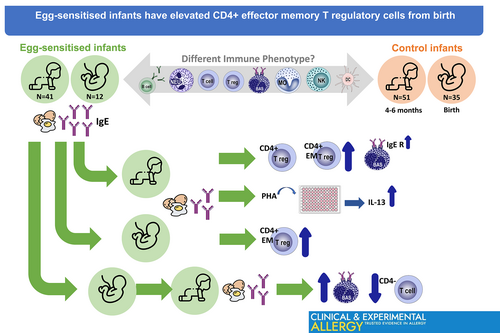
Egg-sensitised infants have elevated CD4+ effector memory T regulatory cells from birth
Cristina Gamez and Jonatan Leffler are joint primary authors.
Debra J. Palmer and Deborah Strickland are joint senior authors.
Abstract
Background
IgE-mediated sensitisation to egg is common in infants. In some cases, the processes leading to egg sensitisation are established in early life, even before introduction to solid foods. The underlying mechanisms remain poorly understood.
Methods
We performed detailed immune cell phenotyping of peripheral blood mononuclear cells and determined in vitro cytokine responses following allergen specific and non-specific immune stimulation. To determine if unique immune profiles were linked to early-life egg sensitisation, we compared 92 infants at 4–6 months of age, with (EggCAP+, n = 41) and without (EggCAP−, n = 51) early egg sensitisation. Additionally, 47 cord blood samples were analysed. For a subset of participants (n = 39), matching cord blood mononuclear cells were assessed by flow cytometry to establish the impact of IgE sensitisation on immune developmental trajectories.
Results
EggCAP+ infants were found to exhibit a unique immune phenotype characterised by increased levels of circulating CD4+ T regulatory cells (Treg), CD4+ effector memory (EM) Treg and increased expression of the IgE receptor, FcεR1, on basophils. The increased CD4+ EM Treg profiles were already present in cord blood samples from EggCAP+ infants. A general Th2-skewing of the immune system was observed based on increased IL-13 production following phytohemagglutinin stimulation and by comparing immune developmental trajectories, EggCAP+ infants displayed an expansion of basophils and reduced levels of CD4− T cells compared to EggCAP− infants.
Conclusions
Immunological profiles associated with egg sensitisation are detectable in infant circulation at 4–6 months of age and at birth. Understanding the immune mechanisms underlying early-life sensitisation could provide important insights for future food allergy prevention strategies.
Graphical Abstract
IgE-mediated sensitisation to egg is common in infants but the underlying mechanisms remain poorly understood. We identified different immunephenotypes between the infants who were IgE-egg sensitised and not-IgE-egg sensitised. Immunological profiles associated with egg sensitisation are detectable at 4–6 months-old and even at birth.
Key messages
- Australian infants commonly develop egg allergy, with sensitisation occurring before introducing egg into their diet.
- Egg-sensitised infants at 4–6 months showed elevated effector memory T regulatory cells, present from birth.
- Egg-sensitised infants displayed elevated basophil expansion, with increased IgE receptor expression in these cells.
1 INTRODUCTION
Rising prevalence of food allergy in young children is a major health concern. The worldwide prevalence of food allergy confirmed by oral food challenge (OFC) is close to 8% in young children.1 Some of the highest rates of food allergy, diagnosed by OFC were reported from Australia, where the prevalence of challenge-confirmed IgE-mediated food allergy is 10%–11% at 1 year of age,2, 3 with egg allergy the most common, affecting 5.5%–8.9% of 1-year-old infants.3, 4 For most individuals, ingestion of food allergens leads to induction of immune tolerance.5 If tolerance induction is dysregulated, IgE-mediated sensitisation, and potential progression to food allergy may occur.5 Sensitisation is a process by which the immune system produces IgE against allergens. Food allergy symptoms develop due to the allergic reaction triggered by the immune system in response to the ingestion of potential food allergens. If tolerance induction is dysregulated, IgE sensitisation to specific food allergens occur, which can then progress to the development and expression of food allergy symptoms in some children. The mechanisms underlying IgE sensitisation remain poorly understood. Our group has previously shown that egg sensitisation can be established by 4–6 months of age, even before introduction to solid foods.6, 7
Multiple factors during pre- and post-natal life appear to contribute to the risk of food sensitisation and ensuing development of food allergy, these include immune8 and barrier dysfunction,9, 10 the microbiome (both maternal and infant)11, 12 and environmental exposures.8 Immune status at birth, which is driven by the maternal in utero environment and other factors, including genetics, may also be an intrinsic factor that predisposes to the development of allergic diseases. In addition, immune system developmental changes that occur during early post-natal life likely further contribute to an increased risk of food allergen sensitisation during early life. Atopy, including IgE sensitisation, is characterised by a T helper (Th) 2 skewed immune response and its related production of cytokines IL-4, IL-5 and IL-13, which are involved in the induction and maintenance of the IgE synthesis.13 In atopy development, Th1 cytokines like interferon-γ (IFN-γ) can antagonise the effect of Th2 cytokines. The most common early-life indicators in children who develop allergic diseases, include diminished IFN-γ levels13 and altered Th2 responses that are followed by skewing of immune responses towards a Th2 phenotype.14 There is also evidence that allergic disease and/or sensitisation is associated with alterations in T regulatory cells (Treg). Treg play a role in control of the allergic response by suppressing Th2 activity and proliferation, and attenuated neonatal Treg function or abundance12, 15, 16 have been shown to be associated with allergy development later in life. Recently, it was reported that the proportion of naïve Treg (nTreg) at birth was lower in infants who developed food allergy by 1 year of age.17 In addition, differences in other immune parameters, including the total number of B cells18 and elevated levels of several myeloid populations,19 have also been associated with allergic disease; however, these associations were found in individuals with established food allergy. To date, the immune profile in young infants with food sensitisation and allergy remains less clear.
In this present study, we investigate the phenotypic and functional immune profiles associated with egg sensitisation at 4–6 months of age (prior to egg ingestion in infant solid foods) and explore if these profiles are also present at birth. Understanding immune mechanisms underlying early-life sensitisation could provide important insights for future food allergy prevention strategies.
2 METHODS
2.1 Study population
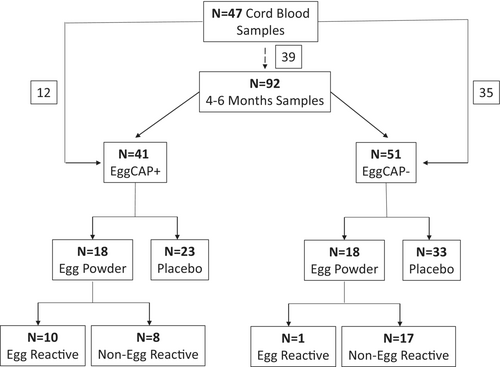
The study population comprised of a subset of infants (n = 92) who had participated in three different randomised controlled trials (RCTs).7, 20, 21 Two of these RCTs7, 20 investigated the effects of early regular egg consumption in solid food on the development of IgE-mediated egg allergy, where infants with (RCT-1)20 or without (RCT-2)7 symptoms of eczema, and no known prior ingestion of egg, were recruited at 4–6 months of age. The third RCT21 investigated the effects of maternal dietary egg intake during the first 6 weeks of lactation and included a follow-up assessment at 4 months of age prior to infant solid foods introduction.
Participating infants had peripheral blood collected at 4–6 months of age. Egg-specific IgE plasma levels were measured using the ImmunoCAP 250 system (Phadia AB, Uppsala, Sweden). Infants with an egg-specific IgE level >0.1 kUA/mL were classified as EggCAP+. The distribution of EggCAP+/− individuals are summarised in Figure 1. As part of the RCT1-2,5 but without prior knowledge of sensitisation status, infants were randomised to ingest pasteurised raw whole egg powder under medical supervision at 4–6 months of age. In the EggCAP+ group, 10/18 (55.5%) infants reacted to egg powder ingestion under medical supervision and were diagnosed with egg allergy. An allergic reaction was defined as at least three concurrent non-contact urticaria persisting for at least 5 min and/or generalised skin erythema and/or vomiting (forceful/projectile) and/or anaphylaxis (as defined by multisystem involvement that must include circulatory and/or respiratory involvement).6, 7 This was in comparison to 1/18 (5.6%) in the EggCAP− group (Figure 1). Cord blood samples were also available from 47 individuals, 12 who became EggCAP+ and 35 who were EggCAP− (Figure 1). Out of those 47 cord blood samples, 39 samples matched a 4–6-month sample. Ethical approval was obtained from the Princess Margaret Hospital Human Research Ethics Committee (approval numbers 1635/EP, 1782/EP and 2060/EP), and written informed consent was obtained from all parents/guardians.
Further methods are described in Appendix S1.
3 RESULTS
3.1 Study population characteristics
Table 1 describes the characteristics of the infants included in this study. No difference in demographic data was observed between the EggCAP+ and EggCAP− infants (Table 1).
| Early egg sensitised (EggCAP+) group | Early non-egg sensitised (EggCAP−) group | p Value | |
|---|---|---|---|
| Infants (n) | 41 | 51 | |
| Birth weight (g)a | 3524.1 (352.8) | 3548.5 (368.2) | .75† |
| Gestational age at birth (weeks)b | 39.3 (38.3–40.1) | 39.5 (39.0–40.4) | .30‡ |
| Infant male sex, n (%) | 24 (58.5) | 27 (52.9) | .75§ |
| Caucasian infant, n (%) | 36 (87.8) | 47 (92.2) | 1.00§ |
| Maternal history of allergic disease, n (%) | 36 (87.8) | 48 (94.1) | 1.00§ |
| Age at blood sample collection (months)a | 4.9 (0.9) | 5.0 (0.7) | .47† |
| Egg powder ingested (intervention group), n (%) | 18 (43.9) | 18 (35.3) | .41§ |
| Egg powder allergic reaction at 4–6 M, n (%) | 10/18 (55.5) | 1/18 (5.6) | <.01§ |
- Note: Data are displayed as amean ± standard deviation or bmedian (inter-quartile range). p-Values of statistical differences between groups using either Student's t test†, Mann–Whitney U test‡ or Pearson's chi-square test§ depending on characteristics of the data.
3.2 Immune phenotype at 4–6 months of age is associated with early egg sensitisation
To identify immune profiles associated with egg sensitisation, two antibody panels were used, focussing on either (i) identifying a large range of immune cell subsets (panel 1, Table S1) or (ii) expression of markers associated with atopy (panel 2, Table S2). Using FlowSOM clustering and UMAP projection, 11 immune cell populations were identified in panel 1 (Figure 2A). Using manual gating (Figure S1) and subsets defined as per Table S3, a total of 33 immune cell subsets were identified. Invariant natural killer T cells (iNKT) were identified using panel 2 (Figure 2B and Figure S2 as well as Table S4). All analysis was performed on manually gated subsets.
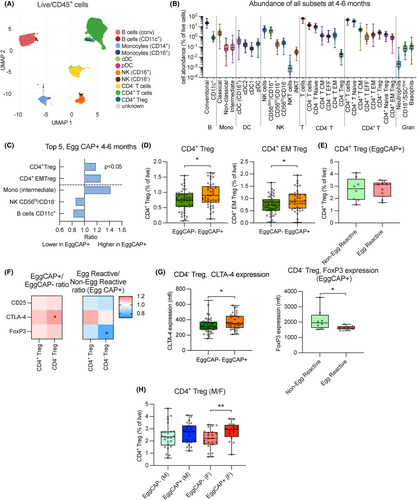
We first compared the abundance (as a proportion of total viable white blood cells) of immune cell subsets between EggCAP+ and EggCAP− infants. The EggCAP+/EggCAP− ratio of the top 5 most different subsets are displayed (Figure 2C). From this analysis, we identified that EggCAP+ infants had higher levels of CD4+ Tregs and specifically CD4+ effector memory (EM) Treg compared to EggCAP− infants (Figure 2C,D) and no difference in CD45RA+ nTregs was observed (data not shown). Comparing levels of CD4+ Tregs within EggCAP+ infants who reacted or not to the egg powder, as part of their RCT, no further difference was observed (Figure 2E). We next analysed expression of markers associated with regulatory activity in both CD4+ and CD4− (majority CD8+) Tregs, such as CD25, CTLA-4 and Foxp3 and observed increased expression of CTLA-4 in CD4− Tregs from EggCAP+ infants compared to EggCAP− infants (Figure 2F,G). Among EggCAP+ infants exposed to egg, decreased expression of FoxP3 in CD4− Tregs was observed in infants who displayed food allergic symptoms following the egg ingestion (Figure 2F,G). Finally, we also assessed if the observed difference in CD4+ Treg abundance differed between male and female infants and identified that the difference between EggCAP+ and EggCAP− infants was only significant in female infants (Figure 2H). No difference was observed in expression of Treg functional markers (CD25, CTLA-4 and Foxp3) between male and female infants (data not shown).
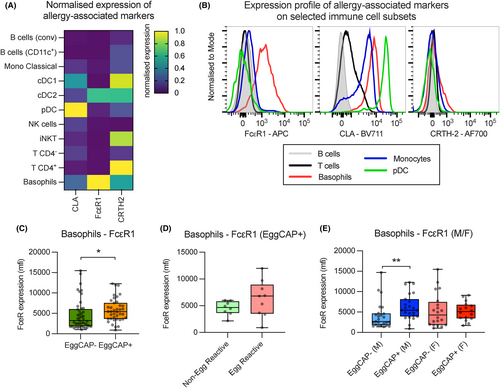
After establishing that only a few differences in immune cell subset abundance were detectable between EggCAP+ and EggCAP− infants, the expression of allergy-associated markers such as cutaneous lymphocyte associated-antigen (CLA), the high-affinity IgE receptor (FcεR1) and Prostaglandin DP2 receptor (CRTH-2), across the main immune cell subsets was assessed. Variable expression was observed across immune cell subsets in all the samples (Figure 3A,B). We observed that FcεR1 was upregulated on basophils in EggCAP+ compared to EggCAP− infants (Figure 3C); however, no additional difference in expression was observed in EggCAP+ infants who reacted to ingestion of egg power or not (Figure 3D). When comparing expression of FcεR1 on basophils between female and male infants, only male infants displayed an upregulated expression in EggCAP+ compared to EggCAP− infants (Figure 3E).
3.3 Immune function at 4–6 months associated with early egg sensitisation
To assess differences in immune system function, PBMC from a subset of samples (n = 91) at 4–6 months of age were cultured with a range of stimulants, including egg allergens (ovalbumin (OVA), ovomucoid (OVM) and lysozyme (LYS)), house dust mite (HDM) and phytohemagglutinin (PHA), and cytokine production was assessed (Figure 4A). Comparing all responses to stimuli across EggCAP+ and EggCAP− infants, EggCAP+ infants displayed elevated IL-13 production following PHA stimulation compared to EggCAP− infants (Figure 4B,C). Increased IFNγ production following OVA exposure was also observed in cultures from EggCAP+ infants who reacted to egg ingestion compared to those who did not react (Figure 4D,E). To identify if cytokine production was associated with the abundance of a particular immune cell subset, cytokine responses were correlated to immune cell abundance. We observed that IL-13 production, following PHA stimulation, positively correlated with basophil abundance, as well as iNKT cells (Figure 4F,G) and that IFNγ production following OVA stimulation positively correlated with increased CD4+ T EM cells (Figure 4F,G). To assess the ability of the findings to separate EggCAP+ and EggCAP− individuals at 4–6 months of age, we included the abundance of CD4+ Treg, CTLA-4 expression on CD4− Treg, FcεR1 expression on basophils and IL-13 production following PHA-stimulation into a principal component analysis with clear separation between EggCAP+ and EggCAP− individuals on principal component 2 (Figure 4H,I). In addition, we assessed the impact of sex and birth weight on the majority of findings without observing any confounding effects compared to an unadjusted regression model (Table S5).
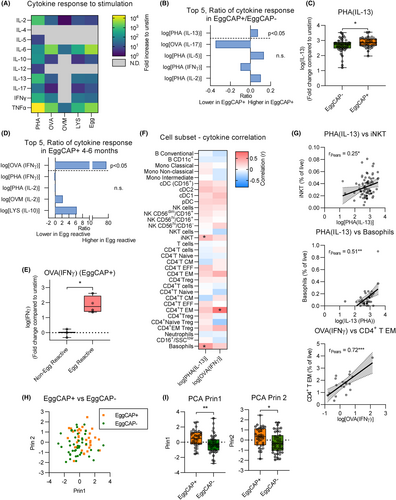
3.4 Immune phenotype at birth may predict early egg sensitisation
We next explored if immune profiles, associated with EggCAP positivity, were also detectable at birth. For this, immune cell subsets were analysed from cord blood samples (n = 47) and compared based on egg-sensitisation status at 4–6 months of age. As expected, the abundance of several immune cell subsets differed between the two timepoints (Figure 5A). Comparing the abundance of immune cells in cord blood based on EggCAP status at 4–6 months, EggCAP+ infants showed elevated levels of CD4+ T EM (Figure 5B,C). We also observed increased levels of CD4+ EM Tregs (Figure 5B,C), suggesting that the increased levels of Tregs observed in EggCAP+ infants at 4–6 months of age may already be present at birth. Assessing expression of allergy-relevant markers, expression of CRTH2 was increased on FcεR1− cDC (cDC1) in infants who were EggCAP+ at 4–6 months compared to those who were not (Figure 5D). We did not observe any differences in CD4+ T EM or cDC1 CRTH-2 expression between male and females (data not shown). Only two cord blood samples were available from EggCAP+ infants who ingested egg powder hence we could not explore this further.
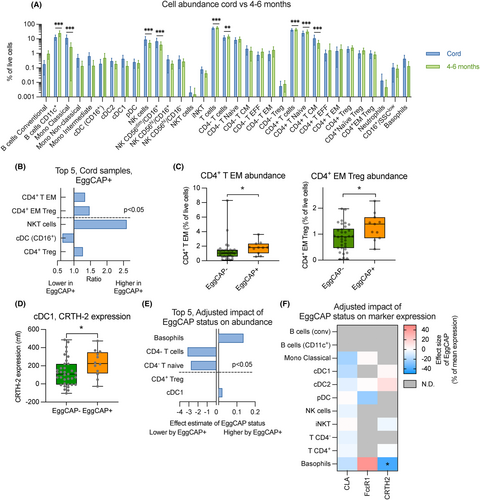
For a subset of individuals (n = 39), cord blood samples that matched with 4–6 months sample infants were available (EggCAP+ n = 8, EggCAP− n = 31). As expected, there was considerable correlation of subset abundance between the two timepoints (Table S6). Using a linear regression model, we assessed the impact of egg sensitisation on changes to immune cell subsets between cord blood and 4–6 months to adjust for potential baseline differences. We identified that infants who were EggCAP+ at 4–6 months, displayed a significant increase in basophil levels between birth and 4–6 months of age, whereas also displaying a reduction in total CD4− T cells and CD4− T naïve cells (Figure 5E). When assessing the impact of allergy markers, EggCAP+ infants showed a significant decrease in CRTH2 expression on basophils (Figure 5F).
4 DISCUSSION
Studies focusing on identifying immune profiles associated with IgE sensitisation during the first 6 months of life are sparse. Here we showed for the first time that infants with early egg sensitisation display elevated levels of circulating CD4+ Tregs, and specifically CD4+ EM Tregs, as well as increased expression of the IgE receptor, FcεR1 on basophils. We also found that the increased levels of circulating CD4+ EM Tregs were already present at birth. Using functional assays, a general Th2-skewing of the immune system was observed based on increased IL-13 production following PHA stimulation. By adjusting for levels at birth, we also found that EggCAP positivity was associated with increased levels of basophils and reduction of CD4− T cells.
Treg deficiency and dysfunction have previously been associated with development of allergic diseases.12, 15, 16, 22-26 It has been postulated that tolerance to food antigens and suppression of the antigen-specific allergic response arises from Tregs induced in the periphery,26 and a diminished capacity to produce these cells could result in the development of food allergy. However, this may not necessarily be reflected in levels of circulating Tregs.27 Indeed, some findings suggest that allergic asthma in children is associated with increased levels of circulating Treg,28 and individuals allergic to birch pollen appear to have functionally intact Tregs albeit an expansion of conventional T cells.29 Indeed, a large recent study has observed increased levels of CD4+ memory Treg in 10-year-old atopic children. Like conventional CD4+ T cells, memory Tregs represent a pool of antigen experienced Tregs which quickly expand to efficiently regulate immune responses following re-exposure to an antigen.30 How memory Tregs are involved in controlling IgE sensitisation is currently not clear although it has been speculated that they increase in response to the chronic inflammation associated with atopic disease.29 Memory Treg populations may also be subject to Th2-mediated reprogramming, as observed in children with food allergy.31 In the present study we observed increased levels of circulating CD4+ Tregs and particularly CD4+ EM Tregs, in egg-sensitised infants; however, when levels were compared between those EggCAP+ individuals who were either clinically egg reactive or not, no difference was observed. This finding may suggest that increased levels in CD4+ Tregs may only be a feature of sensitisation but not for clinical symptomatic allergic disease. We did not observe any difference in Treg maturity, activation, or level of regulatory program, as based on FoxP3, CD25 and CTLA-4 expression, suggesting no functional deficiency. However, increased levels in circulation may suggest a reduced ability to migrate to the tissue. Increased CD4+ EM Treg levels were consistent at both birth and 4–6 months with no changes observed. This suggests that increased levels may stem from inherent or pre-natal mechanisms, which requires investigation in larger longitudinal studies. Indeed, by adjusting for levels at birth, egg-sensitised infants displayed a relative decrease in CD4− Naive T cells between birth and 4–6 months. As most CD4− T cells are likely CD8+, it is reasonable to assume that EggCAP+ infants generate less CD8+ cells compared to EggCAP− infants, irrespective of birth levels. In experimental models, CD8+ T cells have been shown to ameliorate some forms of allergic disease.32 However, in children with peanut allergy, CD8+ T cells appear to become activated when exposed to peanut antigens in vitro.33 We also observed an impact of egg sensitisation on basophils. Basophils are essential for driving the acute phase of allergic reactions through the release of histamines and other inflammatory mediators. Although, no difference was observed in absolute levels of circulating basophils when compared at 4–6 months, if levels were adjusted for levels at birth, a significant increase in basophil abundance was observed in the EggCAP+ infants. This suggests that basophils expand in EggCAP+ infants and our findings also show that these basophils display evidence of increased sensitivity to IgE stimulation, already at 4–6 months of age, through increased expression of FcεR1. These findings are well established in older atopic children.34 Interesting this increased expression of FcεR1 is not appreciated in EggCAP+ infants who reacted to egg powder ingestion at 4–6 months of age although data on egg exposure was limited to a subset of individuals and numbers are thus relatively small.
Biological sex and sex hormones are important in development of atopic disease.35 Although the sample size in this study is limited for sex-specific comparisons, it is interesting to note that only females displayed an increase in circulating Treg levels whereas the increased expression of FcεR1 on basophils was mainly observed in male infants. Whether the immunological processes driving atopy development during early life differs between male and female infants remains to be evaluated, but differences are likely to stem from genetic differences as exposure to endogenous sex hormones at this age is limited.
In addition to assessing the immune phenotype, we also performed functional cytokine production in peripheral blood mononuclear cells. Although no impact of EggCAP status was observed on allergen-specific cultures, we observed increased levels of IL-13, after stimulation with PHA in the EggCAP+ infants and this appeared to correlate with iNKT cells and basophil levels. However, we did not find any cytokine response differences between infants who reacted compared to those who did not clinically react to egg ingestion at 4–6 months of age. Our team has previously shown elevated IL-13 response to OVA, OVM and LYS in 4-month-old infants with eczema who subsequently developed egg allergy at 12 months of age.6 Due to the characteristics of this study, mix of infants from 3 different RCTs7, 20, 21 and from different egg/placebo groups (following baseline sample), we were unable to perform any analysis regarding egg allergy outcomes at 12 months.
There are some limitations to this study. Allergic reactions are highly specific and therefore methodologies to identify and phenotype allergen reactive adaptive immune cells are likely to generate further insights in addition to the non-specific immune profiles assessed in the current study. Although the cytokine response to egg allergen specific cultures showed no difference between EggCAP+ and EggCAP− infants, it is important to consider that none of these analyses were performed on isolated egg specific cells. Furthermore, we must consider that by assessing differences at a such young age, systemic immune responses may be less established or show greater plasticity. Indeed, circulating levels of IgE are highly variable during the first years of life36 and analysis of local tissue responses are likely important to further highlight differences in immune profiles involved in developing IgE sensitisation. Another potential limitation of this study was that the known sensitisation status of the participating infants at 4–6 months of age was limited to egg and peanut only. This study was, however, only focussed on egg sensitisation, as this is the most common food allergen in Australia in infancy, and in this study cohort of infants there were no additional peanut-sensitised infants at 4–6 months of age who were not also egg sensitised. Furthermore, as infants were not excluded if they had already had any direct peanut ingestion any interpretation of peanut sensitisation data would have been more complex. This study was aimed at exploring immune profiles of egg-sensitised compared to non-egg-sensitised infants, when blood samples were collected prior to egg ingestion in solid foods. However, future studies which have sample sizes powered to examine infants with and without sensitisation, and with and without clinical food allergy reactions at 4–6 months of age (when first introduced to those foods) would further enhance our mechanistic understanding in this field. In this study we have 8 infants who did not react to the egg powder but were egg sensitised at 4–6 months of age. It would have been interesting to compare the immune populations of these infants with those who reacted to the egg powder; however, due to limited participant numbers, we could not undertake this analysis.
In summary, by assessing the changing immune profiles in early life, we have identified immunological factors that are associated with development of IgE egg-sensitisation by 4–6 months of age, including inherently elevated levels of CD4+ EM Tregs, an expansion of basophils and induced expression of FcεR1 as well as deficient expansion of CD4− Naïve T cells. This study contributes to our understanding on the immune mechanisms underlying early-life sensitisation that could provide important insights for future food allergy prevention strategies.
AUTHOR CONTRIBUTIONS
C. Gamez and J. Leffler designed the research. D. Palmer and D. Strickland contributed to the design of the research. S. Clark and K. Corscadden performed research. C. Gamez and J. Leffler analysed data and wrote the paper. S. Prescott reviewed the manuscript. All the authors have critically read the paper and approved the final manuscript.
ACKNOWLEDGEMENTS
We would like to sincerely thank all the families who participated in this research and acknowledge the research nurses and research assistants involved. The authors would also like to acknowledge Dr Phil Stumbles for assisting to develop the flow panels and the manual gating strategy. Financial support was obtained from Telethon-Perth Children's Hospital Research Fund, Australia and the EMCR COVID-19 Relief Grant from Telethon Kids Institute, Australia. Open access publishing facilitated by The University of Western Australia, as part of the Wiley - The University of Western Australia agreement via the Council of Australian University Librarians.
FUNDING INFORMATION
Telethon-Perth Children's Hospital Research Fund, Australia and the EMCR COVID-19 Relief Grant from Telethon Kids Institute, Australia.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.