Quality of life decrements in men with prostate cancer undergoing androgen deprivation therapy
Summary
Objective
While androgen deprivation therapy (ADT) has been associated with decreased quality of life (QoL), controlled prospective studies are lacking. We aimed to assess QoL during ADT using two validated questionnaires and determine contributing factors.
Design
Prospective controlled study.
Patients
Sixty-three men with nonmetastatic prostate cancer newly commencing ADT (n = 34) and age- and radiotherapy-matched prostate cancer controls (n = 29).
Measurements
QoL was measured by Short-Form 12 version 2 survey (SF-12) and Aging Males’ Symptoms (AMS) score at 0, 6 and 12 months. Generalized linear models determined the mean adjusted difference (MAD) (95% confidence interval) between groups during follow-up.
Results
Compared to controls over 12 months, men receiving ADT had decreased SF-12 physical component score [MAD −3·61 (−6·94, −0·29), P = 0·013] reflecting worsening QoL but no change in the mental component (P = 0·74). Total AMS score increased [MAD 9·35 (5·65, 13·07), P < 0·001], reflecting worse QoL. Both SF-12 and AMS changes were greater than reported minimum clinically important differences. AMS sub-domains showed increased somatic [MAD 3·96 (1·94, 5·99), P < 0·001] and sexual [MAD 3·80 (2·16, 5·44), P < 0·001] components but not psychological (P = 0·19). Decrements were related to an increase in hot flushes (P = 0·016) but not haemoglobin decrease (P = 0·46).
Conclusions
Within 12 months, ADT is associated with clinically significant decreased QoL, particularly physical and sexual aspects, independent of the confounding effects of a cancer diagnosis or radiotherapy. As QoL is a crucial aspect of prostate cancer treatment, addressing hot flushes, sexual dysfunction and exercise may potentially improve outcomes for men undergoing ADT.
Introduction
Quality of life (QoL) is arguably the most important facet of treating men with prostate cancer, particularly given the excellent long-term survival associated with localized disease. Nearly 50% of patients with high-risk prostate cancer will undergo adjuvant androgen deprivation therapy (ADT), the majority for 2–3 years.1 Adverse effects such as hot flushes, sexual dysfunction, anaemia, muscle weakness, fatigue and change in body image can all contribute to decreased QoL associated with ADT.2
Large retrospective studies from cancer registries have suggested that ADT may be associated with decreased physical, sexual and possibly mental aspects of QoL, however, interpretation is limited by residual confounding, including treatment indications such as cancer diagnosis, radiotherapy or prostatectomy.3-7 The majority of these studies have all been based on data from a single database; the CaPSURE registry. Separating adverse effects of ADT from adverse effects of radiotherapy or prostatectomy such as bowel disturbance, fatigue, wound complication and erectile dysfunction is impossible. Prospective studies have been few and there have only been two controlled studies that suggested decreased overall QoL with ADT.8, 9
To develop appropriate interventional strategies to minimize long-term adverse effects and improve QoL for men undergoing ADT, an understanding of not only the effects on QoL but potential contributors is required.
We hypothesized that ADT would be associated with decreased QoL in all domains and aimed to assess the impact of ADT using two validated questionnaires and determine contributing factors.
Methods
This prospective 12-month case–control study was conducted at a tertiary referral hospital (Austin Health, Melbourne, Australia) and was approved by the Human Research Ethics Committee, Austin Health. All participants provided written informed consent. This is a secondary analysis of predefined QoL end-points of a study assessing the effects of ADT on muscle function (reported separately).
Participants were recruited from prostate cancer outpatient clinics. Inclusion criteria were localized nonmetastatic prostate cancer (Stage T1-3, Nx, M0), aged 55–85 years with an Eastern Co-operative Oncology Group performance status of 0 (fully active and unrestricted in physical activity). Men were excluded if they had any illnesses or other factors predisposing them to androgen deficiency, previous ADT, severe medical comorbidities or any neuromuscular disease. Cases were newly commencing long-term ADT (within 6 weeks of commencement) and prostate cancer controls were not receiving ADT in the 12-month follow-up period. Both groups were matched for age and radiotherapy treatment to delineate specific effects due to ADT.
Assessments occurred at 0, 6 and 12 months. The 12-item Short Form QoL Survey version 2 (SF-12) is a generic measure of health status which is well validated, reliable and clinically useful to compare the relative burden of disease and for differentiating health benefits produced by medical treatments.10 SF-12 is based on the most widely used health survey worldwide, the 36-item Short-Form QoL survey (SF-36), which are calibrated and well correlated (r > 0·94) with each other.10 The SF-12 generates a physical component score (PCS) and a mental component score (MCS) with a decrease in the component score representing impaired QoL in that domain, and normative age-matched mean for an individual being 50 (standard deviation 10).11 The derived score represents the number of points lower than the mean score for an individual's age. Minimum important differences validated across large populations and multiple disease categories are a change in between 2 and 3 points from the population mean of 50.10 The SF-12 was chosen as this is a self-administered, one-page, 2-min questionnaire which was feasible to achieve for participants having multiple assessments in our study.
The Aging Males’ Symptoms (AMS) Scale generates a total score that can be subdivided into three domains; psychological, somatic and sexual.12 An increase in the AMS score represents impaired QoL. The AMS score was designed to assess symptoms of ageing, to evaluate the severity of symptoms over time, and to measure changes pre and postandrogen therapy, and has been validated internationally.13-15 It is sensitive to detect changes associated with androgen treatment with reported minimally important differences in between 10% and 15% change in score.16, 17 Total AMS score and two sub-scales; somatic and psychological scales, correlate well with the physical and mental components of SF-12, respectively,13, 18 but the AMS provides additional assessment of sexual aspects of QoL, an important side-effect of ADT, and for which there is no SF-12 comparator.
Hot flushes per day were recorded and severity rated as previously described.19 Severity was classified as mild symptoms (<3 min, light physical symptoms, no emotional symptoms and no action needed), moderate (up to 5 min, moderate physical symptoms, mild anxiety, some irritability and loss of concentration, need to use a fan, loosen clothing and remove bedding), severe (up to 10 min, physical symptoms described as feeling hotter or very hot, heavy perspiration, dizziness, nausea, shortness of breath, weakness and extreme discomfort; moderate anxiety and irritability; need to loosen clothing, change clothing and change bedding), or very severe (up to 30 min, very significant physical and emotional symptoms and the need to change clothing, bedding, towel off, take a shower and take rest).19
Statistical analysis
Data were not normally distributed and are presented as median and interquartile range (IQR). Comparisons of baseline characteristics were made using Wilcoxon rank sum test for continuous variables or chi square test for frequencies. P values <0·05 were considered significant. Comparison of some variables was adjusted for the influence of covariates using a Generalized Linear Model, as implemented by Deducer 0·7–6 with current dependencies.20 In case of repeated measurements such as a comparison of baseline vs follow-up levels, the model was extended to a Generalized Linear Mixed Model with baseline values incorporated as a fixed covariate and repeated measure by subject as random effect, which is also robust against regression to the mean. As a quantitative measure, mean adjusted difference (MAD) plus 95% CI between the groups from baseline to 12 months is provided. Kendall's tau correlation was used to measure the strength of the relationship between AMS score with hot flushes and haemoglobin. Statistical analyses were performed using r statistical package (version 3·02 for Mac).21
Results
Study subjects
Subject numbers and flow of participants are shown in Fig. 1.
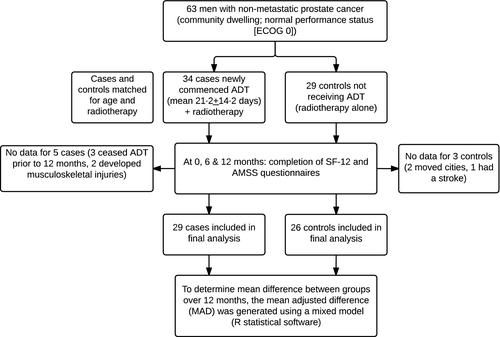
Baseline characteristics
Baseline characteristics are shown in Table 1. Participants were matched for age, body mass index, medical co-morbidities, radiotherapy and baseline testosterone level. The ADT group were predominantly receiving ADT and radiotherapy as treatment for high-risk disease, hence baseline Gleason scores and PSA levels were higher. Controls were predominantly receiving radiotherapy alone (without ADT) as indicated for intermediate risk disease.
| Baseline characteristic | ADT group N = 34 | Control group N = 29 | P value |
|---|---|---|---|
| Age | 67·6 (64·6, 72·0) | 70·6 (65·3, 72·9) | 0·482 |
| Prostate Cancer Gleason Score | 9·00 (8·00, 9·00) | 7·00 (7·00, 7·00) | <0·001 |
| Concurrent radiotherapy treatment | 1·00 (1·00, 1·00) | 1·00 (1·00, 1·00) | 0·517 |
| Total testosterone (nmol/l) | 14·1 (10·2, 17·6) | 15·0 (11·1, 16·9) | 0·912 |
| PSA (μg/l) | 3·62 (0·21, 18·7) | 0·05 (0·03, 0·28) | <0·001 |
| Haemoglobin (g/l) | 149 (140, 157) | 150 (142, 155) | 0·66 |
| Medical comorbidities | |||
| Ischaemic heart disease | 17·6% | 17·2% | 1·00 |
| Diabetes mellitus | 14·7% | 17·2% | 1·00 |
| Liver disease | 0% | 0% | 1·00 |
| Chronic kidney disease | 0% | 0% | 1·00 |
| Hypertension | 58·8% | 58·6% | 1·00 |
- PSA, prostate-specific antigen.
- Data presented are median (interquartile range) or proportions (%). Gleason score <7 = low-moderate risk, 7 = intermediate risk, 8–10 = high risk prostate cancer.
At baseline, all men were clinically eugonadal and had age-appropriate normal testosterone levels which remained unchanged in controls (Table 1). As expected over 12 months of ADT, total testosterone, oestradiol and PSA decreased (Table 2). Haemoglobin also decreased significantly.
| Biochemical parameters | ADT group (n = 34) | Controls (n = 29) | Mean adjusted difference (95% CI) | P value |
|---|---|---|---|---|
| Total testosterone (nmol/l) | ||||
| 0 months | 14·1 (10·2, 17·6) | 15·0 (11·1, 16·9) | −13·0 (−15·4, −10·7) | <0·001 |
| 6 months | 0·40 (0·30, 0·57) | 14·3 (9·90, 17·2) | ||
| 12 months | 0·40 (0·30, 0·50) | 14·8 (11·2, 15·6) | ||
| Prostate-specific antigen (μg/l) | ||||
| 0 months | 3·62 (0·21, 18·7) | 0·05 (0·03, 0·28) | −21·3 (−35·1, −8·2) | 0·002 |
| 6 months | 0·03 (0·03, 0·11) | 0·03 (0·03, 0·21) | ||
| 12 months | 0·03 (0·03, 0·04) | 0·03 (0·03, 0·28) | ||
| Oestradiol (pmol/l) | ||||
| 0 months | 105 (73, 143) | 86 (76, 104) | −86·5 (−98·9, −62·5) | <0·001 |
| 6 months | 25 (19, 38) | 80 (71, 95) | ||
| 12 months | 19 (19, 25) | 72 (56, 93) | ||
| Haemoglobin (g/l) | ||||
| 0 months | 149 (140, 157) | 150 (142, 155) | −14·5 (−19·2, −9·8) | <0·001 |
| 6 months | 136 (131, 143) | 149 (144, 153) | ||
| 12 months | 138 (133, 144) | 152 (146, 158 | ||
| Hot flushes (number/day) | ||||
| 0 months | 0 (0, 0) | 0 (0, 0) | 4·6 (3·1, 6·2) | <0·001 |
| 6 months | 4 (1, 6) | 0 (0, 0) | ||
| 12 months | 3 (2, 7) | 0 (0, 0) | ||
- Medians (interquartile ranges) are presented. Mean adjusted difference refers to the change over 12 months across groups (mixed model). The P value refers to the overall significance of the change between groups during follow-up.
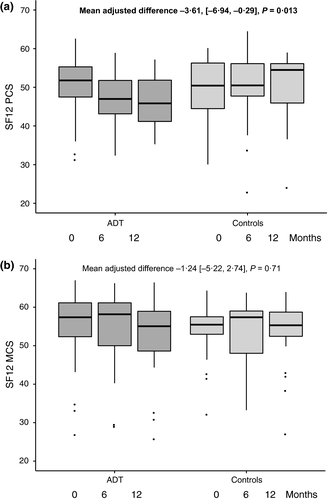
Change in SF-12 score over time
Median SF-12 scores for both groups are shown in Fig. 2. At baseline, the mean (and standard deviation) PCS in the ADT group (50·4 ± 8·0) and the control group (49·6 ± 8·5) were equivalent to the general population mean of 50 ± 10. There was a statistically significant decrease in PCS over 12 months in men undergoing ADT compared with controls, representing worsening QoL [MAD −3·61 (−6·94, −0·29), P = 0·013]. The MCS did not significantly decrease [MAD −1·24 (−5·22, 2·74), P = 0·71].
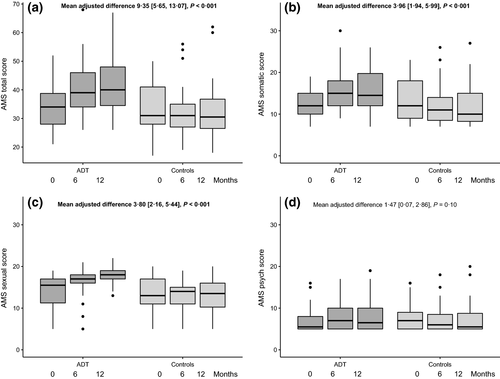
Change in AMS score over time
Aging Males’ Symptoms score for the two groups are shown in Fig. 3. Total AMS score increased over 12 months in men undergoing ADT compared with controls, representing worsening QoL [MAD 9·35 (5·65, 13·07), P < 0·001]. Analysing the three specific domains, somatic [MAD 3·96 (1·94, 5·99), P < 0·001] and sexual [MAD 3·80 (2·16, 5·44), P < 0·001] domains increased but the psychological domain was not statistically different [MAD 1·47 (0·07, 2·86), P = 0·10]. The increase in AMS score in the ADT group was related to an increase in hot flushes (τ = 0·33, P = 0·016), but did not appear to be related to the decline in haemoglobin (τ = −0·10, P = 0·46).
Hot flushes
Over 12 months, the number of hot flushes per day increased in the ADT group compared with controls (Table 2). In the ADT group, the onset of hot flushes occurred at a median 30 [26, 61] days after commencing ADT corresponding to the onset of hypogonadism with GnRH agonists. Hot flush severity also significantly increased in the ADT group compared with controls at 6 and 12 months (Table 3). The number and severity of hot flushes was not related to baseline testosterone level (τ = 0·13, P = 0·32) or the change in testosterone (τ = −0·13, P = 0·32).
| Severity of hot flushes | ADT group n = 34 (%) | Controls n = 29 (%) | P value |
|---|---|---|---|
| 0 months | |||
| None | 34 (100) | 27 (93·1) | 0·208 |
| Mild | 0 | 1 (3·5) | |
| Moderate | 0 | 1 (3·5) | |
| 6 months | |||
| None | 5 (14·7) | 25 (92·6) | <0·001 |
| Mild | 18 (52·9) | 1 (3·7) | |
| Moderate | 11 (32·4) | 1 (3·7) | |
| 12 months | |||
| None | 6 (19·4) | 24 (92·3) | <0·001 |
| Mild | 13 (41·9) | 1 (3·9) | |
| Moderate | 12 (38·7) | 1 (3·9) | |
- Mild severity was defined as <3 min, light physical symptoms, no emotional symptoms and no action needed. Moderate was defined as up to 5 min, moderate physical symptoms, mild anxiety, some irritability and loss of concentration, need to use a fan, loosen clothing, and remove bedding. No participants scored hot flushes as severe or very severe.
Discussion
In this controlled prospective study, we found that when sex steroids were lowered from normal to castrate levels with ADT in men with prostate cancer, QoL decreased with predominant effects on physical components, occurring within the first 12 months of treatment. No significant changes were noted in the mental or psychological components of QoL. Decrease in QoL was associated with an increase in hot flushes but not the fall in haemoglobin. As a matched control group was included, we infer that the decrement in QoL is a direct consequence of ADT, and not due to confounding effects of having a cancer diagnosis or radiotherapy.
Although the SF-12 PCS MAD of −3·6 points was statistically significant, this is also a clinically important difference, greater than published minimally important differences in 2–3 points.10 Based on population-based Medicare surveys of 434 947 individuals, a three-point decline was associated with a 20% increase in mortality, which was consistent across multiple disease groups.22 Furthermore, a three-point decline from baseline PCS score has been shown to be associated with an odds ratio (OR) of 1·44 for being unable to work (approximately 40% higher risk), and an OR of 1·16 for being hospitalized in the subsequent year,23 which is alarming, and reinforces the need to understand and abate contributing factors to decreased QoL in men undergoing ADT.
Similarly, the increase in AMS score of approximately 30% from baseline in androgen deprivation is clinically meaningful and is greater than the reported minimum important difference of 10–15%.13
Many would regard quality as more important than quantity of life, and cancer survivorship has been the focus of recently developed guidelines for men undergoing prostate cancer treatment.24 Separating the exact components of QoL affected by ADT have been challenging, as data has been based predominantly on retrospective registry data. Two prospective controlled studies have been performed. Herr and colleagues followed 79 men on ADT and 65 men not on ADT, however, these were not matched controls, with participants self-selecting whether to receive ADT which limits interpretation.9 Alibhai et al.8 found that PCS of SF-36 declined in men starting ADT, however, remained stable in prostate cancer controls and healthy controls over 36 months. However, the reference population was the healthy cohort, and prostate cancer controls recruited were not matched for radiotherapy which can significantly contribute to QoL decrements. Nevertheless, their findings mirror our matched, carefully controlled data that ADT leads to a decrease in physical components of QoL and do not appear to significantly affect mental components.
In addition to mitigating cardiovascular effects and bone decay in patients undergoing ADT, recommendations for which, have been relatively widely published,2, 25 attention needs to shift towards improving QoL. We found that AMS decrements were significantly related to vasomotor hot flush number and frequency but not the drop in haemoglobin. Hot flushes occur in up to 80% of men undergoing ADT and whilst most intense after initial commencement, can persist for the duration of the therapy.26 Hot flushes may contribute to poor QoL by causing discomfort, interruptions to daily routines, sleep disturbance or avoidance of triggers including exercise. Moreover, the simple presence of a hot flush may be a recurrent reminder of an individual's illness which may impact QoL. Hot flushes did not appear to be a marker of more severe androgen deprivation with no correlation seen with testosterone levels. However, this may not be unexpected, given that only men with a clearly normal testosterone were enrolled leading to an eligibility-driven restricted range of testosterone values being correlated. Despite no evidence-based guidelines, treatment strategies for hot flushes should be discussed and offered to patients.19 Further research is clearly required to investigate the mechanism of episodic hot flushes and also to develop effective, well-tolerated treatments.
Sexual domains of QoL were also significantly reduced in our population of elderly men (mean age 70 years). ADT may compound effects on erectile dysfunction induced by surgery or radiotherapy. The impact on QoL of a loss of erectile function and libido varies depending on the individual, however, should not be underestimated, with potential consequences on masculinity, self-perceived body image, intimacy and relationships with partners. Support should be provided to patients and their partners with discussions regarding medical options for erectile dysfunction as well as psychosocial strategies.27 A willingness to explore other nonerection-dependent sexual practices and acceptance of alternative perspectives on sexual function and intimacy may improve sexual aspects of QoL.28, 29
Few treatments specifically target QoL, but exercise may be an effective strategy to improve physical aspects of QoL and a meta-analysis has recently provided evidence for this.30 However, implementation and sustainability of exercise programmes in clinical practice remain challenging, especially in older men receiving ADT, who commonly have a substantial comorbid burden and may experience ADT-associated fatigue and low mood.31 Motivation to exercise may well be another contributing factor. Whilst we did not find a decrease in self-reported physical activity using the Minnesota Leisure Time Physical Activity Score,32 this tool may well be too insensitive, and it is possible that ADT may reduce motivation to engage in healthy lifestyle measures. Future adequately powered studies will need to focus on developing treatment strategies to target physical function and QoL in men undergoing ADT.
Limitations of this study include that QoL was a secondary outcome, albeit prespecified, and QoL is inherently subjective and influenced by many factors. Assessment of sexual function was limited to the AMS questionnaire which provides fewer details on erectile dysfunction than other scales such as the International Index of Erectile Function. However, erectile dysfunction is more closely related to neurovascular pathology rather than testosterone levels, and androgen deficiency and erectile dysfunction are two overlapping conditions with distinct pathophysiology. This study was also not specifically powered to detect differences in mental or psychological aspects of QoL which may be affected by ADT but to a smaller degree than physical aspects. Furthermore, SF-12 and AMS score, which are not prostate cancer specific, may potentially have been insensitive to detect specific changes in mental health. Mood and cognitive effects of ADT, require further research with more sensitive methodology. Finally, the results of this cohort by virtue of not only their willingness to participate in a study requiring significant participant commitment, and the inclusion criteria required for participation in the gait study (e.g. normal performance status, unaided ambulation), may not be generalizable to more frail prostate cancer patients with higher comorbidity burden, and poorer baseline quality of life. Our findings are, however, notably, consistent with those shown by Alibhai et al.8
Conclusions
Overall, QoL is significantly affected within 12 months of commencing ADT for prostate cancer in men undergoing ADT, above that of a cancer diagnosis alone, prostate cancer progression or radiotherapy. Physical and sexual aspects of QoL are predominantly affected and may be mitigated by exercise. Moreover, adequately treating hot flushes and addressing sexual dysfunction may potentially improve QoL in a substantial number of men undergoing ADT. QoL should be a consideration for all health providers recommending ADT and considerations must be made between balancing quantity with quality of life when treating prostate cancer.
Acknowledgements
The study was funded by a National Health and Medical Research Council (NHMRC) of Australia Project Grant (#1006407). Ada Cheung is supported by a NHMRC Medical and Dental Postgraduate Research Scholarship (#1017233) to undertake this project as part of PhD studies. Mathis Grossmann is supported by a NHMRC Career Development Fellowship (#1024139).
Author contributions
MG, JDZ and ASC designed the research study. MG acquired the funding for the research study. MG and JDZ supervised the overall research project. ASC and DLJ recruited all participants. ASC performed assessments and acquired the data. CD and RH performed statistical analysis of the results. ASC wrote the original draft of the manuscript. All co-authors revised and approved the current manuscript.
Funding
National Health and Medical Research Council of Australia.
Conflict of interest
The authors have nothing to declare.




