NOD2 signalling in hidradenitis suppurativa
Conflict of interest: The authors declare that they have no conflicts of interest.
T. Gambichler and S. Hessam contributed equally to this work and should be considered joint first authors.
Summary
Background
Hidradenitis suppurativa (HS) is associated with dysregulated immune responses including altered expression of cytokines, chemokines, and antimicrobial peptides and proteins (AMPs).
Aims
To evaluate the expression of nucleotide-binding oligomerization domain-containing (NOD)2 and related factors in HS skin samples and keratinocyte cultures.
Methods
We performed real-time PCR for NOD2, receptor-interacting serine/threonine-protein kinase (RIP)2, cyclic amine resistance locus (CARL), skin-derived antileukoproteinase (SKALP)/elafin, human β-defensin (hBD)2, LL37, psoriasin and RNAse7 in lesional and nonlesional skin of 19 patients with HS and in keratinocyte cultures [unstimulated, muramyl dipeptide (MDP)-stimulated or Pam2CSK4 (Pam2)-stimulated] from and nonlesional skin.
Results
We observed significantly elevated mRNA expression for NOD2 (P < 0.01), hBD2 (P = 0.02), RNase7 (P < 0.001), psoriasin (P < 0.01) and SKALP/elafin (P = 0.02) in lesional compared with nonlesional skin. We found a significant correlation between NOD2 mRNA and hBD2 (r = 46; P = 0.04), psoriasin (r = 0.67; P < 0.01) and SKALP/elafin (r = 0.65; P < 0.01). In unstimulated, Pam2-stimulated and MDP-stimulated normal keratinocytes, NOD2, RIP2, CARL and SKALP/elafin expression significantly (P < 0.05) increased from 6 to 48 h, whereas in unstimulated, Pam2-stimulated and MDP-stimulated HS keratinocytes, RIP2, CARL and SKALP/elafin expression significantly (P < 0.05) declined from 6 to 48 h. mRNA expression of NOD2 (unstimulated, Pam2-stimulated, MDP-stimulated), CARL (unstimulated, Pam2-stimulated, MDP-stimulated) and SKALP/elafin (unstimulated, Pam2-stimulated) at 6 h was significantly increased in HS compared with normal keratinocytes.
Conclusion
We have shown for the first time that NOD2 signalling is activated in HS and might contribute to the pathogenesis via induction of AMPs and activation of other pathways such as nuclear factor κB signalling.
Introduction
Hidradenitis suppurativa (HS) is a common chronic inflammatory condition of intertriginous skin. Hyperplasia of the hair follicle epithelium, follicular hyperkeratosis and interfollicular epidermal hyperplasia are considered to be the early events in HS. In addition to a dysregulated immune-mediated inflammatory response, genetic predisposition is frequently discussed as an important risk factor. Predominantly loss-of-function mutations have been reported in genes of the γ-secretase complex, namely NCSTN, PSEN1, PSENEN and PSTPIP1.1-4 Nucleotide-binding, oligomerization domain (NOD)2 mutations have been described in familial HS and HS-associated autoinflammatory conditions, such as PASH (pyoderma gangrenosum, acne vulgaris and suppurative hidradenitis) syndrome. Moreover, polymorphisms within the NOD2 gene have often been reported in Crohn disease (CD), which is not infrequently associated with HS and shares similar pathophysiological mechanisms. Notably, genetic variation in NOD2 and cigarette smoking, a significantly associated factor in HS, are also well-established risk factors for the development of CD.5-8 We aimed to analyse the expression profiles of NOD2 and related factors in samples of patients with HS and in keratinocyte cultures.
Methods
The study was approved (no. 5076-14) by the ethics committee of Ruhr-University Bochum, and conducted according to the Declaration of Helsinki. Informed consent was obtained from all study subjects.
Patients
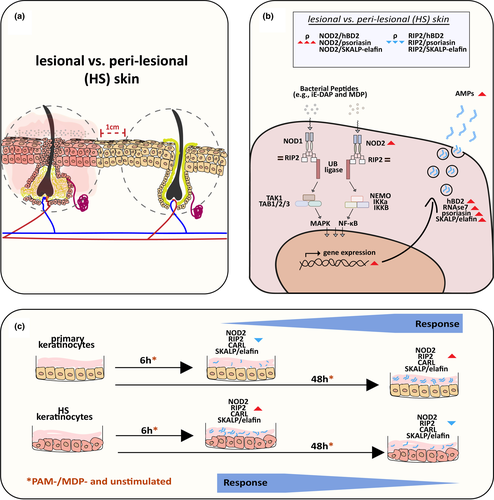
This prospective study was performed on skin tissues from 19 patients with HS. Skin samples were taken from representative inflammatory HS lesions and from neighbouring nonlesional skin (10 mm from the lesional skin border; Fig. 1). Patient characteristics are detailed in Table 1. None of the patients had systemic therapy with antibiotic or immunosuppressive agents within 4 weeks before inclusion. Skin samples were obtained by 5-mm punch biopsy and immediately stored in RNAlater solution (Qiagen, Hilden, Germany) at −80 °C.
| Parameter | (N = 19) |
|---|---|
| Age, years; mean ± SD | 41.63 ± 9.34 |
| Sex, n (%) | |
| Female | 12 (63.2) |
| Male | 7 (36.8) |
| Current smoker, n (%) | 13 (68.2) |
| BMI, kg/m2; mean ± SD | 28.86 ± 6.09 |
| BMI categories, n (%) | |
| Normal weight (≤ 24.9) | 4 (21.1) |
| Overweight (≥ 25) | 4 (21.1) |
| Obesity (≥ 30) | 11 (57.8) |
| Hurley stage, n (%) | |
| I | 3 (15.8) |
| II | 9 (47.4) |
| III | 7 (36.8) |
| Positive family history | 6 (31.6) |
| Biopsy site, n (%) | |
| Axilla | 9 (47.4) |
| Groin | 7 (36.8) |
| Genital | 3 (15.8) |
Cell cultures
Skin biopsies from the lesional skin of three patients with HS were used for cell isolation. After an overnight incubation period with 1% Proteinase K at 4 °C, the epidermis was detached from the dermis. Following enzymatic digestion of the epidermis with 1% collagenase, a single cell suspension was prepared. Isolated keratinocytes were cultured (Dermal Cell Basal Medium and Keratinocyte Growth Additive; American Type Culture Collection, Manassas, VA, USA) at 37 °C, 5% CO2 and 97% humidity. When cells reached 80–90% confluency, they were passaged in six-well plates and used for further experiments. As a control group, a commercially available primary keratinocyte (foreskin) cell line (American Type Culture Collection) was used. Both cell lines were treated with 50 ng/mL Pam2CSK4 (Pam2) and 10 µg/mL muramyl dipeptide (MDP) for 6 and 48 s. After treatment, cells were lysed and frozen at −20 °C (Fig. 1).
RNA isolation and real-time PCR
Total RNA was extracted from cell lysates and skin samples (RNeasy Lipid Tissue Kit; QIAGEN, Chatsworth, CA, USA). The mRNA expression levels were quantified by real-time PCR (PowerSYBR Green PCR Master Mix) (ThermoFisher, Waltham, MA, USA) on a PCR analyser (Cobas z480; Roche, Mannheim, Germany) in accordance with Minimum Information for Publication of Quantitative Real-Time PCR Experiments Guidelines.9Three widely used reference genes (GAPDH, β2-microglobulin and RPL38) were also tested; RPL38 exhibited the most stable expression levels and was used as the housekeeping control. Target mRNA expression results were normalized to corresponding RPL38 mRNA transcript levels (2−ΔΔCT method).10 The primer pairs for NOD2, receptor-interacting serine/threonine-protein kinase (RIP)2, cyclic amine resistance locus (CARL), skin-derived antileukoproteinase (SKALP)/elafin, human β-defensin (hBD)2, LL37, psoriasin and RNAse7 were designed using Primer Express software (PE Applied Biosystems, Foster City, CA, USA).
Statistics
MedCalc software (V19.1.7; MedCalc, Ostende, Belgium) was used for statistical analysis. Analysis of data distribution was performed by the D’Agostino–Pearson test. For non-normally distributed variables, the Wilcoxon test (dependent samples) and the Mann–Whitney U-test (independent samples) were used. Correlations were calculated using Spearman rank correlation coefficient (ρ). P < 0.05 was considered significant.
Results
Patient data
Patient data (median and range) are detailed in Table 2.
| Protein | Skin samples, median (range) | P a | |
|---|---|---|---|
| Nonlesional | Lesional | ||
| NOD2 | 1.22 (0.55–3.1) | 2.91 (0.6–4.13) | < 0.01 |
| RIP2 | 4.68 (3.1–9.62) | 4.27 (2.6–10.1) | 0.89 |
| CARL | 2.9 (1.5–6) | 2.4 (0.88–4.1) | 0.06 |
| hBD2 | 0.65 (0.16–10) | 3 (0.08–161.5) | 0.02 |
| RNase7 | 37.2 (0.12–96.1) | 109.6 (4.8–291.2) | < 0.001 |
| Psoriasin | 84.2 (3.1–790) | 624.2 (3.1–3226) | < 0.01 |
| SKALP/elafin | 1.6 (0.12–8.96) | 6.98 (0.32–47.6) | 0.02 |
- CARL, cyclic amine resistance locus; hBD, human β-defensin; NOD, nucleotide-binding oligomerization domain-containing; RIP, receptor-interacting serine/threonine-protein kinase; SKALP, skin-derived antileukoproteinase.
- a Wilcoxon test.
mRNA expression
We observed significantly elevated mRNA expression for NOD2 (P < 0.01), hBD2 (P = 0.02), RNase7 (P < 0.001), psoriasin (P < 0.01) and SKALP/elafin (P = 0.02) in lesional skin compared with nonlesional skin samples from patients with HS.
Correlation between proteins in lesional skin samples
We found a moderate to strong correlation between NOD2 mRNA and hBD2 (r = 46; P = 0.04), psoriasin (r = 0.67; P < 0.01) and SKALP/elafin (r = 0.65; P < 0.01) in lesional HS skin samples.
RIP2 mRNA expression inversely correlated with hBD2 (r = −0.48; P = 0.04), psoriasin (r = −0.55; P = 0.02) and SKALP/elafin (r = −0.48; P = 0.03) in lesional skin (Table 3).
| Comparators | ρa | 95% CI | P |
|---|---|---|---|
| NOD2 and hBD2 | 0.46 | 0.03 to 0.77 | 0.04 |
| NOD2 and psoriasin | 0.67 | 0.31 to 0.86 | < 0.01 |
| NOD2 and RNase7 | 0.44 | −0.02 to 0.74 | 0.06 |
| NOD2 and SKALP/elafin | 0.65 | 0.28 to 0.85 | < 0.01 |
| RIP2 and hBD2 | −0.48 | −0.77 to −0.03 | 0.04 |
| RIP2 and psoriasin | −0.55 | −0.80 to −0.13 | 0.02 |
| RIP2 and RNase7 | −0.42 | −0.74 to 0.04 | 0.07 |
| RIP2 and SKALP/elafin | −0.48 | −0.77 to −0.03 | 0.03 |
- hBD, human β-defensin; NOD, nucleotide-binding oligomerization domain-containing; RIP, receptor-interacting serine/threonine-protein kinase; SKALP, skin-derived antileukoproteinase.
- a Spearman coefficient of rank correlation.
Cell culture
In unstimulated, Pam2-stimulated and MDP-stimulated normal keratinocytes, NOD2, RIP2, CARL and SKALP/elafin expression significantly (P < 0.05) increased from 6 to 48 h, whereas in unstimulated, Pam2-stimulated and MDP-stimulated HS keratinocytes, RIP2, CARL and SKALP/elafin expression significantly (P < 0.05) declined from 6 to 48 h (Table 4).
| Parameters | Keratinocytes | P | |
|---|---|---|---|
| Normal | HS | ||
| NOD2 (unstimulated) | |||
| 6 h | 0.03 (0.01–0.1) | 0.16 (0.13–0.22) | < 0.01 |
| 48 h | 0.17 (0.1–0.51) | 0.055 (0.03–0.24) | 0.04 |
| 6 h vs. 48 h | P < 0.05 | P > 0.05 | |
| NOD2 (Pam2) | |||
| 6 h | 0.085 (0.04–0.14) | 0.23 (0.07–0.43) | < 0.01 |
| 48 h | 0.26 (0.14–0.38) | 0.17 (0.1–0.21) | < 0.05 |
| 6 h vs. 48 h | P < 0.05 | P > 0.05 | |
| NOD2 (MDP) | |||
| 6 h | 0.09 (0.05–0.12) | 0.16 (0.08–0.53) | 0.01 |
| 48 h | 0.16 (0.06–0.34) | 0.11 (0.02–0.22) | 0.29 |
| 6 h vs. 48 h | P < 0.05 | P > 0.05 | |
| RIP2 (unstimulated) | |||
| 6 h | 8.9 (6.6–13.2) | 10.9 (5.8–14.6) | 0.29 |
| 48 h | 22.3 (14.6–33.7) | 2.9 (2.1–3.6) | < 0.001 |
| 6 h vs. 48 h | P < 0.05 | P < 0.05 | |
| RIP2 (Pam2) | |||
| 6 h | 9.5 (7.1–12.2) | 13.1 (4.1–23.2) | 0.11 |
| 48 h | 20.7 (16.9–34.1) | 3.1 (1.7–4.2) | 0.002 |
| P < 0.05 | P < 0.05 | ||
| RIP2 (MDP) | |||
| 6 h | 10.4 (8.3–16.3) | 12.7 (5.3–16.8) | 0.76 |
| 48 h | 28.6 (15.3–38.2) | 3.2 (2.1–5.4) | < 0.001 |
| 6 h vs. 48 h | P < 0.05 | P < 0.05 | |
| CARL (unstimulated) | |||
| 6 h | 13.7 (7.3–19.6) | 37.7 (18.1–54.6) | < 0.01 |
| 48 h | 19.1 (11.6–30.3) | 7.8 (4.9–12.9) | < 0.01 |
| 6 h vs. 48 h | P < 0.05 | P < 0.05 | |
| CARL (Pam2) | |||
| 6 h | 10.9 (7.5–21.3) | 31.3 (10.1–57.4) | < 0.01 |
| 48 h | 32.4 (13.7–42.9) | 8.5 (4.6–20.3) | < 0.01 |
| P < 0.05 | P < 0.05 | ||
| CARL (MDP) | |||
| 6 h | 13.1 (8.1–28.9) | 37.7 (15.1–45.3) | < 0.01 |
| 48 h | 28.4 (12.8–44.1) | 8.5 (4.3–15) | < 0.01 |
| 6 h vs. 48 h | P < 0.05 | P < 0.05 | |
| SKALP/elafin (unstimulated) | |||
| 6 h | 1.9 (0.8–3.4) | 4.3 (2.3–5.4) | 0.02 |
| 48 h | 2.5 (0.6–5.4) | 0.5 (0.1–1.2) | < 0.01 |
| 6 h vs. 48 h | P < 0.05 | P < 0.05 | |
| SKALP/elafin (Pam2) | |||
| 6 h | 3.1 (0.7–4.1) | 5.7 (1.9–13) | < 0.01 |
| 48 h | 2.9 (0.4–6.9) | 1.5 (0.9–1.8) | 0.25 |
| 6 h vs. 48 h | P < 0.05 | P < 0.05 | |
| SKALP/elafin (MDP) | |||
| 6 h | 2.7 (0.65–5.7) | 3.8 (2.7–9.5) | 0.07 |
| 48 h | 3.6 (0.85–6.3) | 1.1 (0.37–2.2) | 0.02 |
| 6 h vs. 48 h | P < 0.05 | P < 0.05 | |
- hBD, human β-defensin; HS, hidradenitis suppurativa; MDP, muramyl dipeptide; NOD, nucleotide-binding oligomerization domain-containing; Pam2, Pam2CSK4; RIP, receptor-interacting serine/threonine-protein kinase; SKALP, skin-derived antileukoproteinase.
At 6 h, mRNA expression of NOD2 (unstimulated, Pam2-stimulated, MDP-stimulated), CARL (unstimulated, Pam2-stimulated, MDP-stimulated) and SKALP/elafin (unstimulated, Pam2-stimulated) was significantly increased in HS compared with normal keratinocytes. By contrast, at 48 h, mRNA expression of NOD2 (unstimulated, Pam2-stimulated), RIP2 (unstimulated, Pam2-stimulated, MDP-stimulated), CARL (unstimulated, Pam2-stimulated, MDP-stimulated) and SKALP/elafin (unstimulated, MDP-stimulated) was significantly decreased in HS keratinocytes compared with normal keratinocytes (Table 4).
Discussion
NOD2 is an intracellular sensor of intracellular and extracellular pathogens such as bacteria, and is expressed in barrier cells of the skin, where it plays a significant role in wound healing and defence against pathogens. NOD2 is a member of the Nod-like receptor family and recognizes MDP, which is part of the cell wall of almost all bacteria. In keratinocytes, NOD2 regulates the expression of antimicrobial peptides and proteins (AMPs) through the activation of an intracellular signalling cascade that requires RIP. Once activated, NOD2 induces proinflammatory gene transcription via activation of the nuclear factor (NF)-κB signalling pathway (Fig. 1b). In addition to the NFκB pathway, NOD2 stimulation also activates the mitogen-activated protein kinases p38, extracellular signal-regulated kinase and Jun kinase. These cascades are rapidly induced after stimulation with Pam2 and MDP, leading to activation of proinflammatory genes.11-14 Thus, dysfunction of NOD2 signalling pathway can lead to a dysregulated inflammatory response and has been described in other chronic inflammatory diseases, such as inflammatory bowel disease.8
There is a wealth of evidence that AMP expression is significantly altered in HS.15-17 Many AMPs, including hBD2, psoriasin and LL37, are overexpressed in lesional skin of patients with HS, which might be the result of a general overactivation of the innate immune system in response to pathogens or other stimuli. Alternatively, this AMP overexpression could also be due to an altered microbiome in lesional skin of patients with HS. Whether dysregulation in AMP expression is inherent to HS or secondary to alterations in the HS microbiome remains elusive. However, altered AMP expression probably contributes to HS pathogenesis and/or plays an important role in the progression and maintenance of HS.15-17
In this paper, we provide evidence that NOD2/RIP2 signalling might be involved in the pathogenesis of HS. We observed significantly elevated mRNA expression for NOD2, hBD2, RNase7, psoriasin and SKALP/elafin in lesional skin compared with nonlesional HS skin (Fig. 1b). In HS lesions we found a positive correlation between NOD2 mRNA expression and hBD2, psoriasin and SKALP/elafin, and a negative correlation between RIP2 mRNA expression and hBD2, psoriasin and SKALP/elafin. This observation can be explained by a gradual induction of NOD2 tolerance due to the chronic overactivation of the NOD2–RIP2 axis in HS lesions. Although high AMP expression is expected to be associated with high RIP2 expression, degradation of RIP2 was previously reported18 to be closely associated with NOD2-induced tolerance through a negative feedback loop, possibly explaining the observed negative correlation in HS lesions. This indicates activation of the NOD2–RIP2 axis to be especially important in the initial antimicrobial response in HS pathogenesis, while mediating immune tolerance in a chronic HS setting.
We used two different methods for cell culture stimulation. In unstimulated, Pam2-stimulated and MDP-stimulated normal keratinocytes (Fig. 1c), expression of NOD2, RIP2, CARL and SKALP/elafin significantly increased from 6 to 48 h. By contrast, in unstimulated, Pam2-stimulated and MDP-stimulated HS keratinocytes, RIP2, CARL and SKALP/elafin expression significantly declined from 6 to 48 h. At 6 h, mRNA expression of NOD2 (unstimulated, Pam2-stimulated, MDP-stimulated), CARL (unstimulated, Pam2-stimulated, MDP-stimulated) and SKALP/elafin (unstimulated, Pam2-stimulated) was significantly increased in HS compared with normal keratinocytes. However, at 48 h, mRNA expression of NOD2 (unstimulated, Pam2-stimulated), RIP2 (unstimulated, Pam2-stimulated, MDP-stimulated), CARL (unstimulated, Pam2-stimulated, MDP-stimulated) and SKALP/elafin (unstimulated, MDP-stimulated) was significantly decreased in HS compared with normal keratinocytes. HS keratinocytes differed from normal keratinocytes with respect to expression behaviour and timing, independently of stimulation. Unlike normal keratinocytes, HS keratinocytes were characterized by early gene upregulation of NOD2, RIP2, CARL and SKALP/elafin (Fig. 1c).
Apart from NOD2 and associated factors, we also investigated SKALP/elafin in HS, which is the first such study, to our knowledge. SKALP/elafin is an inducible keratinocyte-derived proteinase inhibitor with specificity for polymorphonuclear leucocyte-derived elastase and proteinase-3. SKALP/elafin is virtually absent in normal human epidermis but is found in a number of inflammatory skin diseases, including psoriasis.19, 20 It seems to act as a modulator of epidermal inflammation by interfering with polymorphonuclear leucocyte trafficking and could protect structural proteins against elastase-mediated damage. It has also been found to possess antimicrobial activity and is thus part of the cutaneous host defence system. Inflamed epidermis (psoriasis, wound healing, ultraviolet-irradiated skin) contains keratinocytes that are hyperproliferative and display an abnormal differentiation programme, resulting in expression of anti-inflammatory proteins such as SKALP/elafin. Similar to other inflammatory epidermal changes, inflammation in HS is associated with SKALP/elafin gene upregulation, indicating common compensatory mechanisms in these conditions.19, 20
Conclusion
What’s already known about this topic?
- HS is associated with dysregulated immune responses including altered expression of cytokines, chemokines and antimicrobial proteins.
- NOD2 signalling has not been studied in HS.
What does this study add?
- NOD2 signalling is activated in HS and might contribute to the pathogenesis of HS via induction of AMPs and activation of pathways such as NFκB signalling.
Acknowledgement
This work is part of the doctoral thesis of M. Bakirtzi. We thank D. Kasakovski for preparing the graphical figures.