | P-value* |
|---|---|---|---|
| Sample size | 25 eyes of 25 patients | 48 eyes of 48 patients | |
| Age (years; mean ± SD) | 59.2 ± 17.3 | 60.6 ± 15.6 | 0.73 |
| Range | 13–87 | 27–88 | |
| Gender, n (%) | 0.79 | ||
| Female | 11 (44.0%) | 20 (41.7%) | |
| Male | 14 (56.0%) | 28 (58.3%) | |
| Cup-to-disc ratio, n = 14, 40 | 0.90 ± 0.60 | 0.98 ± 0.66 | 0.66 |
| Visual field MD, n = 10, 23 | −13.94 ± 6.32 | −17.33 ± 8.68 | 0.29 |
| Visual field PSD, n = 10, 23 | 6.96 ± 3.05 | 6.71 ± 3.07 | 0.86 |
| Preoperative visual acuity; median (range) | 20/300 (20/20-LP) | 20/300 (20/25-LP) | 0.35 |
- ECP, endoscopic cyclophotocoagulation; GDD, glaucoma drainage device; MD, mean deviation; NLP, non light perception; PSD, pattern standard deviation.
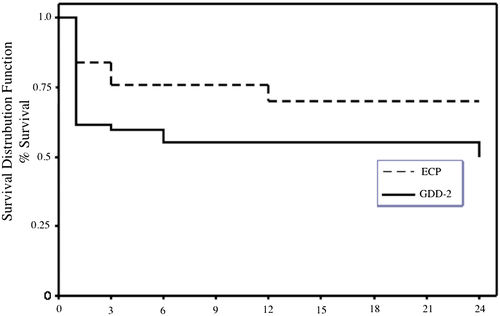
Kaplan–Meier survival functions are shown in Figure 1. Cumulative probabilities of success at 12 months were 0.70 (95% confidence interval [CI], 0.47–0.85) for the ECP group and 0.56 (95% CI 0.40–0.68) for the GDD-2 group. At 24 months, successes were 0.70 (95% CI 0.47–0.85) for the ECP group and 0.51 (95% CI 0.34–0.66) for the GDD-2 group. Overall, there was no significant difference between survival rates of the ECP and GDD-2 groups based on the Kaplan–Meier survival functions (P = 0.12, log rank test; P = 0.09, Breslow generalized Wilcoxon test).
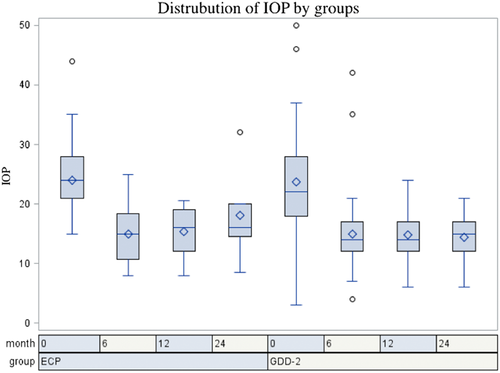
Postoperative IOP was significantly lower than preoperative IOP for both ECP and GDD-2 at all time points for both ECP and GDD-2 groups (P < 0.05 for all time points) (Table 3). There was no significant difference in IOP between ECP and GDD-2 at 6 months (T-value 0.0494, P > 0.5) and 12 months (T-value 0.4924, P > 0.5). Figure 2 illustrates pre- and post-operative IOP by intervention.
| IOP mmHg | ECP (n = 25) | GDD-2 (n = 48) | P-value* |
|---|---|---|---|
| IOP mmHG, (n mean ± SD) | |||
| Preoperative | 25 24.0 ± 6.2 | 48 23.5 ± 8.1 | 0.85 |
| 3 months | 25 13.0 ± 3.4 | 47 14.2 ± 5.5 | 0.19 |
| 6 months | 23 14.9 ± 4.9 | 43 15.2 ± 6.3 | 0.98 |
| 12 months | 19 15.4 ± 3.8 | 30 14.2 ± 4.0 | 0.61 |
| 24 months | 11 18.1 ± 7.4 | 20 14.6 ± 3.8 | 0.14 |
| IOP decrease | |||
| 3 months | 11.0 ± 7.7*** | 9.3 ± 8.9*** | 0.29 |
| 6 months | 8.7 ± 8.6*** | 8.3 ± 11.1*** | 0.64 |
| 12 months | 7.8 ± 6.5*** | 9.3 ± 8.3*** | 0.66 |
| 24 months | 7.0 ± 8.8** | 8.9 ± 7.6*** | 0.52 |
| IOP % decrease | |||
| 3 months | 42.0 ± 20.8*** | 39.6 ± 20.6*** | 0.20 |
| 6 months | 32.4 ± 29.3*** | 35.3 ± 28.6*** | 0.33 |
| 12 months | 30.8 ± 21.6*** | 39.6 ± 30.7*** | 0.56 |
| 24 months | 25.5 ± 34.2** | 38.7 ± 27.8*** | 0.50 |
- Wilcoxon test
- ** P < 0.05.
- *** P < 0.001.
- ECP, endoscopic cyclophotocoagulation; GDD: glaucoma drainage device.
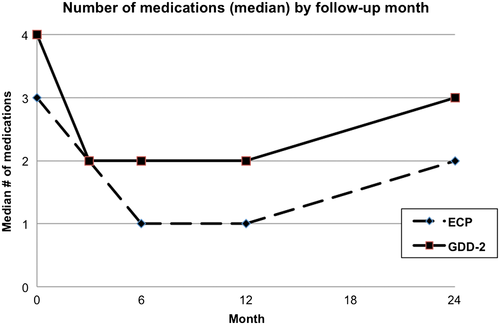
The median number of IOP lowering medication was 3 in the ECP group and 4 in GDD-2 group. There was no significant difference in number of antiglaucoma drops used between ECP and GDD-2 at 6 months (T-value 1.5807, P > 0.5) and 12 months (T-value 0.0674, P > 0.5) (Table 4). Both groups experienced a decrease in the number of medications necessary to achieve adequate IOP after the surgery; this effect lasted throughout the 2-year follow-up (Fig. 3).
| Medications | ECP (n = 25) | GDD-2 (n = 48) | P-value* |
|---|---|---|---|
| Medications, median (range) | |||
| Preoperative | 3 (0–4) | 4 (0–5) | 0.22 |
| 3 months | 2 (0–5) | 2 (0–4) | 0.88 |
| 6 months | 1 (0–4) | 2 (0–5) | 0.13 |
| 12 months | 1 (0–5) | 2 (0–4) | 0.37 |
| 24 months | 2 (0–5) | 3 (0–5) | 0.61 |
| Decrease | Median; mean ± SD | Median; mean ± SD | |
| 3 months | 1 1.4 ± 1.3 | 2 1.6 ± 1.8 | 0.57 |
| 6 months | 1 1.7 ± 1.4 | 2 1.4 ± 1.6 | 0.64 |
| 12 months | 2 1.6 ± 1.5 | 1 1.5 ± 1.8 | 0.74 |
| 24 months | 1 1.5 ± 1.9 | 1 0.9 ± 1.6 | 0.50 |
- ECP, endoscopic cyclophotocoagulation; GDD: glaucoma drainage device.
Failures occurred in both groups because of inadequate pressure control or increased antiglaucoma medications (Table 5). Complications, which were hypotony, corneal oedema, high IOP, inflammation and cystoid macular edema, were seen in both groups and did not show any statistically significant difference (P > 0.05). In the ECP group, seven of the 25 eyes eventually failed, four according to our IOP failure criteria and three because of increased medications following surgery. In the GDD-2 group, 22 of 48 eyes eventually failed, nine because of high pressure, nine because of increased medications and one because of hypotony. No eyes progressed to loss of LP in either group. Cumulative success for ECP and GDD-2 throughout the last follow-up at 24 month did not reach statistically significant difference (Log-Rank test, P = 0.12; Breslow generalized Wilcoxon, P = 0.09).
| ECP (n = 25) | GDD-2 (n = 48) | P-value | |
|---|---|---|---|
| Life-table (survival) analysis | Log-Rank test P = 0.12 | ||
| Cumulative success (%) ± SE, (95% confidence limit) | Breslow generalized Wilcoxon P = 0.09 | ||
| 3 months | 76.0 ± 8.5 (62.8, 93.7) | 60.6 ± 7.4 (45.2, 72.6) | |
| 6 months | 76.0 ± 8.5 (62.8, 93.7) | 56.3 ± 7.1 (40.9, 69.0) | |
| 12 months | 70.2 ± 9.7 (46.8, 84.8) | 55.8 ± 7.2 (39.9, 68.3) | |
| 24 months | 70.2 ± 9.7 (46.8, 84.8) | 51.0 ± 8.0 (34.4, 65.7) | |
| Success | 18 (72.0%) | 25 (52.1%) | |
| Failure | 7 (28.0%) | 22 (45.8%) |
- ECP, endoscopic cyclophotocoagulation; GDD, glaucoma drainage device.
A multivariate Cox proportional hazard analysis was performed to analyse predictors of failure for each group but could not reach a statistically significant difference; thus, ECP and GDD-2 groups were combined, and preoperative IOP < 22 mmHg was found to be a risk factor for failure with a hazard ratio of 3.07 (1.33, 7.08 95% CI, P = 0.0085). In addition, a number of preoperative glaucoma medications < 4 was a significant predictor of failure with a hazard ratio of 2.70 (1.17, 6.23 95% CI, P = 0.0197).
Discussion
When selecting a surgical treatment modality to control IOP after clinical failure of a prior aqueous tube shunt, the risk of postoperative complications and long-term efficacy of the second surgery must be considered carefully. Theoretically, the addition of a procedure that decreases aqueous inflow to an existing procedure that increases aqueous outflow should result in successful reduction of IOP and antiglaucoma medications. Francis et al.11 demonstrated the efficacy and safety of ECP in the treatment of refractory glaucoma with a prior aqueous tube shunt. Using a success definition of IOP reduction ≥ 3 mmHg or a reduction in antiglaucoma medications in the medication-intolerant group with IOP ≤ 21 mmHg, they reported a cumulative success rate of 88% at 6 months, which remained at this level at last follow-up of 2 years.
Lima and colleagues' prospective trial of ECP and Ahmed Valve as a second surgery for refractory glaucoma with a prior trabeculectomy with antimetabolite and IOP ≥ 35 mmHg showed a similar success rate at 2 years between the two groups: 74% for the ECP group and 71% for the Ahmed group.16 A 210-degree ECP treatment was performed via a pars plana approach. Success was defined as IOP between 6 and 21 mmHg with or without medications at 24 months follow-up in this study.
A retrospective evaluation of ECP by Chen et al.9 shows a success rate of 94% at 1 year and 82% at 2 years of follow-up. Mean glaucoma medications decreased from 3.0 ± 1.3 to 2.0 ± 1.3 at last follow-up. The success definition used in this study was ≤21 mmHg with or without glaucoma medications. This study included various glaucoma diagnoses including primary open angle, congenital, chronic angle closure, uveitic, pseudoexfoliation, neovascular and angle recession, and did not differentiate between eyes with prior glaucoma surgery.
In our study of refractory glaucoma with a prior aqueous tube shunt, both ECP and GDD-2 successfully lowered IOP and number of antiglaucoma medications as a second surgery. The GDD-2 group included both 250 and 350 sizes of Baerveldt Glaucoma Implants. Of note, cumulative success was higher for ECP than GDD-2 throughout last follow-up at 24 months. This difference showed a slight trend toward significance but did not reach statistical significance (Log-Rank test, P = 0.12; Breslow generalized Wilcoxon, P = 0.09), perhaps as a result of the smaller sample size of ECP patients compared to GDD-2. A weakness of this study is that no sample size power calculation was made because of the retrospective design. The clinical significance of this trend in our series bears further study in a prospective manner.
It is important to note that this study was retrospective and that patients were not randomly assigned to ECP or shunt implantation as a second surgery. Although the preoperative factors are similar between the two groups, this selection bias does not allow for a fair comparison between the two surgical interventions. Also, our ECP treatment is a near-total 360-degree treatment of all the ciliary processes, which is a more aggressive treatment than in prior studies.
Our study supports the efficacy and safety of both ECP and GDD-2 implantation as a second surgery in eyes with refractory glaucoma with a prior aqueous tube shunt. Both options should be considered for patients requiring a second intervention, with careful attention to the potential postoperative complications caused by each in these patients with multiple prior surgeries.