Exploring immunomodulation by endocrine changes in Lady Windermere syndrome
Summary
Lung disease due to nontuberculous mycobacteria (NTM) occurs with disproportionate frequency in postmenopausal women with a unique phenotype and without clinically apparent predisposing factors. Dubbed ‘Lady Windermere syndrome’, the phenotype includes low body mass index (BMI), tall stature and higher than normal prevalence of scoliosis, pectus excavatum and mitral valve prolapse. Although the pathomechanism for susceptibility to NTM lung disease in these patients remains uncertain, it is likely to be multi-factorial. A role for the immunomodulatory consequences of oestrogen deficiency and altered adipokine production has been postulated. Altered levels of adipokines and dehydroepiandrosterone have been demonstrated in patients with NTM lung disease. Case reports of NTM lung disease in patients with hypopituitarism support the possibility that altered endocrine function influences disease susceptibility. This paper catalogues the evidence for immunomodulatory consequences of predicted endocrine changes in Lady Windermere syndrome, with emphasis on the immune response to NTM. Collectively, the data warrant further exploration of an endocrine link to disease susceptibility in Lady Windermere syndrome.
Introduction
Nontuberculous mycobacteria (NTM) are traditionally defined as species of the genus Mycobacterium that do not cause tuberculosis (TB) or leprosy. Recent phylogenomic analysis has supported division of this genus into an emended genus Mycobacterium and four novel genera. The species of these genera remain members of the family Mycobacteriaceae, and the term ‘mycobacterial species’ remains valid 1. Additionally, ‘NTM’ remains current clinical nomenclature. Therefore, ‘NTM’ will be used in the current review to denote the non-lepromatous and non-tuberculous species of the genera of Mycobacteriaceae and describe disease caused by these species.
NTM are environmental organisms that are widely distributed in water and soil, with a predilection for human-engineered water systems 2. Human infection may manifest as lung disease, defined by the combination of supporting clinical features, nodular bronchiectasis or cavitation on chest imaging and microbiological culture positivity 3. Although the incidence of NTM lung disease is increasing, this condition remains uncommon 4. This disconnect between the ubiquity of NTM and the rarity of disease implicates host susceptibility in pathogenesis.
Risk factors for NTM lung disease include pre-existing structural lung disease, genetic predisposition to bronchiectasis and/or pulmonary infections and immunological deficiencies 5. Reich and Johnson reported a series of patients without clinically apparent predisposing factors who were predominantly elderly females with a fibronodular radiographic pattern of NTM disease affecting the middle lobe and lingula 6. The authors hypothesized that voluntary cough suppression was contributory and offered the descriptor, ‘Lady Windermere's syndrome’, to convey the fastidiousness of such behaviour. This characteristic phenotype is now recognized to include tall stature, reduced body mass index (BMI) and increased frequency of connective tissue-related abnormalities such as scoliosis, pectus excavatum and mitral valve prolapse 7, 8.
The basis for susceptibility to NTM lung disease in Lady Windermere syndrome is yet to be defined. Szymanski and colleagues conducted a candidate gene analysis that associated NTM lung disease with variants of immune, connective tissue, ciliary and cystic fibrosis transmembrane conductance regulator (CFTR) gene sets 9. Compared to controls, patients had more protein-affecting variants across all categories, supporting a multi-genic model of susceptibility. Variants of connective tissue genes may account for physical features of Lady Windermere syndrome and contribute to susceptibility 10. For example, the fibrillin-1 gene mutation, which causes Marfan syndrome, may drive increased signalling by the multi-functional, immunomodulatory cytokine, transforming growth factor (TGF)-β 11. Chan and Iseman postulated that altered adipokine production due to low fat mass or low circulating oestrogen due to postmenopausal status may confer susceptibility to NTM lung disease by modulating immune function 11. The few studies of endocrine changes in Lady Windermere syndrome have investigated leptin, adiponectin, oestrogen and the adrenal androgen, dehydroepiandrosterone (DHEA) 8, 12, 13. This review explores the contribution of immunomodulation to Lady Windermere syndrome with a focus on the capacity of these hormones to modulate the immune response to NTM. Potential roles for other adenohypophysial hormone systems will be considered in light of case reports associating NTM lung disease with panhypopituitarism 14, 15. The hormones discussed, their physiological functions and predicted changes in Lady Windermere syndrome are summarized in Table 1.
| Hormone | Origin | Physiological function | Predicted change | Mechanism of change |
|---|---|---|---|---|
| Leptin | Adipose | Satiety signalling | ↓ | Reduced fat mass |
| Adiponectin | Adipose | Regulation of metabolic processes (e.g. glucose and fatty acid metabolism) | ↑ | Caloric restriction |
| Oestrogens | Ovaries, peripheral conversion of androgens by aromatase | Female sex steroid | ↓ | Menopause |
| Reduced fat mass | ||||
| DHEA | Adrenal cortex (zona reticularis) | Metabolic intermediate in sex steroid synthesis with multiple oestrogenic, androgenic and other functions | ↓ | Senescence |
| Cortisol | Adrenal cortex (zona fasciculata) | Immunosuppression and regulation of multiple metabolic functions, including glucose control, sodium and water balance, cell replication, body composition (muscle and fat mass) | ↑ | Ageing, physiological stress |
| Prolactin | Anterior pituitary | Lactation | ↓ | Menopause – oestrogen deficiency |
| GH/IGF-1 | GH: anterior pituitary | Body composition (muscle and fat mass) | ↓ | Senescence |
| IGF-1: liver | ||||
| Thyroid hormones | Thyroid | Regulation of metabolism in multiple tissues/organs including brain, heart, gastrointestinal tract, muscle, bone | ↓ | Disease/dysfunction |
- DHEA = dehydroepiandrosterone; GH = growth hormone; IGF-1 = insulin-like growth factor 1.
The immune response to NTM
Mycobacterial species comprise obligate intracellular organisms, and the immune response to NTM (Fig. 1) has largely been elucidated through the study of Mycobacterium avium complex (MAC). After phagocytosis by macrophages, M. avium survives within the phagosome, in part by inhibiting phagosome-lysosome fusion and thereby avoiding killing by lysosomal enzymes 16. Phagocytosis triggers the release of proinflammatory cytokines, including tumour necrosis factor (TNF)-α and interleukin (IL)-12 17. These cytokines are associated with the ‘classic’ M1 phenotype of macrophage activation as opposed to the ‘alternative’, anti-inflammatory M2 phenotype, which is associated with secretion of TGF-β and IL-10 18. TNF-α acts in an autocrine fashion to activate bacteriostatic and bactericidal activities of macrophages. Bactericidal mechanisms are mediated by nitric oxide (NO), produced by nitric oxide synthases, and include generation of cytotoxic reactive nitrogen intermediates 19. Neutrophils and natural killer (NK) cells contribute to the innate immune response to MAC 20, 21. NK cell anti-mycobacterial functions include cytotoxicity directed against infected cells and production of immunoregulatory cytokines 22. NK cells respond to TNF-α and IL-12 by releasing interferon (IFN)-γ, which drives activation of macrophages and establishes a positive feedback loop by enhancing IL-12 production 17.
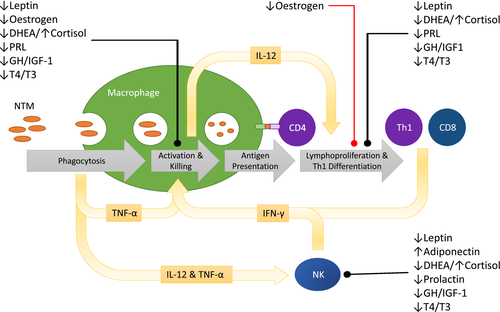
The positive feedback loop involving IL-12 and IFN-γ is the cornerstone of immunity to NTM. Macrophages, neutrophils and other antigen-presenting cells (APCs), such as dendritic cells (DCs), produce IL-12 which drives the differentiation of T cells towards the IFN-γ-producing T helper type 1 (Th1) phenotype and enhances cytotoxic T cell (CTL) function 23, 24. IL-12 also enhances proliferation of human antigen-specific CD4+ and CD8+ T cells in response to M. avium 25. The accumulation of mycobacteria-infected macrophages and antigen-specific T cells is the basis of the granuloma, the hallmark inflammatory structure of mycobacterial infection. The importance of these pathways in humans is illustrated by conditions that result in their deficiency. A number of inherited genetic mutations that impair the IL-12–IFN-γ signalling pathway confer susceptibility to localized or disseminated NTM infection, and are relatively specific to infection by mycobacterial species 5. HIV-infected individuals are susceptible to disseminated NTM infection due to failure of granulomatous inflammation 17.
NTM infection in Lady Windermere syndrome is localized, associated with intact granulomatous inflammation and occurs in the absence of susceptibility to infection by other organisms. Outright failure of the core immune systems therefore seems unlikely. It is proposed that subtle perturbation of the immune response by endocrine changes may contribute to disease susceptibility in patients with Lady Windermere syndrome.
Leptin and adiponectin
Leptin and adiponectin are adipokines, the former being a signaller of satiety. Fat mass normally correlates positively with leptin levels and negatively with adiponectin levels 26. Two studies have investigated serum adipokine concentrations in patients with NTM lung disease. The patients in each study were predominantly slender, postmenopausal women. Tasaka and colleagues, who studied Asian patients with stable lung disease and negative sputum cultures, demonstrated normal relationships between BMI and adipokine levels 12. Adiponectin levels were higher in patients than in BMI-matched control subjects. Although median leptin was 30% lower in patients, this difference did not reach statistical significance. Both groups were characterized by very low leptin levels and a median BMI less than 18·5 kg/m2. Despite BMI matching, it is possible that fat mass differed between the groups. In contrast, Kartalija et al. studied a predominantly Caucasian population that included patients at various stages of disease with a mean BMI of 22·06 kg/m2 8. Control subjects in this study were of significantly greater BMI than patients and the authors controlled for this mismatch by correcting adipokine levels for fat mass, measured using a calliper method. The main finding was that the patient group did not exhibit the normal relationships between fat mass and adipokine concentrations observed in the control group. The positive correlation of leptin levels with fat mass was weak due to elevated leptin in patients with lower fat mass and reduced leptin in patients with higher fat mass. The authors hypothesized that this dysregulation may be a consequence of cytokine signalling in patients with a greater burden of inflammation. On the assumption that premorbid adipokine regulation is normal, the baseline adipokine milieu of Lady Windermere syndrome is likely to be decreased leptin and elevated adiponectin. The following discussion will focus on the immunomodulatory consequences of these changes.
Mice that lack the genes for leptin (ob/ob mice) or the leptin receptor (db/db mice) are susceptible to mycobacterial infection, but also exhibit obesity and a number of hormonal abnormalities that may impair immune function 27. The lung tissue of ob/ob mice infected with M. abscessus 28 or M. tuberculosis 27 was found to exhibit deficient lymphocyte recruitment, reduced production of IFN-γ and disorganized granuloma formation. In addition, mycobacterial killing was impaired. The IFN-γ response to M. tuberculosis was restored by leptin replacement. Similar abnormalities in db/db mice with pulmonary M. tuberculosis infection were potentially caused by abnormal chemokine response and deficient antigen presentation to T cells 29. As a consequence, the expression of inducible nitric oxide synthase (iNOS) by myeloid cells and production of IFN-γ by T cells were delayed.
A number of immunomodulatory mechanisms may link leptin deficiency with susceptibility to mycobacterial infection. Impaired T cell proliferative responses and altered cytokine production have been reported in children with congenital leptin deficiency, including complete suppression of IFN-γ, reduction of IL-4 and IL-10 and increase of TGF-β 30. These abnormalities were reversible with leptin replacement. In vitro incubation of human T cells with leptin is associated with increased production of IFN-γ and IL-2 but reduced production of IL-4 and IL-10, particularly in activated cells 31, 32. In the presence of leptin, human DCs up-regulate production of several cytokines, including IL-12 and TNF-α, down-regulate production of IL-10, and thereby drive polarization of naive T cells towards a Th1 phenotype 33. Leptin may also facilitate proliferation and cytotoxicity of NK cells 34.
The immunomodulatory consequences of elevated adiponectin have been difficult to define due to inconsistencies between studies. Adiponectin indirectly decreases Toll-like receptor (TLR)-mediated IFN-γ secretion by NK cells, due possibly to direct inhibition of APC functions 35. The finding that adiponectin induces proinflammatory responses in primary human macrophages was thought to represent a programme of limited activation which desensitizes cells to further proinflammatory stimuli 36. Additional complexity is introduced by the observation that adiponectin enhances both M1 and M2 macrophage functions, suggesting that the outcome of adiponectin signalling in a given inflammatory situation may depend on the dominant macrophage phenotype 18. Although signalling through adiponectin receptors expressed by human DCs 37 and T cells 38 attenuates cellular immunity, studies of the capacity of adiponectin-treated murine bone marrow-derived dendritic cells (BMDCs) to drive T cell responses have produced conflicting results 39, 40. These discrepancies may be a consequence of variation of experimental conditions, such as the timing of adiponectin exposure, the choice of stimulating agent and the cell model studied.
Perturbation of serum adipokine levels has the potential to negatively modulate innate and adaptive immune responses. The sex bias of Lady Windermere syndrome represents a challenge to this hypothesis, as plasma levels of leptin and adiponectin are significantly greater in females than males 26.
Oestrogens
Oestrogens are synthesized from androgens by aromatase. In men, this process occurs primarily in extra-gonadal sites, such as adipose tissue, and may generate quantities of oestrogen approaching those synthesized in women 41. The dominant form of oestrogen in pre-menopausal women is ovarian oestradiol (E2). After menopause, the quantitatively most significant oestrogen is oestrone (E1), which is primarily produced by extra-glandular aromatization of adrenal androgen precursors, particularly in adipose tissue 42. Aromatization is regulated by the adipokines, with leptin being stimulatory and adiponectin being inhibitory 41.
The association of Lady Windermere syndrome with postmenopausal status suggests a role for oestrogen deficiency. Reduced aromatization due to low fat mass and altered adipokine levels may compound the consequences of ovarian failure. It has not been established whether patients with Lady Windermere syndrome are particularly oestrogen-deficient in comparison to healthy control subjects. Danley et al. published preliminary findings that the levels of oestrone and ultrasensitive oestradiol in postmenopausal women with M. avium lung disease are not significantly different from those in age-matched control subjects 13.
Oestrogen deficiency increases susceptibility to non-tuberculous mycobacterial infection in rodent models. Male mice have poorer outcomes than female mice after intravenous infection with M. intracellulare 43. Superior killing of mycobacteria by female peritoneal macrophages was thought to be the mechanism. In a murine study of pulmonary M. avium infection, ovariectomy was linked to increased pulmonary bacillary burden, a finding reversed by in vivo E2 replacement 44. E2 augmented in vitro macrophage NO production and expression of iNOS mRNA in response to IFN-γ stimulation. It was proposed that E2 enhanced intracellular killing by increasing generation of reactive nitrogen intermediates. E2-mediated up-regulation of NO generation and microbial killing is supported by rodent models of other infections, including Leishmaniasis 45, 46.
Oestrogen deficiency may lead to dysregulation of other aspects of the immune response to NTM. Oestrogen appears to protect premenopausal women from the inflammatory consequences of sepsis by a mechanism involving reduced production of TNF-α by neutrophils 47. In addition, oestrogen up-regulates surface annexin A1 on human neutrophils, producing a phenotype that is associated with reduced activation and inhibition of cell adhesion 48. High concentrations of oestrogen suppress NK cell cytotoxicity, but the effect of oestrogen deprivation is unclear. NK cells purified from healthy premenopausal women exhibit a dose-dependent reduction in cytotoxicity in response to in vitro oestrogen exposure 49. Although high-dose oestrogen therapy increased the proportion of NK cells in splenocytes from ovariectomized mice, cytotoxicity was reduced 50. In contrast, oestrogen replacement therapy increased NK cell cytotoxicity in an ovariectomized rhesus monkey model of menopause 51.
There is considerable evidence that oestrogens modulate adaptive CD4+ T cell responses and cytokine production 52. However, consolidation of these data is difficult, due to variability of methodologies 53, the biphasic dose-dependent nature of oestrogen-mediated effects 54 and the tissue-specific distribution of the multiple oestrogen receptors 55. The effects of oestrogen on pulmonary granulomatous inflammation have been the subject of few studies. Ovariectomy in rats was associated with diffuse, mature granuloma formation in response to heat-killed bacillus Calmette–Guérin (BCG), whereas control animals exhibited sparse, less well-defined granulomas 56. In addition, the bronchoalveolar lavage (BAL) content of ovariectomized animals was characterized by reduced macrophages and increased neutrophils, activated T cells and IFN-γ production. These differences were reversed by E2 and progesterone when administered individually or in combination. The enrichment of pulmonary granulomatous inflammation in ovariectomized rats was validated by a more recent study 57. These results align with a second peak in incidence of sarcoidosis in older women 58. They may be consistent with evidence that human DCs, when matured in the presence of E2, respond to TNF-α stimulation with biased production of IL-10 relative to IL-12p70 and expression of C-C motif chemokine ligand 1 (CCL1), a chemokine defining Th2 cells and regulatory T cells (Tregs) 59. Moreover, oestradiol potentiates the suppressive effects of Tregs, especially at high concentrations 60.
Oestrogen deficiency may therefore contribute to chronic mycobacterial infection by impairing intracellular killing while facilitating dysfunction of other aspects of innate and adaptive immunity. Such a scenario would be consistent with Lady Windermere syndrome, in which clinical and radiological changes reflect inflammatory responses but NTM are not cleared from respiratory specimens.
DHEA
DHEA and its sulphated form, DHEAS, are androgenic steroid hormones produced primarily by the adrenal cortex in response to adrenocorticotrophic hormone (ACTH), with small quantities being produced by the gonads. Blood levels of DHEA and DHEAS are greater in men than women, but decline markedly with ageing in both sexes 61. The relative contribution of the adrenal gland to circulating DHEA/S increases after menopause due to ovarian functional decline. This may render postmenopausal women more susceptible to the effects of impaired adrenal androgen synthesis than younger women or men 61.
The work by Danley et al. demonstrated that blood levels of DHEAS are significantly reduced in patients with NTM lung disease at various stages of disease and treatment 13. There are several potential explanations for this finding. Rifampicin, an antibiotic used to treat NTM lung disease, induces hepatic cytochrome P450 enzymes which metabolize steroid hormones 62. In addition, cytokines produced during the inflammatory response to mycobacterial species may influence adrenal function. The secretion of DHEA by adrenal cell lines is suppressed by the supernatant of M. tuberculosis-infected peripheral blood mononuclear cells (PBMCs), and TGF-β has been implicated as the causative factor 63, 64. Reduced DHEAS may also arise from disorders of the hypothalamic–pituitary–adrenal (HPA) axis, such as adrenal insufficiency 65.
Reduced blood levels of DHEA or DHEAS have also been identified in patients with TB infection 63, 66, 67 and HIV/TB co-infection 68. In these studies, there was accompanying elevation of blood cortisol concentration or cortisol/DHEA ratio. Cortisol, an adrenal glucocorticoid with potent immunosuppressive and metabolic functions, is produced in increased quantities during stress and healthy ageing 69. In vitro data support the immunomodulatory significance of these changes. Bozza et al. demonstrated that stimulation of PBMCs from TB-infected patients resulted in significantly higher IFN-γ production by cells from patients with above-median plasma DHEA or below-median plasma cortisol/DHEA ratio 67. DHEA additionally increased antigen-driven proliferation of PBMCs from tuberculin-responder healthy blood donors. A study of M. tuberculosis-stimulated PBMCs from patients with HIV/TB co-infection associated DHEA exposure with increased proportion of IFN-γ-producing cells and reduced expression of forkhead box protein 3 (FoxP3), a marker of Treg activity 68. Angerami et al. studied human DCs that were stimulated with heat-killed M. tuberculosis in the presence or absence of DHEA or cortisol 70. In contrast to cortisol, DHEA increased IL-12 production and expression of antigen-presenting proteins by DCs, leading to enhanced induction of antigen-specific T cell proliferation and IFN-γ production. DHEA has also been associated with reduced bacterial burden in human macrophage-like THP-1 cells via an autophagic mechanism 71.
Treatment of TB-infected mice with DHEA or its derivative, androstenediol, reduced pulmonary bacterial counts and altered cytokine expression while skewing the inflammatory pattern from pneumonic to granulomatous inflammation 72. Small studies of DHEA supplementation in ageing men and postmenopausal women have demonstrated modulation of immune parameters, including increased NK cell number and cytotoxicity 73, 74. Although DHEA supplementation may represent an opportunity for therapeutic investigation, further research is required to confirm reduced DHEA/S levels in Lady Windermere syndrome and investigate the possibility of HPA dysfunction.
Other hormones of potential significance
Case reports have described NTM lung disease in patients with panhypopituitarism who were receiving hormone replacement therapy with corticosteroids and thyroid hormone 14, 15. The hormones of the lactotrope, somatotrope and thyrotrope axes have not been investigated in Lady Windermere syndrome, but are likely to be altered in comparison to younger, healthy women. The production of prolactin is inhibited by dopamine and stimulated by oestradiol 75. Thus, basal serum concentrations of prolactin decrease in women after menopause but may remain stable or increase in ageing men 76. Most of the biological effects of growth hormone (GH) are mediated by insulin-like growth factors (IGFs) 77. Pituitary GH secretion and serum IGF-1 concentration reduce during ageing in men and women 78. The thyroid hormones, triiodothyronine (T3) and thyroxine (T4), are secreted by the thyroid gland in response to adenohypophysial thyroid-stimulating hormone (TSH). T4 is converted to the more active T3 in peripheral tissues by deiodinases 79. Although circulating T3 and T4 do not change during healthy ageing 76, the prevalence of thyroid dysfunction increases 80. The incidence of primary hypothyroidism, especially due to autoimmune thyroiditis, increases with age and women are more commonly afflicted than men 80. Data support beneficial effects of these hormones on immune functions that are pertinent to the control of mycobacterial infection, especially during physiological stress and ageing.
Prolactin is produced by human PBMCs and functions in an autocrine fashion to stimulate lymphoproliferation 81. Effects of prolactin on other cell types include up-regulation of murine macrophage phagocytosis and NO generation 82, 83 and human NK cell cytotoxicity and IFN-γ secretion 84, 85. Treatment of mice with bromocriptine, a dopamine receptor agonist, decreased production of IFN-γ from T cells and impaired activity of macrophages against Listeria monocytogenes and M. bovis 86. Replacement of prolactin reversed these effects. Dorshkind and Horseman 87 argued that, rather than being necessary for normal immune function, the role of prolactin is to mitigate the immunosuppressive effects of glucocorticoids during physiological stress. In physiological or stress-induced concentrations, prolactin enhances IL-12 and IFN-γ production by phytohaemagglutinin and lipopolysaccharide (PHA+LPS)-stimulated whole blood from healthy human donors and opposes the effects of cortisol 88. It may be that any immunomodulatory consequences of postmenopausal prolactin deficiency adopt particular significance in Lady Windermere patients, who are subject to physiological stress driven by infection and low BMI.
GH deficiency in adults and children has been associated with reductions in NK cell number, NK activity and the phagocytic activity of monocytes and neutrophils 78, 89. In vitro studies have demonstrated that GH and IGF-1 facilitate a number of innate and adaptive immune functions. IGF-1 enhances the activity of NK cells from both GH-deficient adults and healthy individuals 90. GH up-regulates the production of NO and TNF-α by murine peritoneal macrophages 83 and IGF-1 stimulates production of TNF-α by human monocytes 91. In addition, GH acts via an autocrine or paracrine mechanism to enhance production of IFN-γ by human PBMCs stimulated with IL-12 92. GH treatment reverses ageing-related reductions of leucocyte chemotaxis, mitogen-induced lymphoproliferation and NK cell activity in male 93 and female 94 rats, with particularly marked effect in ovariectomized animals.
Thyroid hormones exert modulatory effects on lymphocytes, macrophages and neutrophils 95. Recent work has demonstrated that expression of deiodinase 2 is up-regulated in murine macrophages during inflammation and that macrophage function is impaired in its absence, implying a role for locally produced T3 96. Application of T3 to differentiated murine macrophages inhibits M2 activation but enhances M1 activation, increasing IFN-γ and TNF-α production and the extent of phagocytosis 97. Murine DCs express thyroid receptors and T3 stimulates maturation marker expression, secretion of IL-12 and ability to drive naive T cell proliferation and IFN-γ production 98. Thyroid hormone supplementation restored mitogen-induced T cell proliferation in mice subjected to chronic mild stress 99. In elderly humans, NK cell activity positively correlates with serum T3 concentration 100. In vitro T3 supplementation significantly increased the activity of NK cells from individuals with a serum T3 concentration at the lower end of the reference range.
The hormones of the lactotrope, somatotrope and thyrotrope axes merit investigation in Lady Windermere syndrome. Given the advanced age of Lady Windermere patients, it seems plausible that pathogenesis may be facilitated by immunomodulatory consequences of senescence or dysfunction of these systems.
Discussion and conclusion
Elucidation of the factors that confer susceptibility to NTM lung disease in Lady Windermere syndrome is a strategic priority due to the increasing incidence and challenging management of this condition. We hypothesize that underlying genetic susceptibility is unmasked by immunomodulatory consequences of physiological endocrine changes arising from ageing, menopause or slender body habitus (Fig. 1). These changes include reduced oestrogen, leptin and DHEA and increased adiponectin and cortisol. Reduced prolactin, relative growth hormone deficiency and thyroid dysfunction may be additional regulators. Non-physiological changes in hormone levels and receptor function are also possible. For example, some genes associated with NTM lung disease in the study by Szymanski et al. affect endocrine systems. Impaired CFTR function reduces follicle-stimulating hormone-driven oestrogen biosynthesis 101. Polymorphisms of the IRF8 immune gene have been associated with autoimmune thyroid disease, although the implicated polymorphisms differ from those that have been associated with NTM lung disease 102. Additionally, Szymanski and colleagues conducted an exome-wide genetic burden test that identified genes of significance involved in oestrogen receptor signalling and lipid metabolism 9.
A caveat is that immunomodulation by hormones has largely been studied in animal models or in vitro environments designed for investigation of isolated hormones. Much of these data are yet to be validated in vivo in humans. Many hormonal effects are dependent on factors such as receptor subtype, cell type and inflammatory milieu. Additional complexity arises from non-linear concentration–response relationships, interdependence of endocrine systems and modification or production of hormones by target tissues.
Further investigation is warranted to define endocrine changes of significance in Lady Windermere syndrome. As illustrated by research on DHEA in TB, demonstration of causality is likely to be challenging due to the bidirectional signalling between the endocrine and immune systems. Certain medical co-morbidities and medications may be additional sources of confounding. For example, rifampicin alters metabolism of steroid hormones. Dopamine antagonists, which are commonly used to treat antibiotic-related nausea, may alter serum prolactin levels 75.
In conclusion, multiple interacting endocrine systems have the potential to contribute to Lady Windermere syndrome by modulating immune responses to NTM. Further research is warranted to explore the role of endocrine changes as independent susceptibility factors and identify new pathways for treatment.
Disclosures
The authors have no personal, professional or financial conflicts of interest relevant to this paper.
Author contributions
M. H. conceptualized and drafted the manuscript. J. M., W. I. and R. T. made significant contributions to the conception and critical revision of the manuscript.




