Comparison of mutation profiles in primary melanomas and corresponding nodal naevi using next-generation sequencing
TG and EMR contributed equally to this work and should be considered joint first authors.
Summary
Background
Nodal naevi (NN) represent aggregates of melanocytes within peripheral lymph nodes. NN are relatively often found in patients with malignant melanoma (MM), and may mimic metastatic disease.
Aim
To study mutation profiles in MM and NN to find out whether NN descend from a primary MM.
Methods
Next-generation sequencing was performed on formalin-fixed paraffin-embedded tissue of 26 pairs of primary MM and corresponding NN detected by sentinel lymph node biopsy, and 29 MM-characteristic genes were investigated.
Results
In this study, 90% of mutations were detected exclusively in either MM or NN, but not both, in the same patient; the percentage of identical NN and MM mutations in the same individual was only 10%. The most frequently discovered shared mutations were a C>G substitution in the CDKN2A gene and in-frame deletion in ARID1A. Oncogenic driver mutations were frequently observed in MM but only rarely in NN. About three-quarters of mutations in both MM and NN were characterized by C>T or G>A substitutions. The detected rate of ultraviolet (UV)-related C>T base changes was comparably high in both primary MM (35%) and NN (32%).
Conclusions
Based on our data, it seems that NN descend from previously UV-exposed BRAF wildtype cutaneous melanocytes, rather than from primary MM or arrested progenitor cells.
Introduction
Nodal naevi (NN) usually consist of intracapsular (more rarely trabecular) melanocytic aggregates, which are most frequently observed in axillary lymph nodes. In patients with malignant melanoma (MM), NN can be observed in about up to 10% of sentinel lymph node (SLN) and in up to 1% of non-SLN samples.1, 2 By contrast, NN are less frequently detected in SLN or complete lymph node dissection specimens of other cancers. In breast cancer, for example, NN are observed in 0.1% of lymph nodes.1 Two concepts of how NN may develop have been suggested: (i) NN are neural cells that settle during embryonal migration of cells from the neural crest, and (ii) NN are the result of benign dissemination of cutaneous melanocytes (mechanical transport, benign metastasis).3 Even though immunohistochemistry and other diagnostic techniques have improved significantly during recent decades, NN may still be misinterpreted as micrometastases, particularly in patients with MM,3-6 while a misdiagnosis of melanoma metastasis as NN may result in false staging and inappropriate treatment. However, it has been speculated that NN might descend from preceding MM and/or benign naevi.1-4, 7 In this study, we aimed to investigate whether an overlap exists between these two entities regarding their molecular–pathological signatures, and used next-generation sequencing (NGS) to assess melanoma-characteristic mutations in MM and corresponding NN.
Methods
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the local ethics committee of the Ruhr-University Bochum (no. 4749-13; 2013). The data presented in this study are not publicly available due to patient privacy, but are available on request from the corresponding author.
Patients
In total, 26 patients with primary MM and associated NN on SLN biopsy (SLNB) were assessed (Table S1). Indications for SLNB were guideline-based according to the current MM classification systems.8-10 We included patients with histopathologically confirmed primary MM or NN on SLNB. Hence, 52 specimens including the primary MM and the corresponding NN of 26 patients were investigated. Clinical patient data were extracted from electronic databases. Follow-up data were evaluated using the electronic databases and contacting the patients, their relatives, dermatologists, resident practitioners and clinicians.
Histology and immunohistochemistry
Routine histology (preparation, sampling, microscopy) was carried out in line with pathology guidelines for the evaluation of SLN of patients with MM.11 Primary MM and SLN were reassessed by two senior dermatohistopathologists. Immunohistochemistry was performed on formalin-fixed paraffin-embedded (FFPE) tissue using commercial antibodies (S100B, Melan-A, HMB-45, Ki-67; DAKO, Hamburg, Germany). Differentiation between benign nodal melanocytic aggregates and metastatic disease was performed in line with the morphological criteria proposed by Scolyer and colleagues.11
DNA isolation
For DNA extraction, we used three sections (10 μm thickness) from each tumour sample (n = 52 samples). Before and after sectioning, we used haematoxylin and eosin (H&E)-stained SLN tissue to assess the localization and size of each NN. Macrodissection was conducted on the basis of the NN localization in the H&E slides. For DNA isolation, we used a commercial kit (QIAamp DNA-Mini-Kit; QIAGEN, Hilden, Germany), following the manufacturer’s instructions for macrodissected specimens.
Library preparation and next-generation sequencing
We used a well-established amplicon-based sequencing panel that includes 29 genes that are frequently mutated in melanocytic tumours.12 Except for DDX3X, the 29-gene panel included all the genes that are significantly mutated in melanoma as proposed by the Cancer Genome Atlas Network.13 The 29 genes investigated were ARID1A, BAP1, BRAF, BRCA1, CDK4, CDKN2A, CTNNB1, ERBB2, EZH2, FBXW7, GNA11, GNAQ, HRAS, IDH1, KIT, KRAS, MAK2K1, MLH1, NF1, NRAS, PIK3CA, PPP6C, PTEN, PTPN11, RAC1, RB1, SF3B1, STK19 and TP53 (Table 1). A commercial kit (GeneRead Library Prep Ki; QIAGEN) was used for library preparation, and 5 ng of DNA was sufficient for the amplification of the target sequences. Targeted amplicon sequencing was conducted on an integrated sequencer (MiSeq 2005; Illumina, San Diego, CA, USA). Amplification of the human telomerase reverse transcriptase promoter was carried out in a separate PCR assay and spiked to the library preparation primers used.
| Patient | Mutated genes |
|---|---|
| 1 | CDKN2A, IDH1 |
| 3 | ARID1A, CDKN2A |
| 7 | ARID1A, CDKN2A |
| 8 | ARID1A, CDKN2A |
| 13 | CDKN2A |
| 17 | CDKN2A |
| 18 | CDKN2A, PIK3CA |
| 20 | ARID1A, CDKN2A, PIK3CA |
| 21 | ARID1A, CDKN2A, TP53 |
| 22 | ARID1A, CDKN2A, PIK3CA |
| 23 | ARID1A, CDKN2A, IDH1 |
| 24 | ARID1A, CDKN2A, IDH1 |
| 25 | CDKN2A, IDH1 |
- Investigated genes: ARID1A, BAP1, BRAF, BRCA1, CDK4, CDKN2A, CTNNB1, ERBB2, EZH2, FBXW7, GNA11, GNAQ, HRAS, IDH1, KIT, KRAS, MAK2K1, MLH1, NF1, NRAS, PIK3CA, PPP6C, PTEN, PTPN11, RAC1, RB1, SF3B1, STK19 and TP53.
Data analysis and base/variant calling
Sequencing analyses workflows were conducted by means of specialized software (Biomedical Genomic Workbench, V5.0; QIAGEN). Mutation types were annotated using several databases, including ClinVar, COSMIC, dbSNP, and the 1000 Genomes Project, as previously described.14 Mutations were filtered for quality using the following critera: overall coverage ≥ 10 reads, ≥ 2 read counts of the mutated allele, ≥ 5% frequency of mutated allele, mutation reported in COSMIC, mutation is nonsynonymous. Detailed assessment was based on characteristic ultraviolet (UV) radiation (UVB C>T, UVA G>T) and FFPE artefacts (C>T) conditioned base changes. Driver mutations were classified in line with recent publications.14-18
Comparison of mutations per mega base pairs (mut/Mbp)
To compare our mutation rate with the data of Tang et al.,19 who investigated the genomic landscapes of individual melanocytes from human skin, we evaluated mutations (mut)/Mbp. By normalizing the number of mutations to the length of the sequencing panel (30 genes, 110 649 bp), we found a mean ± SD of 114 ± 116 mut/Mbp for PM and 76 ± 91 mut/Mbp for NN. As the sequencing panel was small, every individual detected mutation in each sample increased the mutation rate by roughly 9 mut/Mbp. A two-sided paired t-test between both groups was not significant (P = 0.19).
Statistical analysis/illustration
Analysis and visualization of the data was carried out by means of R software (V3.5.1; https://www.r-project.org). We generated bar graphs by means of the R package ggplot220 and heat maps with aid of the R package ComplexHeatmap,21 including the hclust method (ward.D.) and distance (euclidean).
Results
Number of mutations
We detected 553 mutations in the 52 tissue samples studied (mean ± SD 18 ± 18 per sample): 333 mutations in primary MM and 220 in NN, with a mean ± SD of 11 ± 12 and 7 ± 7, respectively (Fig. S1).
Genes affected
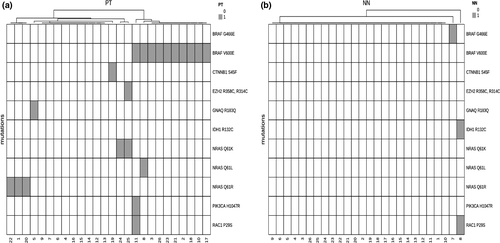
Most of the mutations in the MM samples were detected in the TP53 gene (n = 88), with TP53 mutations making up 16% ± 14% of all mutations in MM samples and 7% ± 13% of all mutations in NN samples. The vast majority (90%) of mutations were detected exclusively in either MM or NN of the same patient (Fig. S1), but not both; the percentage of identical mutations detected both in MM and NN of the same individual was only 10% (Table 1). The most frequent shared mutations (8 of 13; 61.5%) were a C>G substitution in the CDKN2A gene and an in-frame deletion of a single glutamine in the ARID1A gene (Table 2).
| Gene | Chr | Base change | Mutation type |
|---|---|---|---|
| CDKN2A | 9 | C>G | SNV |
| ARID1A | 1 | GCA removed | Deletion |
| IDH1 | 2 | C>T | SNV |
| PIK3CA | 3 | A>G | SNV |
| TP53 | 17 | T>C, G>A | SNV, MNV |
- Chr, chromosome, MNV, multinucleotide variants; SNV, single-nucleotide variants.
Oncogenic drivers
We frequently observed oncogenic driver mutations in MM but only rarely in NN (only 8% of NN specimens had at least one driver mutation; Fig. 1a,b). BRAF.V600 (n = 11; 42% of MM) and NRAS.Q61 (n = 6; 23% of MM) genes were the most frequent driver mutations in MM specimens. We detected a large number of other mutations in both MM and NN, showing great variability in the number found within each patient and between the two tumour types (MM and NN) (Fig. 2).
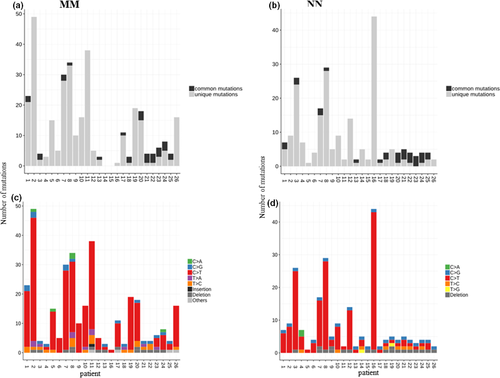
Mutation types
The majority of mutations (78% in MM and 75% in NN) were C>T or complementary G>A substitutions. In MM samples, the basal mutation rate was almost exclusively associated with UV-related C>T base changes (Fig. 2).
Other associations
We did not observe any association between the mutational load and the storage age (between 2002 and 2016) of the FFPE specimens.
Discussion
In the present investigation, we aimed to assess mutations that are frequently detected in MM and compare the mutation profiles of MM and NN. Comparison of the mutation profiles was performed to clarify whether there is a molecular signature indicating that NN may descend from the primary MM within the draining lymph basin. Altogether, 24 melanoma-characteristic driver mutations were found in 17 MM but only 2 NN samples, with the latter having a total of only 3 detected driver mutations in 3 genes (BRAF.G466E, IDH1.R132C and RAC1.P29S in two cases).22 These mutations were not found in the corresponding primary tumours of the same patients. Novel mutations do occur during tumour development/progression, indicating that a minority of NN might have a malignant potential; nevertheless, we did not observe MM recurrence or MM-related death in the two cases mentioned above.5 Furthermore, none of the discovered driver gene mutations were found in both the primary MM and corresponding NN. Hence, our data do not support the concept that NN originate through mechanical transport of MM-associated melanocytes into regional lymph nodes. However, we cannot fully exclude the possibility that pre-existing benign melanocytes of MM reached lymph nodes via mechanical transport. Hence, the aforementioned results on driver mutations also confirm the benign nature of NN and argue against a direct origin of NN from the corresponding primary MM.
Compared with common melanocytic naevi (CMN) the proportion of mutations in the BRAF gene appears to be much lower in NN; Pollock et al.23 previously detected mutant BRAF in over 80% of CMN. Additionally, and in contrast to our data, Taube et al.7 detected a mutated BRAF gene in 50% of their NN samples. Obviously, the sole presence of mutant BRAF is insufficient for transformation into malignancy, as detailed by Pollock et al.23 Moreover, the relatively high UV signature mutational load in NN is not in agreement with the hypothesis that NN are neural cells that settle during embryonal migration of cells from the neural crest.3 It is very unlikely that such progenitor cells ever had exposure to UV radiation. The mutation profile, especially with regard to the mutational UV signature, rather suggests descent of the NN from CMN or individual melanocytes from skin. However, the fact that NN are usually observed intracapsularly and usually not found in sinuses gives support to the neural crest hypothesis.3 Nonetheless, a limitation of the present investigation is that we may not have detected all NN mutations, and as we used macrodissected material, we cannot fully exclude that NN were significantly contaminated with lymphocytic tissue.
Cao et al.24 recently investigated two patients with giant congenital naevus (GCN) and corresponding NN. Clonal evolution analysis (whole-exon sequencing) of their first patient, whose tumour mutation burden value was relatively stable, showed that the GCN and nodal naevus had the same initial origin and then diverged into two branches as a result of gene mutations. By contrast, in the other patient, whose tumour mutation burden value was 68.02 per Mb in a GCN but only 17.55 per Mb in an associated NN, these two samples were from different origins in the beginning, each with its own gene mutation profile. Interestingly, Cao et al.24 concluded that their results are consistent with the two aforementioned theories on the pathogenesis of NN at the molecular biological level. Lozada et al.25 investigated the repertoire of somatic genetic alterations affecting 300 key cancer genes in CMN, and confirmed the high prevalence of recurrent hotspot mutations affecting BRAF and NRAS. Furthermore, Shain et al.26 reported that unequivocally benign CMN parts of melanomas harboured BRAF.V600E mutations exclusively, whereas those categorized as intermediate were enriched for NRAS mutations. By contrast, Tang et al.19 recently studied the genomic landscapes of individual melanocytes from human skin, and detected numerous mutations predicted to activate the mitogen-activated protein kinase (MAPK) pathway. These included loss-of-function mutations in negative regulators of the MAPK pathway, affecting the NF1, CBL and RASA2 genes. As expected, melanocytes from UV-exposed sites had UV-signature mutations. In that study, the authors also observed gain-of-function or change-of-function mutations in BRAF, NRAS and MAP2K1; however, BRAF.V600E mutations (the most common mutation in the MAPK pathway occurring in CMN) were not detected.19 With regard to the mutation profile of NN observed in the present study (absence of BRAF.V600E mutations), the findings of Tang et al.19 may indicate that NN rather descend from normal melanocytes of previously UV-exposed skin.
We detected higher mutation rates in our samples compared with the results of Tang et al.19 However, it is difficult to compare mutation rates directly with the data reported by Tang et al.19 for several reasons. First, we used a smaller sequencing panel than theirs (30 vs. 500 genes) limiting the accuracy of our mutation rate. We also used completely different approaches to filter our mutation dataset for somatic mutations. Tang et al.19 detected mutation rates from 0.8 up to 32.2 mut/Mbp (mean 7.9 mut/Mbp) in benign melanocytes that were comparable with respective MM samples. Importantly, mutation rates largely vary between individuals, anatomical sites and individual melanocytes, and also with degree of sun exposure. In addition, our study had gene selection bias because we specifically used genes that are frequently mutated in MM (driver mutations). Because of the small sequencing panel, as mentioned above, comparing signature mutations was also limited, and extracting or fitting mutational signatures was not possible because of the low number of detected mutations. The benign melanocytes studied by Tang et al.19 had a high frequency of UV signatures, indicating a high number of C>T transitions, which were also present in the samples with higher mutation rates in our study. In our MM samples, the basal mutation rate was almost exclusively associated with UV-related C>T base changes.15, 17, 22 However, such mutations are also known to be a result of deamination processes induced by the older age of FFPE material. Such parameters represent a source of false-positive results that could possibly lead to misinterpretation of somatic mutations.27 To distinguish UV-induced from deamination-induced C>T substitutions, we compared mutation load with the age of the FFPE specimens, and did not find any significant association between them, thus excluding the possibility that FFPE artefact-related mutations played a significant role in our study.27
However, in the context of this study, it would also be interesting to compare the mutation profiles of NN with melanomas of unknown primary (MUP), which are sporadically found, for example, in inguinal or axillary lymph nodes. In other words, could NN be the origin of MUP? Indeed, several researchers have observed that the genomic profiles of patients with known cutaneous MM and MUP are considerably concordant.28, 29 Hilke et al.29 demonstrated that the majority of cutaneous MMs and MUPs could be classified in the genomic subtypes BRAF, RAS and NF1. Compared with other melanoma subtypes (mucosal, acral), cutaneous MM and MUP had the highest tumour mutation burden.29 Interestingly, these tumours are also similar in terms of the number of copy number variations and the frequency of genomic subgroups, BRAF-, RAS-, NF1-mutated or triple wild type.27 Taken together, the results of Hilke et al.29 and others support the notion that the cutaneous MM subtype and MUP are similar, and that the latter likely originates from regressed or unrecognized primary cutaneous MMs. Most importantly, however, diagnosis of NN appears to be robustly reliable, as we and other research groups have demonstrated in large study populations that overall survival does not differ between patients with NN and patients with negative SLNB.5, 30, 31 Hence, de Beer et al.30 recently concluded that the current management of patients with NN as the same as those with a negative SLN seems to be appropriate.
Conclusion
Our results indicate that primary MM and corresponding NN do not show significantly overlapping mutation profiles. We suggest that NN descend from previously UV-exposed BRAF wildtype CMN or individual melanocytes, rather than from primary MM or arrested progenitor cells. Hence, the mechanical transport of benign cutaneous melanocytes via the lymph draining basin into lymph nodes appears to result in NN formation. To shed more light on the development of NN, copy number variations should be compared on a single cell level in future studies.
What's already known about this topic?
- NN represent aggregates of melanocytes within peripheral lymph nodes.
- NN have a benign nature; however, their origin is still unclear.
What does this study add?
- It is likely that NN descend from previously UV-exposed BRAF wildtype cutaneous melanocytes rather than from primary MM or arrested progenitor cells.
Acknowledgement
We are very grateful to K. Griewank and S. Horn (Essen) for designing the sequencing panel, and the collaborating histopathological laboratories for providing FFPE samples: K. Fegeler (Münster), H. Kutzner (Friedrichshafen), H.-U. Kasper (Münster), A. Neuber (Wesel), D. Krahl (Heidelberg) and A. Tannapfel (Bochum). This study is part of the doctoral thesis of EMR. Open access funding enabled and organized by ProjektDEAL.
Conflict of interest
JCB has received speaker honoraria from Amgen, Merck Serono, Pfizer, Recordati and Sanofi; advisory board honoraria from 4SC, Amgen, eTheRNA, Merck Serono, Novartis and ReProTher, research funding from Alcedis, Boehringer Ingelheim, Bristol-Myers Squibb, IQVIA, and Merck Serono; and travel support from 4SC and Incyte. TG has received speaker and/or advisory board honoraria from BMS, Sanofi-Genzyme, MSD, Novartis Pharma, Roche, AbbVie, Almirall, Janssen, Lilly, Pfizer, Pierre Fabre and Merck Serono, outside the submitted work. DS has received personal fees from Amgen, Array, AstraZeneca, Boehringer Ingelheim, Incyte, 4SC, Leo Pharma, Pfizer, Pierre Fabre, personal fees and other monies from Merck-EMD, BMS, Roche, Novartis, MSD, Sanofi/Regeneron and Philiogen. ES has received grants and/or personal fees from Almirall, Galderma, Pierre Fabre and Leo Pharma, outside the submitted work. The other authors declare no conflicts of interest.