Oligomeric amyloid β facilitates microglial excitotoxicity by upregulating tumor necrosis factor-α and downregulating excitatory amino acid transporter 2 in astrocytes
Abstract
Objectives
Soluble oligomeric amyloid β (oAβ) directly causes synaptic dysfunction and neuronal death, which are involved in the pathogenesis of Alzheimer's disease. In contrast, several lines of evidence have shown that glia-mediated excitotoxicity is also involved in the disease progression of Alzheimer's disease. However, it is still unclear how oAβ induces glia-mediated excitotoxicity. Therefore, we tried to clarify the mechanism of oAβ-induced glia-mediated excitotoxicity using mouse primary cultures.
Methods
Glial glutamate production was assessed using a colorimetric assay kit. Glial production of tumor necrosis factor-α (TNF-α) was evaluated using a specific enzyme-linked immunosorbent assay kit. Microglial survival was examined using the MTS assay. mRNA and protein expression levels of excitatory amino acid transporter 2 were determined using quantitative polymerase chain reaction and western blotting, respectively.
Results
We showed that oAβ-stimulation induces glutamate release from astrocyte/microglia co-cultures, but not from single culture of astrocytes or microglia. oAβ increased TNF-α release from astrocytes, but not from microglia, and the astrocyte-derived TNF-α enhanced glutamate release from microglia. TNF-α elongated microglial survival and triggered sustained microglial glutamate release in a positive feedback mechanism through TNF-α receptor 1. Furthermore, treatment of oAβ decreased astrocytic expression of excitatory amino acid transporter 2, which plays a pivotal role in homeostasis of the extracellular glutamate level.
Conclusions
The present findings suggest that oAβ contributes to glia-mediated excitotoxicity through the two-hit mechanism by suppressing astrocytic glutamate uptake through excitatory amino acid transporter 2 and enhancing microglial glutamate release, and triggers chronic glial neuroinflammation through the TNF-α/TNF-α receptor 1 positive feedback loop in Alzheimer's disease.
Introduction
Alzheimer's disease (AD) is the most common cause of dementia in the elderly.1 A pathological hallmark of AD is senile plaque composed of fibrillar amyloid β (Aβ). Although fibrillar Aβ reportedly induces neuronal dystrophy and tau hyperphosphorylation, recent evidence suggests that soluble oligomeric Aβ (oAβ), rather than fibrillar Aβ, contributes to AD pathogenesis by higher neurotoxicity including inhibition of hippocampal long-term potentiation and disruption of synaptic plasticity.2-7
Progressive activation of astrocytes and microglia is another pathological hallmark of AD, which represents a link between neuroinflammation and neurodegeneration in AD pathogenesis.8 Excessive glutamate from activated glial cells causes excitotoxicity that contributes to the disease progression of AD.9, 10 Furthermore, astrocytes in AD patients showed a decreased expression level of excitatory amino acid transporter 2 (EAAT2), resulting in disruption of homeostasis of the extracellular glutamate level.11 As the proof of the involvement of excitotoxicity in AD pathogenesis, a N-methyl-D-aspartate (NMDA) receptor blocker, memantine, is clinically used for AD treatment.12 We also reported that blockade of microglial glutamate release significantly improved memory impairment in a mouse model of AD.13 However, it is still uncertain whether oAβ directly induces glia-mediated excitotoxicity in AD.
In the present study, we found that oAβ induced tumor necrosis factor-α (TNF-α) release from astrocytes, and the TNF-α activated microglia to release glutamate. oAβ also downregulated EAAT2 expression in astrocytes. Furthermore, TNF-α triggered sustained microglial glutamate release and elongated microglial survival. Our findings showed that oAβ induces glia-mediated excitotoxicity through the two-hit mechanism by suppressing astrocytic glutamate uptake and enhancing microglial glutamate release, and triggers chronic glial neuroinflammation through the TNF-α/TNF-α receptor 1 (TNFR1) positive feedback loop in AD.
Materials and methods
Reagents
Recombinant mouse TNF-α, goat monoclonal anti-mouse TNF-α neutralizing antibody, hamster monoclonal anti-mouse TNFR1 neutralizing antibody and hamster monoclonal anti-mouse TNF-α receptor 2 (TNFR2) neutralizing antibody were purchased from R&D Systems (Minneapolis, MN, USA). Synthetic human Aβ1–42 was obtained from Peptide Institute (Osaka, Japan).
Preparation of oligomeric Aβ1–42
Soluble oligomeric amyloid β1–42 (oAβ1–42) was prepared as described previously.14 Briefly, synthetic human Aβ1–42 was dissolved in 100% 1,1,1,3,3,3-hexafluoro-2-propanol at a concentration of 1 mmol/L. This solution was completely dried by a vacuum desiccator. The obtained film was resuspended in dimethyl sulfoxide to a concentration of 5 mmol/L, and diluted with Dulbecco's Modified Eagle Medium/F12 (Invitrogen, Carlsbad, CA, USA) at a concentration of 100 μmol/L. This solution was incubated at 4°C for 24 h to obtain oAβ1–42. Oligomerization was confirmed using western blotting and transmission electron microscopy as described previously.14 A final concentration of 5 μmol/L oAβ1–42 was used in all experiments.
Cells
All protocols were approved by the Animal Experiment Committee of Nagoya University (approved number: 14018). C57BL/6J mice were purchased from Japan SLC (Hamamatsu, Japan). Primary microglia cultures were isolated from primary mixed glial cell cultures prepared from newborn mice on day 14 using the “shaking off” method as described previously.15 The purity of the cultures was >99%, as determined by anti-CD11b immunostaining. Primary astrocyte cultures were purified from primary mixed glial cultures by three to four repetitions of trypsinization and replating as described previously.16, 17 The purity of astrocytes was >95%, as determined by glial fibrillary acidic protein-specific immunostaining. The cultures were maintained in the culture medium (Dulbecco's modified Eagle's minimum essential medium; Sigma-Aldrich, St. Louis, MO, USA) supplemented with 10% fetal bovine serum (Equitech-Bio, Kerrville, TX, USA), 5 μg/mL bovine insulin (Sigma-Aldrich) and 0.2% glucose. Microglia and astrocytes were plated at a density of 1 × 105 cells/well respectively in 48-well multidishes. To prepare astrocyte/microglia co-culture, 1 × 105 of microglia were added to 1 × 105 of astrocyte cultures.
Enzyme-linked immunosorbent assay of TNF-α
Cells were treated with 5 μmol/L oAβ for 0–48 h. Then, TNF-α concentration in the culture supernatants was assessed using a specific enzyme-linked immunosorbent assay kit (R&D Systems). Assays were carried out in three independent trials.
Measurement of glutamate release
To assess the effect of oAβ on glial glutamate release, astrocytes and microglia were treated with 5 μmol/L oAβ for 0–48 h. To evaluate the effect of conditioned media on glial glutamate release, astrocyte-conditioned media (ACM) or microglia-conditioned media (MCM) were collected 24 h after stimulation. Then, microglia were incubated with ACM and astrocytes were incubated with MCM for 24 h, respectively. To confirm the effect of TNF-α on microglial glutamate release, microglia were treated with ACM in the presence of 10 μg/mL anti-TNF-α neutralizing antibody, 20 μg/mL anti-TNFR1 neutralizing antibody or 20 μg/mL anti-TNFR2 neutralizing antibody for 24 h. To examine the effect of TNF-α on the sustained glutamate release from microglia, microglia were treated with 10 ng/mL TNF-α for 4 days. After gentle washing with phosphate-buffered saline three times, microglia were incubated in the new culture medium with or without 10 ng/mL TNF-α. To neutralize TNF-α signaling, 10 μg/mL anti-TNF-α neutralizing antibody, 20 μg/mL anti-TNFR1 neutralizing antibody or 20 μg/mL anti-TNFR2 neutralizing antibody were also added into the culture medium. The medium exchange was carried out every 4 days until day 15. Extracellular glutamate concentrations in the culture supernatants were determined everyday using a colorimetric Glutamate Assay Kit (Yamasa Corporation, Tokyo, Japan) as described previously.18 Assays were carried out in three independent trials.
Assessment of microglial survival
Microglial survival was assessed using the 3-(4,5-dimethyl-thiazol-2yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay with CellTiter 96 Aqueous one solution assay (Promega, Madison, WI, USA) as described previously.19 Assays were carried out in five independent trials.
RNA extraction and reverse transcription polymerase chain reaction
Total RNA was extracted from astrocytes using the RNeasy Mini Kit (Qiagen, Valencia, CA, USA) and reverse transcribed into cDNA using SuperScript II (Life Technologies, Carlsbad, CA, USA), as described previously.20 Expression levels of genes encoding EAAT2 and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were measured using quantitative polymerase chain reaction, which was carried out on cDNA on a Rotor-Gene Q using the Rotor-Gene SYBR Green PCR Kit (Qiagen). Relative expression levels were determined using the ΔΔCT method; the genes of interest were normalized to the geometric mean of GAPDH. The following specific primer sets were used:
- Mouse EAAT2 sense: 5′-ATCAACAGAGGGTGCCAACA-3′;
- Mouse EAAT2 antisense: 5′-GTTTCGGTGCTTTGGCTCAT-3′;
- Mouse GAPDH sense: 5′-TGTGTCCGTCGTGGATCTGA-3′;
- Mouse GAPDH antisense: 5′- CCTGCTTCACCACCTTCTTGA-3′.
Assays were carried out in three independent trials.
Western blotting
Astrocytes were lysed in TNES buffer (50 mmol/L Tris-HCl at pH 7.5, 150 mmol/L NaCl, 1% Nonidet P-40, 2 mmol/L ethylenediaminetetraacetic acid and 0.1% sodium dodecyl sulfate) with protease inhibitor mixture (Complete Mini EDTA-free; Roche Diagnostics, Basel, Switzerland). Cell lysate proteins dissolved in Laemmli sample buffer (20 μg/well) were separated on 4–20% sodium dodecyl sulfate-polyacrylamide gels (Mini-Protean TGX; Bio-Rad, Hercules, CA, USA) and transferred to Hybond-P polyvinylidene difluoride membranes (GE Healthcare, Piscataway, NJ, USA) as described previously.16, 21 The membranes were blocked for 1 h at room temperature with 5% skim milk in Tris-buffered saline containing 0.05% Tween-20, and then incubated overnight at 4°C with rabbit polyclonal anti-EAAT2 antibody (1:50; Oncogene, Cambridge, MA, USA) and mouse anti-β-actin monoclonal antibody (Sigma-Aldrich). After an overnight incubation with primary antibodies at 4°C, each blot was probed with horseradish peroxidase-conjugated anti-mouse immunoglobulin G (GE Healthcare). Blots were then visualized with SuperSignal West Dura Extended Duration Substrate (Thermo Fisher Scientific, Waltham, MA, USA), and quantitated using a CS Analyzer 3.0 system (Atto, Tokyo, Japan). Assays were carried out in five independent trials.
Statistical analysis
Statistical significance was analyzed with one-way analysis of variance followed by post-hoc Tukey's test, using GraphPad Prism version 6.0 (GraphPad Software, La Jolla, CA, USA).
Results
oAβ-treated astrocyte-conditioned media induced glutamate release from microglia
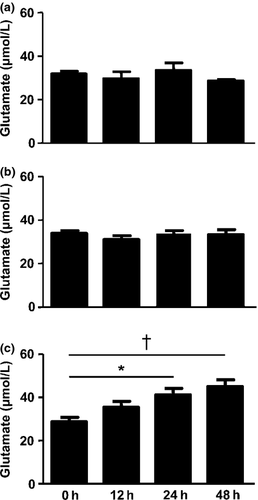
First, we examined whether oAβ affects glutamate release from astrocytes and microglia. Stimulation with oAβ induced glutamate release from astrocyte/microglia co-culture in a time-dependent manner (Fig. 1c), whereas it did not induce glutamate production in single culture of astrocytes (Fig. 1a) and microglia (Fig. 1b).
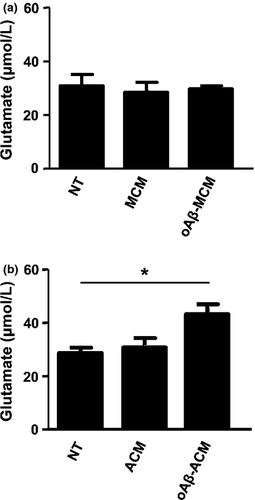
Next, we assessed the glutamate release from astrocytes incubated with oAβ-treated or untreated microglia-conditioned media. As shown in Figure 2a, microglia-conditioned media did not affect glutamate release from astrocytes. We also evaluated the glutamate release from microglia incubated with oAβ-treated or untreated astrocyte-conditioned media. oAβ-treated astrocyte-conditioned media significantly increased glutamate release from microglia (Fig. 2b). These results showed that the soluble factor released by oAβ-treated astrocyte induced microglial glutamate release.
oAβ-treated astrocyte-conditioned media induced glutamate release from microglia
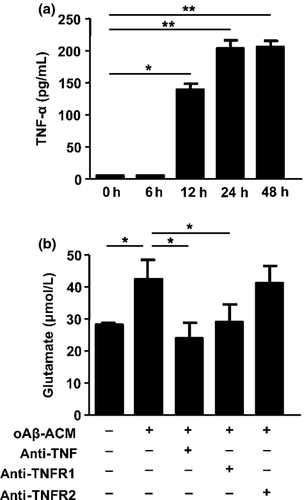
As we identified that TNF-α is a key inducer of glutamate release from microglia, we then assessed whether oAβ induces TNF-α in astrocytes.19 As shown in Figure 3a, oAβ induced astrocytes to release TNF-α whose level reached a plateau at 24 h after stimulation. Next we examined whether TNF-α released from oAβ-treated astrocytes is critical for microglial glutamate production. As expected, blockade of TNF-α or TNFR1 abolished glutamate release from microglia treated with oAβ-treated astrocyte-conditioned media, whereas blocking TNFR2 did not affect microglial glutamate release (Fig. 3b). These results showed that oAβ induce glutamate release from microglia through astrocytic TNF-α.
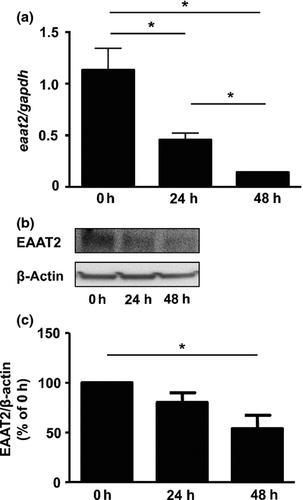
oAβ downregulated EAAT2 in astrocytes
Astrocytes effectively incorporate excessive glutamate released from microglia through EAAT2, and maintain homeostasis of the extracellular glutamate level.16, 22 Therefore, we examined whether oAβ-treatment affects the expression level of astrocytic EAAT2. oAβ significantly decreased both the mRNA and protein expression level of EAAT2 in astrocytes (Fig. 4a–c). We then assessed whether a decrease in astrocytic EAAT2 by oAβ depends on TNF-α. However, blockade of TNF-α did not restore the EAAT2 expression level in oAβ-treated astrocytes (data not shown). These data showed that oAβ downregulates astrocytic EAAT2 in a TNF-α-independent manner.
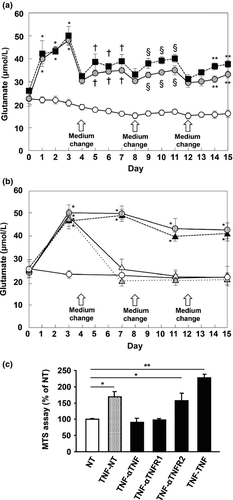
TNF-α triggered sustained microglial glutamate release and elongated microglial survival
As we previously showed that initial stimulation with TNF-α induces subsequent TNF-α release in microglia through TNFR1 signaling in an autocrine manner, we next confirmed whether TNF-α stimulation triggers sustained microglial glutamate release.9, 23 Intermittent TNF-α stimulation naturally induced oscillated glutamate release from microglia (Fig. 5a; filled squares). Interestingly, a single stimulation with TNF-α also triggered sustained glutamate release from microglia (Fig. 5a,b; gray circles). These effects were canceled by addition of neutralizing antibodies against TNF-α and TNFR1 (Fig. 5b; open triangles and gray triangles), but not by anti-TNFR2 neutralizing antibodies (Fig. 5b; filled triangles). These data showed that initial stimulation with TNF-α triggers sustained glutamate release from microglia in a positive feedback mechanism through TNF-α/TNFR1 signaling. Furthermore, TNF-α-treated microglia also showed significant elongated survival as well as activation, which were abrogated by neutralization of TNF-α or TNFR1 (Fig. 5c). These findings showed that the TNF-α/TNFR1 signaling drives a self-activation loop in microglia by a positive feedback mechanism.
Discussion
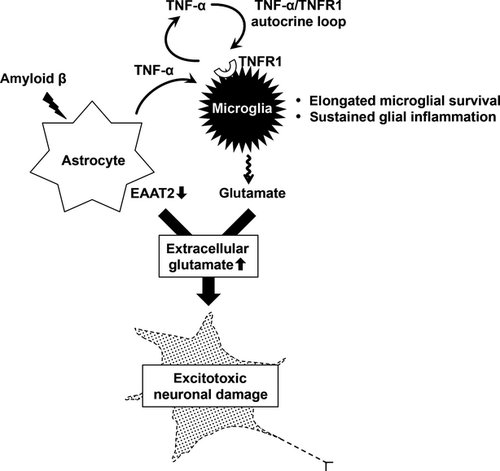
In the present study, we propose that oAβ induces glia-mediated excitotoxicity through the two-hit mechanism by suppressing astrocytic glutamate uptake and enhancing microglial glutamate release, and triggers chronic glial neuroinflammation through the TNF-α/TNFR1 positive feedback loop in AD. As shown in Figure 6, oAβ stimulates astrocytes to release TNF-α. Then, released TNF-α induces microglia to produce an excessive amount of glutamate. oAβ also downregulated EAAT2 protein expression in astrocytes, leading to the disruption of excessive extracellular glutamate scavenging. This combination results in excitotoxic neuronal damage. Furthermore, TNF-α stimulation drives sustained TNF-α release in microglia through TNFR1 signaling in a positive feedback manner.19, 23 This microglial self-activation in an autocrine manner leads to sustained microglial glutamate release and elongated microglial survival, which cause chronic glial neuroinflammation. Indeed, AD patients show the elevated serum level of TNF-α,24, 25 and several lines of evidence corroborated that TNF-α/TNFR1 signaling and excitotoxicity play important roles in the pathogenesis of AD mouse models.13, 26-29 Although a recent study reported that oAβ transiently induced astrocytic glutamate release and subsequent synaptic dysfunction, our proposed mechanism seems plausible to explain sustained chronic neuroinflammation in AD.30 Activated glial cells act as an amplifier of neuroinflammation in various neurological disorders including AD.31, 32 TNF-α/TNFR1 signaling might be a potential therapeutic target to halt the glia-mediated vicious spiral of neuroinflammation in AD.
TNF-α-neutralizing antibody therapy has already shown clinical benefits for patients with rheumatoid arthritis and other peripheral inflammatory diseases; however, several serious problems exist for the application to AD.33, 34 First, these agents are largely unable to penetrate the blood–brain barrier, which severely limits their use in the setting of neuroinflammation in the central nervous system. Second, blockade of TNF-α increases the risk for malignancy and infection, especially tuberculosis. Third, pan-TNF-α inhibition might exacerbate the disease progression of AD through inhibition of TNFR2 signaling.35 Recent studies showed that thalidomide, a blood–brain barrier-penetrable TNF-α inhibitor, ameliorates the disease symptoms of AD mouse models; however, it might also disturb the downstream signaling of TNFR2 as well as TNFR1.36-39 Therefore, more preferable therapeutic agents might be a blood–brain barrier-permeable small molecule that specifically suppresses TNFR1 signaling.
In conclusion, we showed that oAβ facilitates microglial glutamate release by inducing TNF-α release from astrocytes and diminished EAAT2 protein expression in astrocytes. TNF-α also triggered sustained microglial glutamate release and elongated microglial survival, which fuels chronic glial neuroinflammation. We propose that oAβ induces glia-mediated excitotoxicity through two-hit mechanism, and that blockade of TNFR1 signaling could be a good candidate for therapeutic target to halt the chronic glial neuroinflammation in AD.
Acknowledgments
This work was supported in part by a Grant-in-Aid for Scientific Research on Innovative Areas from the Ministry of Education, Culture, Sports, Science and Technology of Japan; a grant from the Advanced Research for Medical Products Mining Program of the National Institute of Biomedical Innovation (NIBIO) of Japan; and grants from the Ministry of Health, Labor and Welfare of Japan.
Conflict of interest
None declared.