Design of an Ara h 2 hypoallergen from conformational epitopes
Jungki Min and Tarun Keswani contributed equally to this work.
Abstract
Introduction
Adverse reactions are relatively common during peanut oral immunotherapy. To reduce the risk to the patient, some researchers have proposed modifying the allergen to reduce IgE reactivity, creating a putative hypoallergen. Analysis of recently cloned human IgG from patients treated with peanut immunotherapy suggested that there are three common conformational epitopes for the major peanut allergen Ara h 2. We sought to test if structural information on these epitopes could indicate mutagenesis targets for designing a hypoallergen and evaluated the reduction in IgE binding via immunochemistry and a mouse model of passive cutaneous anaphylaxis (PCA).
Methods
X-ray crystallography characterized the conformational epitopes in detail, followed by mutational analysis of key residues to modify monoclonal antibody (mAb) and serum IgE binding, assessed by ELISA and biolayer interferometry. A designed Ara h 2 hypoallergen was tested for reduced vascularization in mouse PCA experiments using pooled peanut allergic patient serum.
Results
A ternary crystal structure of Ara h 2 in complex with patient antibodies 13T1 and 13T5 was determined. Site-specific mutants were designed that reduced 13T1, 13T5, and 22S1 mAbs binding by orders of magnitude. By combining designed mutations from the three major conformational bins, a hexamutant (Ara h 2 E46R, E89R, E97R, E114R, Q146A, R147E) was created that reduced IgE binding in serum from allergic patients. Further, in the PCA model where mice were primed with peanut allergic patient serum, reactivity upon allergen challenge was significantly decreased using the hexamutant.
Conclusion
These studies demonstrate that prior knowledge of common conformational epitopes can be used to engineer reduced IgE reactivity, an important first step in hypoallergen design.
Graphical Abstract
Key messages
- Structural data of human IgG complexed with Arah2 were utilized to rationally design a hypoallergen.
- Hypoallergenicity of an Arah2 hexamutant was demonstrated by reduced IgE serum inhibition in 16 patients.
- The Arah2 hexamutant showed reduced anaphylaxis in a mouse model using pooled allergic patient sera.
1 INTRODUCTION
IgE-mediated peanut allergy is a severe and chronic condition currently affecting 1–2% of the population.1 Peanut is the most common cause of food-induced anaphylaxis, resulting in the highest number of emergency room visits.2 Peanut allergy usually emerges in childhood, and in contrast to other food allergies like milk and eggs, it does not typically resolve with age.3, 4 Thus, therapeutics to prevent anaphylaxis in peanut allergy have been under development.
Peanut oral immunotherapy (OIT) has recently emerged as a promising treatment option that can desensitize peanut allergic individuals. However, as our current therapies involve the delivery of unmodified allergens to patients with a known low threshold for said allergens, it inherently carries the risk of IgE cross-linking and anaphylaxis. Indeed, clinical trials have shown an increased risk of systemic allergic reactions. Moreover, gastrointestinal adverse events and eosinophilic esophagitis are among the most common side effects in oral immunotherapy, resulting in cessation of therapy.5 To improve the safety of peanut OIT, modifications to the treatment such as lower dosing, or different routes of exposure such as sublingual or epicutaneous have been explored.6, 7 These methods reduce the risk of anaphylaxis; however, these modifications have been less successful in generating sustained unresponsiveness, or durable clinical efficacy. In addition, these improvements have not fully mitigated the risk of anaphylaxis.
Thus, a way to simultaneously reduce the risk of a severe and systemic allergic reaction while maintaining or enhancing clinical efficacy is urgently needed. The most clinically relevant biomarker that correlates with adverse outcomes to peanut OIT is the level of specific IgE directed against the immunodominant peanut allergen Ara h 2.8, 9 Hence, reducing IgE binding by modifying the allergen Ara h 2 has been suggested as a way to make peanut OIT safer.10, 11 Recent studies of anti-Ara h 2-specific B cells cloned from patients undergoing oral immunotherapy found a surprising convergence of immunoglobulin sequences suggesting that Ara h 2 antibody paratopes recognize common epitopes on Ara h 2.12-14 These studies primarily identified IgG sequences but other studies have identified shared Ara h 2-specific IgE and IgG antibody clones,12, 15, 16 which supported a paradigm of sequential switching from IgG to IgE. A confirmation of this class switch was implied by Ota et al who again cloned similar public IgG immunoglobulin sequences and noted the memory of pathogenic IgE in IgG1 producing B-cells.17 Finally, sequential epitope mapping of serum IgE and IgG4 induced during peanut OIT have shown significant overlap.18 Altogether, these studies suggest that both IgE and IgG antibody repertoires to Ara h 2 are focused on constrained set of epitopes.
Utilizing structural knowledge of these epitopes, a few carefully selected mutations could be effective at reducing peanut OIT adverse outcomes. Ideally, the proposed modifications to the allergen would remove the antigenic determinants that drive pathogenicity while preserving its therapeutic benefit of retraining the immune system. In this work, we utilized our previous studies on the epitopes of Ara h 2,14 along with the crystal structures of an additional conformational epitope, to test whether we can engineer a hypoallergen with mutations that would abrogate IgE binding. Further, we tested if this hypoallergen would reduce IgE binding in vitro and reduce the risk of anaphylaxis in a mouse model of passive cutaneous anaphylaxis. This represents a key first step in hypoallergen design and, potentially, a pathway for the creation of customizable Ara h 2 therapeutics to shape the B-cell repertoire.
2 METHODS
2.1 Monoclonal Ara h 2-specific antibody cloning
Monoclonal antibodies were produced from Ara h 2 affinity-selected B cells as previously described using a fluorescent multimer from peripheral blood mononuclear cells (PBMCs) isolated from patients undergoing high-dose peanut oral immunotherapy in two clinical trials (NCT01324401, NCT01750879).14 Participants in both clinical trials (aged 7–55, positive skin prick test) were recruited with informed consent, and the studies were approved by the institutional review board (IRB) of Mass General Brigham Healthcare (protocols 2010P000609 and 2012P002153). Briefly, Ara h 2-specific single B cells were sorted into a 96-well plate for amplification of paired heavy and light chain sequences, for subsequently cloning into IgG1 heavy chain and kappa light chain vectors.12 Recombinant antibodies were produced by transient transfection of HEK293 T cells (ATCC, CRL-3216), for Protein G purification and quantification using biolayer interferometry, as below.14
2.2 Recombinant Ara h 2 cloning, expression and purification
For crystallography, Ara h 2.01 (31–160) was fused with an N-terminal 6His-thioredoxin tag and a TEV cleavage site to create a gene construct called HisTRX(TEV)A2 that was bacterially expressed and purified. HisTRX(TEV)A2 was transformed into E. coli Origami B cells in the presence of antibiotics (100 μg/mL ampicillin, 50 μg/mL kanamycin, and 12.5 μg/mL tetracycline). For ELISA, BLI, and PCA, Ara h 2.01 was cloned into pMal vector with the NotI and EcoRI restriction sites to create a construct called MBP(TEV)A2 having N-terminal maltose binding fusion protein followed by a TEV cleavage site. MBP(TEV)A2 was transformed into E. coli Origami B cells harbouring a TRX plasmid in the presence of antibiotics (100 μg/mL ampicillin, 50 μg/mL kanamycin, 35 μg/mL chloramphenicol, and 12.5 μg/mL tetracycline). Glycerol stocks were prepared and inoculated into 25 mL Luria broth containing the antibiotics for overnight culture, which was transferred into 1 L Terrific broth with the corresponding antibiotics. Cells were grown at 37°C until the OD600 reached 0.6 when 500 μM IPTG was added to induce protein expression, and cells were incubated at 18°C for overnight. Cells were harvested by centrifugation at 4000 g for 15 min, and the pellet was lysed by sonication in the resuspension buffer (25 mM Tris pH 8.0, 500 mM NaCl). The soluble fraction was separated by centrifugation at 47,900 g and loaded onto 5 mL Ni-NTA for HisTRX(TEV)A2 or 5 mL amylose resin for MBP(TEV)A2 in batch at 4°C. The resins were washed with the buffer three times, followed by elution by resuspension buffer containing 400 mM imidazole for HisTRX(TEV)A2 and 40 mM maltose for MBP(TEV)A2, respectively. Concentrated proteins were loaded onto Superdex 200 26/60 equilibrated with the resuspension buffer, and the peak fractions were pooled, frozen, and kept at −80°C until used. For crystallography the TRX tag was eventually cleaved (See Appendix S1), but for ELISA, BLI, and PCA the MBP tag remained attached.
2.3 Recombinant antibody expression
Heavy and light chain antibody plasmids (22S1, 13T1, 23P34 and 13T5) were prepared using Qiagen®Plasmid plus Giga Kit as described in the manufacturer's manual. Recombinant antibodies were expressed using the ExpiCHO expression system (Thermo Fisher Scientific, Carlsbad, CA) with Max titre protocol. Five hundred miliitlitres of ExpiCHO-STM cells were prepared in ExpiCHO expression media as a suspension culture using Thomson Optimum Growth™ flasks (Oceanside, CA) with 8% CO2 and 80% humidity at 37°C while shaking at 130 rpm at a density of 6 × 106 cells/mL. An equal amount (0.5 mg each) of heavy and light chain vectors were added into the final 20 mL of OptiPRO SFM media and incubated for 5 min at RT. 1.6 mL of ExpiFectamine were added to 18.4 mL of OptiPRO SFM media and incubated for 5 min at RT. These two media containing DNAs and ExpiFectamine were mixed and slowly added to the ExpiCHO-STM cells (density of 6 × 106 cells/mL) while swirling the flask gently. Cultures were incubated in a shaker for 22 h (Day 0). To enhance the antibody expression, 3 mL ExpiCHO enhancer mixed with 80 mL ExpiCHO feed media was added to the culture gently. The cells were incubated with 5% CO2 and 80% humidity at 32°C while shaking at 130 rpm (Day 1). On the 5th day, an additional 80 mL ExpiCHO feed media was added to the culture and continued growth. On the 14th day, cells were harvested by centrifugation at 500 g for 10 min. The supernatant was then mixed with diatomaceous earth, passing through a 0.2 μm filter.
3 RESULTS
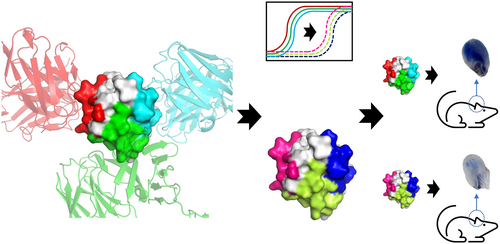
Previous studies cloned 74 antibodies to Ara h 2 from patients undergoing oral immunotherapy for peanuts.14 Roughly a third of the antibodies recognized linear epitopes similar to those observed previously. However, the other 2/3 failed to bind linear epitopes but blocked a greater fraction of patient IgE than antibodies recognizing linear epitopes. Further, competitive binding experiments established that there were three bins of these antibodies that recognized conformational epitopes on Ara h 2. A schematic of this workflow appears in Figure 1A. In order to determine what differentiates the linear epitopes from the conformational epitopes we utilized X-ray crystallography. Note that bin refers to this empirical clustering of antibodies, while epitope describes specific residues on the antigen. Examples of antibodies from bins 1 and 3 were observed in a ternary crystal structure, published previously.14 The antibodies 13T1 (bin 1) and 22S1 (bin 3) bind to epitopes on Ara h 2 approximately 180 degrees apart, as seen in Figure 1B.
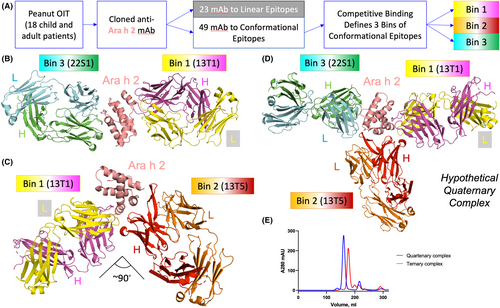
Herein, we report a second ternary crystal structure of Ara h 2, 13T1 (bin 1) and 13T5 (bin 2), shown in Figure 1C with key parameters described in Table 1 and Material and Methods. There are several novel features of this ternary structure. 13T1 and 13T5 make a more acute angle with respect to the epitopes they recognize, approximately 90 degrees. A simulated quaternary structure of Ara h 2, 22S1, 13T1, and 13T5 did not show any obvious clashes that would prevent formation (Figure 1D). Confirming this hypothesis, a quaternary structure was suggested by gel filtration chromatography (Figure 1E), however attempts to crystallize this complex were unsuccessful. Returning to the ternary complex with 13T1 and 13T5, the antibodies also make contact with each other via the heavy chain of 13T5 interacting with the light chain of 13T1. This requires some minor conformational changes of the 13T1 light chain compared to the complex of T1:Ara h 2: 22S1. Another interesting observation of the 13T5:Ara h 2 interactions is that the paratope is composed completely of the 13T5 heavy chain. This is unique among the anti-Ara h 2 human antibodies studied so far. This suggested that other light chains complexed with the 13T5 heavy chain would still bind to Ara h 2. This was confirmed by swapping in the light chain from 22S1 with 13T5HC, which bound to Ara h 2 without a change in the response rate by biolayer interferometry (BLI) (Figure S1). The vast majority of the 13T5 epitope is comprised of residues within helix 3, although a few discontinuous residues make contact with the complimentary determining regions (CDRs).
| 13T1Fab/Arah2/13T5Fab (PDB ID:8G4P) | |
|---|---|
| Space group | C2 |
| Cell dimensions | |
| a, b, c (Å) | 136.302, 136.794, 96.819 |
| α, β, γ (°) | 90.00, 127.34, 90.00 |
| Resolution (Å) | 50.0–2.25 (2.29–2.25)a |
| No. unique reflections | 65,663 |
| Redundancy | 3.6 (3.6) |
| Completeness (%) | 98.1 (98.5) |
| R sym | 0.064 (0.636) |
| I/σI | 7.2 (1.5) |
| CC1/2 | 0.993 (0.782) |
| Refinement | |
| Resolution (Å) | 2.25 |
| Rwork/Rfree (%) | 18.9/21.8 (26.7/30.1) |
| No. atoms | |
| Protein (non-hydrogen) | 7152 |
| Water | 250 |
| Other | 65 |
| Wilson B-factor | 48.60 |
| Ramachandran favoured/allowed/outliers (%) | 97.9/1.99/0.10 |
- a Data from the highest resolution shell are shown in parenthesis.
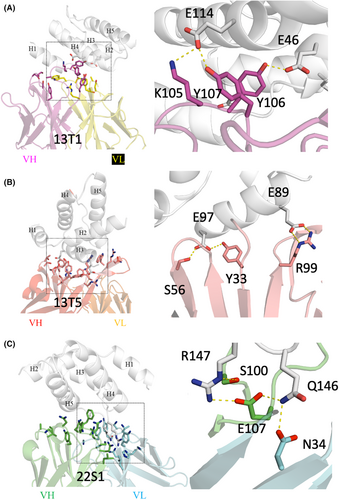
The precise interactions between the allergen and antibodies can be studied for residues that might be modified to reduce binding in a hypoallergen. Figure 2 shows a close examination of the epitope-paratope interactions for all three antibodies. With regards to bin 1, Ara h 2 residues E114 and E46 appeared to interact with tyrosine residues in the heavy chain of 13T1 that were frequently conserved in bin 1 antibodies (Figure 2A). We identified that Ara h 2 residues E97 and E89 form two salt bridges or two hydrogen bonds, respectively, with 13T5 representing bin 2 (Figure 2B). With regards to bin 3, Ara h 2 residues R147 and Q146 make multiple hydrogen bond interactions with 22S1 (Figure 2C). The aforementioned six residues on Ara h 2 were chosen as candidate residues for mutagenesis in the hypoallergen design based on apparent key energetic interactions. In order to disrupt binding, six double mutations were designed that either reduced the Ara h 2 sidechain to an alanine, or reversed the charge of the amino acid residue and subjected to empirical testing.
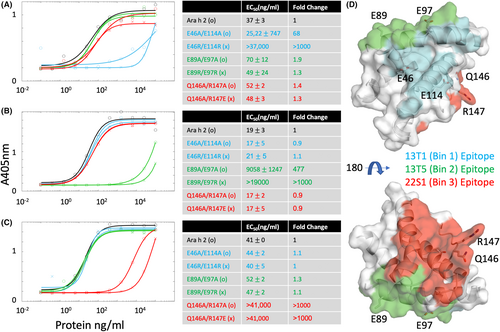
These Ara h 2 mutant proteins were tested for antibody binding using BLI and a direct ELISA. Note that the rAra h 2 constructs in these and subsequent experiments contains an MBP tag, which we find increases solubility for long term storage and minimally perturbs IgE binding.19 Figure S2 first demonstrates that the engineered double mutations abrogate binding to the expected antibody by BLI. Figure 3 extends this observation and shows that in each permutation of antibody and double mutant, the designed mutations reduced mAb binding of the intended epitope but did not perturb binding to the other epitopes using ELISA. First, this confirms the location of the epitopes as determined by crystallography. Second, it also confirms that the site-directed mutations do not affect the conformation of Ara h 2 substantially, since two other monoclonal antibodies which recognize conformational epitopes will still bind. For mutants binding to 13T1 and 13T5 only one double mutant showed a >1000 fold change in EC50; hence those respective mutations were selected for further study. In the case of 22S1, both double mutants had equivalent effects. We selected the change in charge mutation (Q146A/R147E) as likely to have better solubility and proceeded with further studies on this.
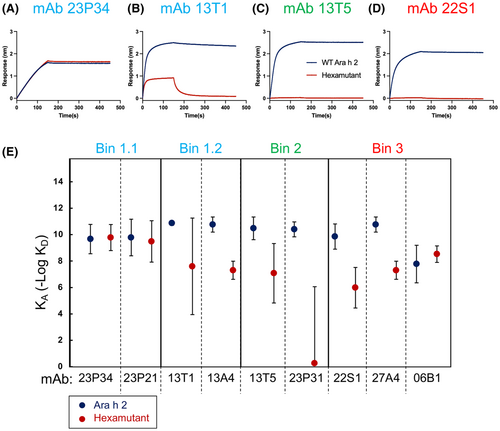
For a general purpose hypoallergen with reduced IgE reactivity to all three bins, we created a hexamutant of Ara h 2 with mutations E46R, E89R, E97R, E114R, Q146A, and R147E. To test if the binding by Arah 2-specific mAbs is reduced to the hexamutant, we used BLI to measure dissociation constants (Figure 4 and Figure S3). In a previous study of these Ara h 2-specific mAb, bin 1 was subdivided into 2 sub-bins (1.1 and 1.2). Bins 1.1 and 1.2 localized to different regions based on competitive binding experiments using other mAb that recognized sequential peptides: 121QGRQQEQQ128, which blocks bin 1.1 and 30DRRCQSQLER39, which blocks bin 1.2.14 In Figure 4, the affinity of antibodies to bin 1.1 were largely unaffected by the hexamutant, but antibodies in bin 1.2 showed reduced affinity. Similarly, antibodies to bins 2 and 3 were consistently reduced in affinity. Two important points can be inferred from this result. First, the conformational epitope of bin 1.1 is still recognized, indicating that the overall protein structure of the hexamutant is similar to native Ara h 2. Second, antibodies in the same bins show a consistent response to the targeted mutations. This makes sense, considering that antibodies belonging to the same bins had highly similar CDR sequences, even though they were cloned from different patients. This suggests that antibodies converge on common epitopes with similar paratopes, consistent with previously published data.14
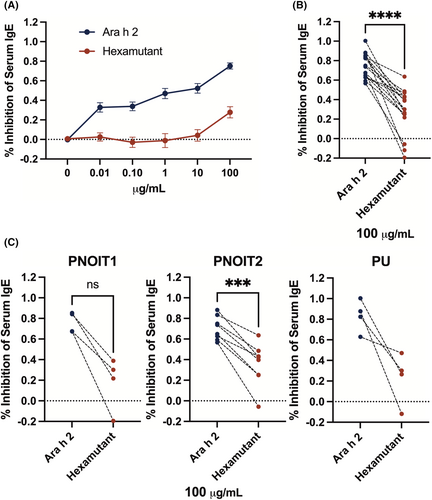
We hypothesized that the Ara h 2 mutations may reduce IgE binding if the IgE paratopes were similar to those found in the IgG antibodies. To test if IgE binding to the hexamutant would be reduced, a competitive ELISA was designed to compare inhibition of serum IgE binding to either Ara h 2 or hexamutant. We used sera from 16 peanut allergic patients and plotted the results as percent inhibition across all patients in Figure 5A (variability within individual patient data in Figure S4; patient statistics in Figure S5). The percent inhibition by hexamutant was significantly lower than to rAra h 2 at the top concentration (paired comparison Figure 5B), and in all 3 cohorts, including paediatric (PNOIT1) and adult peanut-allergic patients (PNOIT2) with low thresholds of reactivity and adult peanut-allergic patients with a high threshold of reactivity (PU, Figure 5C).
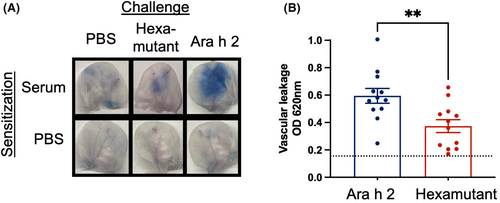
The next important question for a hypoallergen is whether or not it is able to reduce the potential for anaphylaxis by reducing IgE cross-linking. A mouse model of passive cutaneous anaphylaxis (PCA) was selected as a surrogate for human histamine-mediated vascular leakage during anaphylaxis. The experiment was designed to test if the hexamutant would be less effective at cross-linking human anti-Ara h 2 IgE, thereby reducing allergic activation. Transgenic mice with a human FcεRI were sensitized intradermally in the ears with pooled serum from peanut allergic patients (n = 6), to effectively transfer human IgE repertoires onto cutaneous mast cells. The mice were subsequently challenged with Ara h 2, hexamutant, or PBS mixed with Evans blue dye. Vascular leakage was measured by the amount of Evans blue dye that infiltrated the surrounding tissue. Figure 6A shows representative images of excised mouse ears demonstrating that the extent of dye leakage is greater for WT challenged compared to the hexamutant challenged or the PBS control. The amount of extravasated dye was measured for 12 mice per challenge group as shown in panel B, confirming the photographic evidence. There was a significant difference in absorbance at 620 nm (Student's t-test, p < .006) between Ara h 2 and the hexamutant, demonstrating that the hexamutant has reduced anaphylactic potential.
4 DISCUSSION
In this study, we characterized conformational epitopes of the immunodominant allergen Ara h 2. The data were used to engineer an example hypoallergen, whereby a number of minimal mutations to the allergen are made to reduce the risk of anaphylaxis yet maintain enough of the native structure that the immune system can recognize the antigen and initiate further adaptive immune changes in allergen recognition.10, 20, 21 Other studies have inferred dominant epitopes from peptide arrays,22 murine antibodies,23 mutational analysis,24 or chimeric allergens.25 Our current work presents a unique approach, with the use of atomic details derived from structural studies of human monoclonal antibodies identified from peanut allergic patients and applied to the design of hypoallergens.14 For example, our structural studies have identified critical challenges in the design of previous hypoallergens. In a previous proposal, out of the six residues identified for mutagenesis, only three are now known to be surface available and likely to affect antibody binding.11, 19 Other efforts to design hypoallergens have attempted to circumvent the issue of dominant epitopes by denaturing the allergen, effectively eliminating all the epitopes. This has been accomplished by designing allergen fragments or mosaics that lack IgE reactivity.20, 26, 27 Yet other hypoallergens have been proposed that destabilize the protein either by mutagenesis28, 29 or chemical modifications.30, 31 In contrast, our study sought to be more surgically selective to mutate immunodominant epitope residues utilizing the crystal structure data.
The fact that we based our hypoallergen design off of our analysis of three bins of conformational IgG epitopes provokes the question as to whether these epitopes are the same as IgE epitopes. As discussed above, many studies have suggested that this is likely.13, 17 Indeed, the hexamutant mutations that reduced binding to the IgG cloned antibodies analysed in this study clearly also reduce IgE reactivity in the competitive ELISA and the PCA (Figures 5 and 6). We conclude that there is substantial overlap in the IgE and IgG paratopes, mostly likely due to sequential class switching, and as such, that our utilization of IgG antibodies to design the hypoallergen does not diminish its relevance to IgE.
Several challenges remain in the design of hypoallergens for future therapy. Among them, we wonder whether the mutated hypoallergen would be processed similarly to induce protective adaptive responses in treated patients. We also wonder whether a customization of the therapy will be necessary for patients to develop the particular antibody clones necessary for blockage of their unique IgE repertoire. For example, in the case of peanut allergy, antibodies recognizing epitope 1.2 were suggested to be important for durable tolerance after peanut immunotherapy.14 Therefore, it may be advantageous to mutate other residues adjacent to these epitopes so that key epitopes for successful therapy are created. Thus, perhaps the very commonly recognized epitope 1.1 should be targeted for reduced IgE binding. On a broader level, then, one could imagine shaping the B-cell repertoire of individual patients depending on their existing IgG at certain points during OIT. Besides the hexamutant, the double mutants described in Figure 3 may be useful diagnostic probes of whether or not a patient has substantial antibodies recognizing specific epitopes, which could be indicative of whether therapy should continue, or can be stopped. For example, a competitive inhibition ELISA with the Q146A/R147E mutant could assess if a patient has enough of the important bin three antibodies for sustained tolerance.
Another question is whether the hypoallergen will effectively modulate allergen-specific T cell responses, which may be clinically relevant to the development of sustained unresponsiveness after immunotherapy.32-34 Numerous hypoallergen proposals have tested for T-cell activity: A previous proposal for a Der p 2 hypoallergen showed reduced skin-prick test size in the hypoallergen yet equivalent T-cell proliferation.35 Similarly, a triple epitope Bla g 2 mutant with reduced IgE antibody binding had T-cell modulatory capacity.36 Lastly, the Ara h 2 hypoallergen proposed by King et al.11 reduced IgE binding, but did not affect T-cell proliferation. These studies suggest that hypoallergens designed to reduce or eliminate IgE-mediated activation may not modulate allergen-specific T cell responses in immunotherapy. Yet, hypoallergens may be particularly useful during the early induction phase of immunotherapy,37 when IgE-crosslinking is highest in effector cells and before significant modulation of T cell responses. While the timing, selective use, and ultimate design of hypoallergens will require careful clinical study, the ability to intentionally tailor hypoallergens that preserve the structural integrity of the allergen is a significant advancement for future therapies.
5 CONCLUSION
In this paper we have demonstrated that structural knowledge of the important B-cell epitopes can be used to tailor IgE reactivity to Ara h 2. We focused on the problem of designing hypoallergens and future studies will be needed to find the right compromise between reduced risk and therapeutic benefit. We believe this is an important step in developing site-specific hypoallergens for peanut and other allergic diseases.
AUTHOR CONTRIBUTIONS
All authors designed experiments. JM, TK, NAL, IRL, OM-R, LA, SLS, LLE, RMP, LCP performed experiments. All authors analysed data. Jungki Min, Tarun Keswani, Orlee Marini-Rapoport, Sarita U. Patil, and Geoffrey A. Mueller wrote the manuscript. The authors would like to thank Taylor Cosey for technical assistance.
ACKNOWLEDGEMENTS
This research was supported in part by the Intramural Research Program of the National Institutes of Health (NIH), National Institute of Environmental Health Sciences, ZIA-ES102906 (GAM), ZIC-ES102645 (LCP) and ZIC-ES043010 (LP). This work was funded by NIH, National Institute of Allergy and Infectious Disease (NIAID) grant 5R01AI155630 (SUP) and R01AI077653 (AP), and the Food Allergy Science Initiative (FASI) (SUP). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. X-ray data were collected at Southeast Regional Collaborative Access Team (SER-CAT) 22-ID (or 22-BM) beamline at the Advanced Photon Source, Argonne National Laboratory. SER-CAT is supported by its member institutions, and equipment grants (S10_RR25528, S10_RR028976 and S10_OD027000) from the National Institutes of Health. Use of the Advanced Photon Source was supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences, under Contract No. W-31-109-Eng-38.
CONFLICT OF INTEREST STATEMENT
Jungki Min, Lars C. Pedersen, Sarita U. Patil, and Geoffrey A. Mueller filed U.S. Provisional Patent 63/486,570 regarding the Ara h 2 mutant proteins discussed herein. SUP filed patent PCT/US2020/052875 related to the antibodies used in this work.
ETHICS STATEMENT
Participants in clinical trials were recruited with written informed consent, and the studies were approved by the Institutional Review Board of Massachusetts General Brigham Healthcare (protocols 2010P000609 and 2012P002153). All animal experiments were conducted under protocols approved by the Massachusetts General Hospital Institutional Animal Care and Use Committee.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available in Protein Data Bank at http://rcsb.org, reference number 8G4P.