Safety and effectiveness of a milk ladder for managing children with IgE-mediated milk allergy
Summary Box
- 148/171 (87%) infants with IgE-mediated milk allergy successfully introduced milk using a home milk ladder.
- Allergic reactions were common, but the three anaphylaxis cases were triggered by accidental milk exposures.
IgE-mediated cow's milk protein allergy (CMPA) is managed in Ireland using a milk ladder, a method of gradual baked milk introduction.1 A recent review explored the introduction of baked milk as a treatment for IgE-mediated CMPA and demonstrated that the number of patients who became tolerant to unheated milk during the study periods was greater in the gradual milk introduction group (range 54%–88.1%, mean 62.5%) compared to those who underwent complete avoidance of milk (range 0%–66.7%, mean 21.42%).2
The aim of this study is to assess the introduction of milk in IgE-mediated CMPA using an adapted home introduction milk ladder strategy.
This was a retrospective chart review undertaken in Cork University Hospital, Ireland and in a local primary healthcare facility with a special interest in allergy between 2011 and 2020.
Inclusion criteria were children with a diagnosis of IgE-mediated CMPA which met the diagnostic criteria of the presence of symptoms consistent with IgE-mediated CMPA and a Skin Prick Test (SPT) positive for cow's milk (≥3 mm) and/or specific IgE levels for cow's milk >0.35 k U/L.
All ranges of severity, including those individuals who had anaphylactic reactions, or were already tolerant to baked milk, irrespective of their SPT weal size and specific IgE level were included for analysis. Cases were excluded if data were considered incomplete.
The Milk Allergy in Primary Care (MAP) Guideline,1 also known as the milk ladder was used, and all doses were introduced at home.
The main outcome was the introduction of more than 150 mL of cow's milk or the equivalent intake of 4.5 g milk protein daily without any symptomatology. Failure to complete the ladder was defined as the failure to introduce liquid cow's milk after 36 months of follow-up.
Data were analysed with Stata version 14.
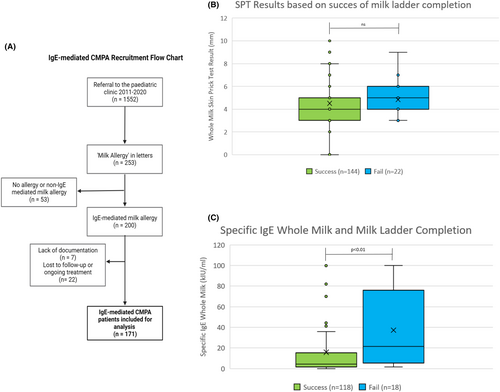
The total cohort of patients in the 10-year analysis included 200 patients with IgE-mediated CMPA. A flow diagram of the patient recruitment process is summarized in Figure 1A. Of these, 7 were excluded due to information missing from the charts, and 22 patients had insufficient evidence of success of completion of the ladder as they were lost to follow up by 36 months. Thus, 171 participants were included for analysis.
Of the 171 patients, 140 (81.9%) had atopic dermatitis, 40 (23.4%) had asthma/viral-induced wheeze and 118 (69%) had other food allergies. Food allergies included egg (105 patients, 61.4%), peanut (40 patients, 23.4%), other nuts (13 patients, 7.6%) and other food (32 patients, 18.7%).
Of the 171 included patients, 148 (86.6%, (95% confidence interval (CI): 80.6–90.9)) successfully completed the milk ladder. Similarly, Ball and Luyt found that 78/86 (90%) children were able to tolerate all forms of milk by the end of the study period.3 This study provides evidence for the effectiveness of the milk ladder among all ranges of severity of allergic symptoms in young children with IgE-mediated CMPA.
The number who experienced allergic reactions to milk while on the milk ladder was 73 (42.7%), while 32 (18.7%) patients experienced allergic symptoms due to accidental exposure to milk above their current stage on the ladder), most of which manifested as cutaneous symptoms (urticaria, angioedema). Three patients (1.8%) had anaphylactic reactions to milk products above their current ladder position, none of which was caused by the food they were eating on the milk ladder.
Allergic reactions due to the milk ladder occurred similarly in those who completed the ladder compared to those who did not (41.9% vs. 47.8%, p = .59). Similarly, experiencing allergic symptoms due to accidental exposure to milk did not affect completion vs non-completion of the milk ladder (17.6% vs. 26.1%, p = .33). Therefore, by offering thorough education and support regarding the management of mild allergic symptoms, progression through the milk ladder can be successful and safe.3
SPT wheal size was not associated with non-completion of the milk ladder (mean 4.5 mm completers vs. 4.8 mm non-completers, p = .36; Figure 1B), however, a higher value of whole milk-specific IgE was associated with incomplete introduction (15.8 kIU/L completers vs. 37.2 kIU/L non-completers, p < 0.01; Figure 1C). This reflects the findings of a preceding Irish intervention study.4 Specific IgE to whole milk at baseline may therefore identify children who may require additional support in completing the milk ladder. There was no difference in milk ladder progress between children with or without concomitant atopic conditions.
This study provides evidence for the efficacy and safety of the milk ladder in young children with IgE-mediated CMPA, the lack of which was highlighted in a review of Dietary Advancement Therapies.5 However, families should be informed that no management strategy is ‘risk free’ and that while the risk of allergic reaction is present, there are potential benefits of promoting tolerance to milk at an earlier age.6
Strengths of this study included the largest sample size to date in a study of milk ladder use in IgE-mediated CMPA, with a wide period of analysis and the absence of biased referral of more severe cases of CMPA to other allergy centres due to the limited access to tertiary allergy services in Ireland. Limitations include the retrospective nature of this study, the lack of a placebo group and the lack of performance of oral food challenges to confirm the diagnosis of CMPA, rather, the diagnosis of CMPA was made on a clinical history of IgE-mediated CMPA and a positive SPT and specific IgE.
In conclusion, this retrospective study is the largest analysis to date of the effectiveness and safety of milk ladder use in IgE-mediated CMPA. Future research should compare the effectiveness of the milk ladder to other CMPA treatments, and a prospective cohort study is also recommended to further establish the effectiveness and safety of milk ladders as CMPA treatment.
[Correction added on 17 January 2024 after online publication: Table 1 and its citation have been removed in this version.]
AUTHOR CONTRIBUTIONS
T. A.H.: methodology, software, validation, resources, data curation; C.C.: resources, data curation, writing—review and editing, project administration; L.F.: resources, data curation; F.M.: writing—original draft preparation; M.O'S. methodology; A.M.McG. resources, data curation; S.H.: methodology, software, validation, resources, data curation; A.McK.: data curation; Y.D.: methodology; J.H.: methodology, writing—review and editing; R.V.: conceptualization, methodology, formal analysis, supervision; J.T.: conceptualization, methodology, validation, resources, writing—review and editing, visualization, supervision.
ACKNOWLEDGEMENTS
This research received support from the National Dairy Council and Dairy Research Ireland. The authors wish to thank Ms Jackie OLeary for her expertise and management of quality and regulatory affairs during this study. Open access funding provided by IReL.
CONFLICT OF INTEREST STATEMENT
No conflicts of interest.
ETHICS STATEMENT
The study was conducted in accordance with the Declaration of Helsinki and approved by the Clinical Research Ethics Committee of the Cork Teaching Hospitals ECM4(e) 13/4/2021.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




