Non–heat inactivated autologous serum increases accuracy of in vitro CFSE lymphocyte proliferation test (LPT) for nickel
Funding information
Toegepaste en Technische Wetenschappen (TTW) formerly known as Stichting Technische Wetenschappen (STW). Grant number: 13382.
Abstract
Background
Skin patch testing is still seen as the gold standard for the diagnosis of allergic hypersensitivity. For several metals and for patients with a suspected adverse reaction to their medical device implant material, patch testing can be unreliable. The current alternative to metal allergy patch testing is the in vitro lymphocyte proliferation test (LPT) using tritiated thymidine. This method is well-established but requires handling of radioactive material, often uses heat-inactivated allogenic human pooled serum and cannot determine T cell subsets.
Objective
To develop a radioactive free LPT by using carboxyfluorescein succinimidyl ester (CFSE) and to evaluate the influence of serum source (heat-inactivated human pooled serum [HI HPS] vs autologous serum) on the sensitivity and specificity of the nickel-specific LPT.
Methods
Peripheral blood mononuclear cells derived from nickel-allergic patients and healthy controls were collected, labelled with CFSE and cultured in medium containing 10% HI HPS or 10% autologous serum with or without additional T cell skewing cytokine cocktails (Th1: IL-7/IL-12, Th2: IL-7/IL-4 or Th17: IL-7/IL-23/IL-1β) in the absence or presence of NiSO4. The stimulation index (SI) was calculated as the ratio of divided cells, that is the percentage of CFSElow/neg CD3+CD4+ T-lymphocytes upon nickel stimulation compared to the percentage of CFSElow/neg CD3+CD4+ T-lymphocytes without antigen. These results were compared with the history of Ni allergy, patch test results and the MELISA test.
Results
Autologous serum positively influenced Ni-specific proliferation while HI HPS negatively influenced Ni-specific proliferation. The test protocol analysing CD4+ cells and autologous serum without skewing cytokines scored the best diagnostic values (sensitivity 95%; specificity 93%; and overall accuracy 94%) compared to the parallel test using HI HPS (accuracy 60%). Cytokine supplements did not further improve the test protocol which used autologous serum. The protocol using HI HPS could be further improved by addition of the cytokine skewing cocktails.
Conclusions
Here, we describe an optimized and highly accurate flow cytometric LPT which comprises of CFSE-labelled cells cultured in autologous serum (not heat inactivated) and without the presence of T cell skewing cytokines.
Clinical relevance
The sensitivity and specificity of LPT is enhanced, compared to HI HPS, when autologous serum without skewing cytokines is used.
1 INTRODUCTION
Metal exposure is a known cause of allergic contact dermatitis (ACD).1 The prevalence of metal allergy has decreased since governments regulated the incorporation of sensitizing metals in jewellery and other articles that come into long-lasting contact with the skin. However, metal ions are still common sensitizers resulting in contact allergy.2 Nickel, cobalt and chromium are all present in the standard patch test series and are still ranked in the top 10 allergens with the highest sensitization rates in the European patch test population.3, 4
Patch testing is a simple and reproducible procedure for the diagnosis of contact allergy to metal and is therefore the diagnostic test of first choice.3 However, insufficient allergen penetration may result in limited inflammation of the skin at the test site resulting in false-negative ratings, whereas irritant reactions can induce false-positive ratings.5 Also, there is a risk of the patient becoming sensitized by the patch test. Furthermore, patch tests are mainly validated for allergic contact dermatitis, rather than for internal exposure to metal allergens (eg in implant patients). For metals such as titanium and palladium, the patch test salt preparation, concentration and vehicle choice are still subject to improvement.6
The lymphocyte proliferation test (LPT) for in vitro detection of metal sensitization was developed to overcome the above-mentioned shortcomings of the patch test.7, 8 Over the last two decades, the metal LPT has been adapted and improved, especially for the detection of nickel sensitization, as it is the most predominant metal allergy.9, 10 However, despite this test being promising, it comes with high cost since it uses radioactive tritiated thymidine (3H) and requires a specialized facility to run the test. No less important is the environment impact due to the long half-life of tritiated thymidine (3H). The LPT is therefore still not practical as long-term answer for large-scale diagnostic use and requires further adaptation.9, 10
A non-radioactive alternative to monitor lymphocyte transformation is carboxyfluorescein succinimidyl ester (CFSE).11 CFSE is a versatile tool for the fluorescent intracellular labelling of living cells. The dye is long-lasting and well retained within labelled cells. When a CFSE-labelled cell divides, the dye is equally divided between sister cells. Therefore, divided cells can be tracked by the CFSE signal intensity dilution via flow cytometry. Also, in contrast to 3H incorporation assays, flow cytometry can be used to determine T cell subsets and quantify cell proportions.11 Flow cytometric evaluation by CFSE dilution has been mainly described for demonstrating effector cell functions of lymphocytes and autoantigen-specific lymphocyte proliferation.12-15 Reports on CFSE performance in metal hypersensitivity are scarce.11, 16 From the available data, it can be concluded that assay conditions may be further optimized to increase the diagnostic performance of these tests. Particularly for cases where there is a long lag period between exposure and the in vitro test where the frequency of circulating specific T cells may have gone down, tests with higher sensitivity may be needed. We therefore aimed to further develop the radioactive free LPT using CFSE for diagnostic testing for metal ACD, by evaluating the influence of serum source (heat-inactivated human pooled serum [HI HPS] vs autologous serum) and addition of T helper cell skewing cytokines on the sensitivity of the assay. We have previously reported that metal-specific T cell proliferation in the 3H-based assay was improved by the addition of Th1 and Th2 skewing cytokines in line with their role in the pathogenesis of ACD.7 Th17 cells may be involved in the pathogenesis as well.17-19 We therefore investigated the effects of Th1, Th2 and Th17 skewing cytokine cocktails on the diagnostic performance of the proliferation assay.
Since nickel is still considered the number one allergen, with 11.9%-25.2% of the European patch test population being sensitized,4 we used Ni as a model metal allergen. We included in our study, Ni-sensitized patients with a history of Ni-allergy and non Ni-sensitized controls and used the patient's history of intolerance to nickel-containing particles (nickel allergy) combined with the patch test as a the diagnostic reference. Additionally, our results were compared with MELISA® (MEmory Lymphocyte Immuno Stimulation Assay), a patented modification of the radioactive LPT.
2 METHODS
2.1 Patients
This study was approved by the medical ethical committee of VU University Medical Centre (Central Committee on Research Involving Human Subjects protocol number: NL52668.029.15), and written informed consent was obtained from all the study participants. The study was conducted in compliance with ethical rules for human experimentation that are stated in the Declaration of Helsinki. A total of 52 patients participated in this study after informed consent had been obtained (Table 1). The patients were selected based on their patch test results: 27 patients (93% females) had a positive patch test reaction to 5% NiSO4 petrolatum (pet.) after 2, 3 or 6 days; 25 patients (72% females) had a negative patch test. The history of Ni allergy was recorded (nickel-induced eczematous skin reactions, nickel exposure, eczema after contact with piercings, dental braces, jewellery, jeans) for all patients. Thereafter, patients were divided into a Ni-allergic group (positive patch test and history) or a control group (negative patch test and history). The age range in the true-positive group was 25-75 years (median 54), and in the true-negative group, it was 40-78 years (median 61). In the remaining patients, the patch test did not confirm the history. Patients were tested for nickel sensitization with flow cytometric LPT using CFSE (instead of radioactive 3H) and with MELISA. The diagnostic values of the flow cytometric LPT and the MELISA were evaluated against the patient's history of nickel allergy combined with the patch test (“gold standard” for this study). None of the patients had recently used systemic immunosuppression or underwent UV radiation. Peripheral blood samples were taken at the VU Medical Centre Department of Dermatology within 5 years (mean 14 months) after epicutaneous patch testing. The general characteristics of the participants are shown in Table 1.
| Characteristic | Control patients | Ni-allergic patients | PT- H+ | PT + H- |
|---|---|---|---|---|
| Age (y) (range) | 61 (40-78) | 54 (25-75) | 60 (28-71) | 55 (30-81) |
| Sex, n | 9 (F), 6 (M) | 20 (F), 0 (M) | 9 (F), 1 (M) | 5 (F), 2 (M) |
| PT result | − | 11 (+), 9 (++/+++) | − | 5 (+), 2 (++/+++) |
| Mean time since PT (SD) (mo) | 13.3 (15.2) | 11 (11.8) | 21 (33.4) | 12 (5.4) |
- Abbreviations: H, medical history of nickel metal allergy; PT, patch test.
2.2 Patch tests
All patients had been patch tested with at least the 30 allergens of the European baseline series of contact allergens, including 5% nickel sulphate (NiSO4) in petrolatum.20 Application time was 48 hours, and test readings were performed at 48, 72 and 168 hours (7 days). Patch tests were positive if at least at one reading a positive reaction to NiSO4 (5%) occurred. Positive reactions were rated as +, ++ or +++, in accordance with the International Contact Dermatitis Research Group (ICDRG) reading criteria. The patch tests were read by one of two dermatologists with expertise in allergology in our outpatient clinic and in accordance to the European society of contact dermatitis guideline for diagnostic patch testing in order to limit observer bias.20
2.3 Lymphocyte proliferation test
Peripheral blood samples (40 mL) were collected in Vacutainer heparin tubes (3 × 10 mL) and Vacutainer serum tubes (1 × 10 mL) (Becton Dickinson GmbH). The blood was processed at the Oral Cell Biology Laboratory of the Academic Centre for Dentistry Amsterdam (ACTA). Peripheral blood mononuclear cells (PBMCs) were isolated on Ficoll Histopaque (Sigma-Aldrich Chemie GmbH) immediately upon arrival in the laboratory and labelled with CFSE (CellTrace™; ThermoFisher) at a final concentration of 3 µmol/L. The cells were cultured at a slight angle in flat-bottom 48-well culture plates at 37°C in a 5% CO2-containing humidified atmosphere in medium (IMDM, 100 IE/mL Na-penicillin and 100 gµ/mL streptomycin, 2 mmol/L L-glutamine [Biochrom seromed] and 50 mol/Lµ β-mercapto-ethanol) containing either 10% heat-inactivated human pooled serum (Merck), 10% human male AB serum (Sigma-Aldrich), 10% heat-inactivated human male AB serum (Sigma-Aldrich), autologous serum or heat-inactivated autologous serum. Culture media were supplemented with several cytokines: IL-7 (0.1 ng/mL; Strathmann Biotec AG), IL-12 (1 ng/mL; PeproTech), IL-4 (3 ng/mL), IL-1β (10 ng/mL) and IL-23 (10 ng/mL), all from R&D systems. The following combinations were used: for Th1 skewing IL-7 and IL-12, for Th2 skewing IL-7 and IL-4 and for th17 skewing IL-7, IL-23 and IL-1β, as described by Moed et al (2005) and de Jong et al (2010).7, 21 The final cell density was 7.5 × 105 cells/mL/well. Duplicate wells were stimulated over a period of 7 days with or without 50 µmol/L NiSO4 (Merck). Nickel concentration was based on the study by Spiewak et al (2007).22 The T cell mitogen phytohaemagglutinin (PHA; Merck) (3 μg/mL) was used as positive control. Unstimulated cells were defined as negative controls.
2.3.1 T cell proliferation
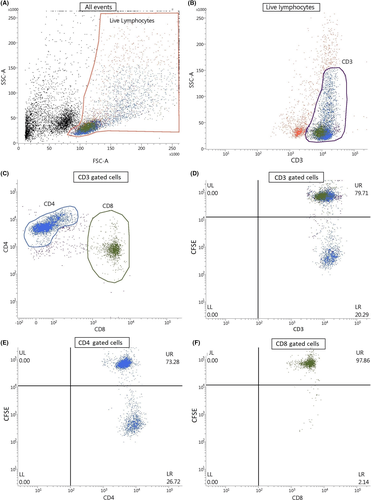
Nickel-specific T cell proliferation was assessed by flow cytometry. Acquisition and analysis were performed on a BD FACSVerse flow cytometer and associated FACSuite software using the gating strategy depicted in Figure 1. Ten thousand events were collected. Lymphocytes were gated for CD3+, CD3+CD4+ and CD3+CD8+ T cells. Cell surface marker staining was performed using the following three monoclonal antibodies: CD3 BV510 (BD), CD4 PerCP-Cy5.5 (Thermo Fisher) and CD8a APC-eFluor 780 (Thermo Fisher). The results were expressed as the stimulation index (SI), which is calculated as the ratio of the percentage of CFSElow/neg CD3+, CD3+CD4+ or CD3+CD8+ T-lymphocytes upon nickel stimulation to the percentage of CFSElow/neg CD3+, CD3+CD4+ or CD3+CD8+ T lymphocytes without antigen.
2.4 LPT-MELISA®
The LPT-MELISA is a patented modification of the LPT. It was performed by Invitalab using a standardized and validated protocol as described by Stejskal et al (1994) and Valentine-Thon et al (2006)8, 23 in which HPS was replaced with autologous serum. Blood samples were transported in vacutainer citrate and vacutainer serum tubes by courier. The blood samples were processed within 24-48 hours after blood withdrawal.
2.5 Statistical analysis
SPSS (version 18.0.2, IBM SPSS Inc., SPAW Statistics), MedCalc® (version 11.6.1.0, MedCalc Software bvba) and GraphPad Prism (version 7.00, GraphPad Software) were used for statistical analyses. To determine optimal SI cut-off values to distinguish positive from negative patients, receiver operator characteristics (ROC) curve analyses were performed for each in vitro parameter. To test for normality, the Shapiro-Wilk test was used. Friedman's one-way ANOVA followed by Dunn's multiple comparison test was used to assess differences in cell proliferation. Differences in sensitivity and specificity between tests were calculated with the McNemar test. Spearman's rank correlation (Rho) was used to describe the strength of the association between the LPT and the MELISA test. Statistical significance was defined as P < .05.
3 RESULTS
3.1 CFSE-labelled CD4+ T cells are the dominant proliferating population responding to nickel
To develop the flow cytometric CFSE LPT, we first investigated the Ni-specific T cell proliferation in CD4+ and CD8+ T cell subsets (Figure 1). CD4+ T cells demonstrated the best proliferative response and SI in the nickel-allergic population (Figure 1D,E for gated CD3+ and CD4+ T cells showing significant population of proliferated cells in lower right quadrant and Figure 1F for gated CD8+ T cells showing low proportion of proliferated cells in lower right quadrant; example of a nickel-allergic patient), resulting in consistently better diagnostic values compared to CD8+ cells. This was true for all cultures, regardless of serum choice or addition of cytokine cocktails (data not shown). Therefore, from here on only the results of the LPT analysing CD4+ cells are described.
3.2 Comparison of autologous serum with heat-inactivated human pool serum in LPT
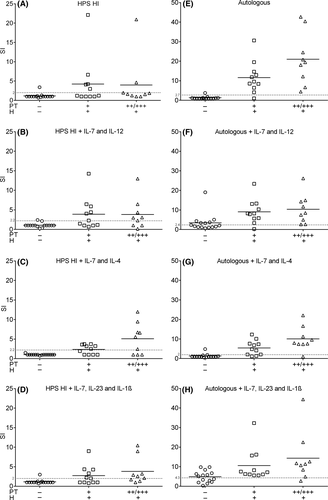
Heat-inactivated human pool serum (HI HPS) is usually used to test in vitro specific T cell recall responses. It is suitable for standardized tests, logistically easy to use and quality controlled. However, there is a possibility of cell activation by xenogeneic antigens. Also, the presence of possible auto-antibodies (eg HLA or AB antigens) and the activation of complement components can result in non-specific cell activation and complement-mediated lysis in cell cultures. To decrease the effects of complement and allo-specific antibodies, serum is heat-inactivated (HI). In autologous serum, no antibodies against autologous cells are present and heat inactivation is not mandatory. Therefore, we evaluated both autologous serum and HI HPS in our assays. Patients were allocated in a Ni-allergic group (truly positive, N = 20) and a group without Ni allergy (truly negative, N = 15), the latter served as a control group. Unless otherwise specified, these groups were used for all test results. Either HI HPS, autologous serum or autologous serum HI was used in the LPT. In all test protocols, strong proliferative responses to PHA were observed, which were comparable between Ni-allergic and the control group patients (data not shown). The mean proliferation response of CD4+ cells to 50 µmol/L NiSO4 in nickel-allergic patients was significantly higher using autologous serum compared to HI HPS and autologous HI serum, respectively, 20.8 ± 15.9% vs 4.3 ± 6.1% vs 6.2 ± 8.8% (Figure 2A). The increased Ni-induced proliferation in autologous serum conditions did not coincide with an increase in background cellular proliferative responses (without Ni). This is reflected when the CD4+ proliferation is expressed as SI. The mean SI in nickel-allergic patients using autologous serum was 15.8 ± 11.2, compared to a mean SI of 4.1 ± 6.0 using HI HPS and 3.6 ± 3.6 using autologous HI serum (Figure 2B). In contrast, the corresponding nickel-specific SI in the control group was comparable in all serum groups, respectively, 1.3 ± 0.7%, 1.2 ± 0.6 and 1.4 ± 1.0.
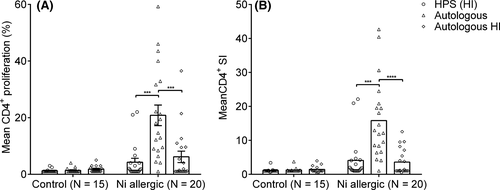
As there is a large variation in time between the last exposure (patch test) and LPT, we analysed whether the SI was lower in patients with a longer time lapse between patch test and LPT. In Ni-allergic patients from whom the blood was collected within 6 months after patch testing, the mean SI (15.9, SD = 10.7, n = 8) was similar to the mean SI (15.8, SD = 11.6, n = 12) in patients tested for the LPT later than 6 months after patch testing, indicating that the time since last exposure did not influence the strength of the proliferative response.
3.3 Autologous serum increases the Ni-specific proliferation and accuracy of the LPT
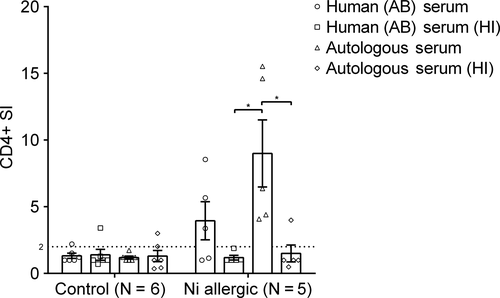
Interestingly, HI autologous serum caused a significant decrease in Ni-specific proliferation. To validate the role of HI on Ni-specific proliferation, 11 patients were additionally tested with human pooled AB serum and human pooled AB HI serum. Non-HI allogenic serum should be preferably from AB male donors to reduce the chance of lymphocyte cytotoxicity. No differences in mean SI was seen in the control group (N = 6). In allergic patients (N = 5), the use of autologous serum resulted in a significantly higher mean SI compared autologous HI and human pooled AB HI serum (Figure 3). All five patients from the allergic group showed a SI >2 when tested with autologous serum. Although there was no significant difference in mean SI between human AB serum and human AB serum HI, in the non-HI group three of the five patients tested positive (SI > 2), compared to zero in the HI group. This indicates that autologous serum increases the Ni-specific proliferation and accuracy of the LPT compared to human pooled serum, while HI serum results in a decrease of LPT accuracy.
3.4 Influence of Th1, Th2 and Th17 cytokines on LPT
Previously, we have shown that supplementing skewing cytokines improves the diagnostic value of the radioactive 3H LPT.24 Therefore, we next investigated the influence of four different skewing conditions (none, Th1, Th2 and Th17 cytokines) on the LPT results for nickel-allergic or non-allergic patients. Autologous serum was compared with HI HPS. To discriminate between a positive and a negative LPT result and to determine the diagnostic values of the LPT using either of the sera, optimal SI cut-off values were established using ROC-curve analysis (Figure S1). The highest accuracy (94.3%) was found for the autologous serum protocol without any skewing cytokines (Figure 4). Using the optimal SI cut-off value (2.7), only one false-positive and one false-negative test result was observed. This cut-off resulted in a sensitivity of 95%, specificity of 93.3%, positive predictive value of 95% and a negative predictive value of 93.3%. Furthermore, there was a good coherence with the strength of the patch test reaction. Supplementing skewing cytokines to autologous serum did not increase LPT accuracy, partly due to an increase in a-specific proliferation in control group donors without a significant increase in specific proliferation in Ni-allergic donors. In contrast, all skewing cocktails increased the LPT accuracy in HI HPS cultures, although not to the extent of autologous serum. For HI HPS, the best diagnostic values were found when using Th2 skewing cytokines. This reduced both the numbers of false positives (from one to zero) as false negatives (from 12 to 8). The sensitivity was 60%, the specificity 100%, the positive predictive value 100% and the negative predictive value 65.2%. These findings strongly indicate that autologous serum without skewing cytokines is the optimal test condition in CFSE-labelled lymphocytes cultures. These results emphasize the possibility to enhance the accuracy of the LPT. The results of the CFSE LPT using CD4+ T cells and subsequent test characteristics are summarized in Table 2.
| LPT test protocol | SI cut-off (ROC) | Area under the curve (AUC) | Sensitivity (95% CI) | Specificity (95% CI) | Positive predictive value (95% CI) | Negative predictive value (95% CI) | Accuracy |
|---|---|---|---|---|---|---|---|
| HPS—no skewing | 2 | 0.79 (0.65-0.94) | 35.00 (15.39-59.22) | 93.33 (68.05-99.83) | 87.50 (49.01-98.08) | 51.85 (43.17-60.42) | 60.00 (42.11-76.13) |
| HPS—Th1 skewing | 2.2 | 0.81 (0.66-0.96) | 60.00 (36.05-80.88) | 93.33 (68.05-99.83) | 92.31 (63.60-98.80) | 63.64 (50.15-75.27) | 74.29 (56.74-87.51) |
| HPS—Th2 skewing | 2.2 | 0.83 (0.71-0.97) | 60.00 (36.05-80.88) | 100.0 (78.20-100.0) | 100.0 | 65.22 (52.29-76.23) | 77.14 (59.86-89.58) |
| HPS—Th17 skewing | 2 | 0.79 (0.63-0.94) | 60.00 (36.05-80.88) | 93.33 (68.05-99.83) | 92.31 (63.60-98.80) | 63.64 (50.15-75.27) | 63.64 (56.74-87.51) |
| Autologous—no skewing | 2.7 | 0.96 (0.89-1.00) | 95.00 (75.13-99.87) | 93.33 (68.05-99.83) | 95.00 (74.04-99.22) | 93.33 (67.35-98.96) | 94.29 (80.84-99.30) |
| Autologous—Th1 skewing | 2.4 | 0.84 (0.70-0.99) | 95.00 (75.13-99.87) | 60.00 (32.29-83.66) | 76.00 (62.83-85.58) | 90.00 (56.04-98.45) | 80.00 (63.06-91.56) |
| Autologous—Th2 skewing | 2 | 0.93 (0.85-1.00) | 85.00 (62.11-96.79) | 93.33 (68.05-99.83) | 94.44 (71.72-99.13) | 82.35 (61.97-93.04) | 88.57 (73.26-96.80) |
| Autologous—Th17 skewing | 4.3 | 0.80 (0.66-0.90) | 95.00 (75.13-99.87) | 53.33 (26.59-78.73) | 73.08 (61.02-82.47) | 88.89 (52.78-98.28) | 77.14 (59.86-89.58) |
Note
- Sensitivity, specificity, positive predictive value, negative predictive value, accuracies and optimal cut-off values with corresponding area under the curve for CD4+ test protocols using various serum sources and skewing conditions.
3.5 Comparison of optimized CFSE LPT with patch test and MELISA
We compared our optimal CFSE LPT test protocol (autologous serum without skewing cytokines and CFSE-labelled CD4+ lymphocytes) with the well-established MELISA test. Unfortunately, due to the low number of viable T cells after transport, the MELISA test could not be performed in six true-negative and six true-positive patients. The missing data were not related to other variables (missing data are completely at random) and were handled using list-wise deletion. Using the remaining data, there was a high positive correlation between the optimized CFSE LPT and the MELISA test (ρ = .74, P = .0001). The sensitivity (100%) and specificity (77.8%) of the MELISA test do not significantly differ (P = 1.0, P = 1.0) compared to the sensitivity (95%) and specificity (93.3%) of the CFSE LPT (Figure 5).
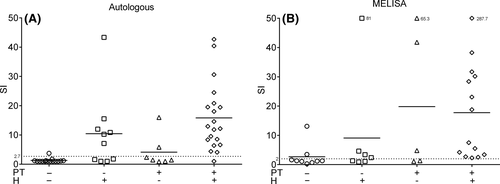
In order to compare our optimal LPT with the patch test as a diagnostic tool, we next tested an additional 17 patients in which the patch test did not confirm the history of Ni allergy (Table 1; Figure 5A). In a total of 52 patients (20 true positive, 15 true negative, 17 mentioned above) the history of Ni allergy was used as a reference to calculate sensitivity and specificity (Table 1). The patch test has a sensitivity of 67% and a specificity of 68%. Our optimized CFSE LPT has a sensitivity of 83% and a specificity of 86%. The sensitivity and specificity of the patch test and the LPT are not significantly different (P = .13 and P = .22, respectively). Twelve of the 17 patients were also tested with the MELISA test, using the history as a reference the MELISA has a sensitivity of 86% and a specificity of 64%.
4 DISCUSSION
Our study describes major advancements above the traditional LPT which makes use of HI HPS and radioactive 3H. The optimized LPT used CFSE-labelled cells cultured in autologous serum (not HI). In contrast to HI HPS cultures, Th subset skewing cytokines could not further enhance the performance of the test with autologous serum. Therefore, from a clinical perspective autologous serum without cytokines is preferable because it has a better performance and is a cheaper test.
We found that the proliferative potential of all lymphocyte subsets was greatly affected by the serum source. Autologous serum significantly enhances the proliferative response and SI in Ni-allergic donors compared to HPS, HI HPS and autologous HI serum. Interestingly, all sera showed similar proliferation among non-allergic donors and autologous serum did not increase the number of false positives compared to the other sera. Most importantly, use of autologous serum greatly increased the accuracy of the assay, enabling a better discrimination between Ni-allergic and non-allergic patients, without enhancing background proliferative response. Several authors compared sera in “traditional 3H” LPT studies, resulting in various conclusions. Two research groups showed that using 10% autologous serum resulted in the highest nickel proliferation and increased the signal to noise ratio compared to HPS.25, 26 However, no significant differences between allogenic or autologous serum were reported in a MELISA test study.27 Despite the discrepancies among these reports, which might be due to a high level of variability between human serum lots, the use of autologous serum seems to be on the rise,28-30 which is strongly supported by our results.
The use of CFSE for flow cytometric evaluation resulted in a sensitive assay which can replace the use of 3H as it is easy to implement in routine diagnostics laboratories. This is in agreement with Spoerri et al,16 who used a CFSE cytometric LPT using 10% human AB serum and CD4+ cells to detect Ni hypersensitivity. With detailed medical history of metal allergy (patients with a negative history for nickel allergy serving as controls) as a reference, they found a sensitivity and specificity off 40.6% and 82.1%. The sensitivity was significantly lower compared to the sensitivity we found (83%) using autologous serum, the specificity being similar (86%). In our study, there was a large variation in the time range (8.7 months) between diagnostic patch testing (and presumably the last exposure in diagnosed patients) and blood collection for the in vitro tests in Ni-allergic patients. However, we did not observe a correlation between LPT response and time range between patch testing and blood collection. This illustrates another strength of this test as there is often a significant delay between exposure and diagnostic work-up. Our study confirms previous findings by us and others that CD4+ cells are the dominant proliferating population and thereby also verifies a cell-mediated hypersensitivity reaction.7, 31-33 Furthermore, by discriminating CD4+ from CD8+ cells, higher SI’s and better diagnostic values could be found. This highlights the advantage over the “traditional” LPT that lacks the ability to discriminate between subsets and measures proliferation in all peripheral blood mononuclear cells.11 Our results are in line with the current perception of the inflammatory mechanisms in ACD. While CD8+ cells appear to be critical for the development of nickel contact allergy,17 CD4+ cells seem to be the major effector cells,34 and the main cells infiltrating nickel-exposed skin.31
Although this study did not focus on the mechanisms explaining the influence of serum choice and HI, our results do show that lymphocytes proliferated significantly more in the presence of autologous serum compared to autologous HI serum. Allogenic serum is HI primarily to inactivate complement which may cause cell death in combination with potentially present alloreactive antibodies.35 On the other hand, complement factors have been shown to play a role in metabolism of T cells and inactivation may be detrimental as such.36 Furthermore, C5a is described as a critical mediator of allergic contact dermatitis,37 as it is essential for the recruitment of CD4+ cells, and, together with C3a, provides costimulatory and survival signals to CD4+ cells.38, 39 However, complement also interacts with other hematopoietic cells and tissue cells, making it difficult to ascribe the same role to it in a LPT using only PBMC. Another possible explanation of the detrimental effect of HI is that levels of many hormones, growth factors and other proteins change as a result of HI.35 For example, cellular uptake of nanoparticles is negatively influenced by heat-induced protein denaturation.40 Therefore, the behaviour of cells and the haptenization of Ni ions in cultures supplemented with HI serum is likely different than that of cells in cultures with non-HI serum. Analysis of serum before and after HI by mass-spectrometry and proteomics may identify proteins important for haptenized antigen-specific T cell proliferation.
No significant change in diagnostic values for CD4+ proliferation was seen in autologous serum cultures when adding either of the T helper subset skewing cytokines. In contrast, in HI HPS supplemented cultures, addition of all cocktails caused an impressive increase in proliferation and diagnostic values, with Th2-supplemented cultures being the most effective in detecting a Ni-specific response. These findings support previous observations. Spiewak et al22 discussed that Ni-specific in vitro lymphocyte proliferation and cytokine production using human pooled serum can be substantially improved by addition of type 1 or type 2 skewing cytokines. In contrast to the widely accepted involvement of Th1 cytokines (IFN-γ) in Ni allergy,41 they showed that the Th2 immune response has an important role in the in vitro elicitation. Recent research also describes an important role of nickel-specific Th17 cells, as these cells were found in the skin and the blood of nickel-allergic patients.18, 19, 31 The fact that skewing cocktails did not improve autologous serum protocols could indicate that the serum of Ni-allergic patients, in contrast to HPS serum, already contain patient-specific elevated levels of pro-inflammatory cytokines. This will be the subject of further research. A recent study by Summer et al29 using autologous serum reported that 12/15 Ni PT-positive patients also had a positive LPT and all control patients were LPT negative. Our MELISA test results, in which autologous serum is used, also show a strong concordance with the “golden” standard. These differences in performance provide a rationale for a comparative study on serum components.
In conclusion, our results indicate autologous serum positively influenced Ni-specific proliferation while HI serum negatively influenced Ni-specific proliferation. Furthermore, the flow cytometric LPT by CFSE dilution is a promising method for detecting Ni sensitization. Although these results are based on retrospective data, the excellent diagnostic values of the LPT illustrate its clinical value and its potential in patients for which the patch test is inconclusive or not a diagnostic option. It can provide a relatively simple alternative to PT and the “traditional 3H” LPT. Furthermore, it can be performed on commercial flow cytometers and detailed information on lymphocyte subsets can be obtained. The test protocol using autologous serum without skewing cytokines resulted in the best diagnostic values and should be further evaluated for the in vitro diagnosis in patients with ACD to metal allergens and in patients with non-dermal-related allergies such as sensitization to metal-containing implanted devices.
CONFLICT OF INTERESTS
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
de Graaf NPJ and Rustemeyer T collected the patient data. de Graaf NPJ collected the blood samples. de Graaf NPJ and Roffel S performed the in vitro tests. De Graaf NPJ, Roffel S and Bontkes HJ performed the data analysis. Feilzer AJ and Kleverlaan CJ developed the research question. All authors were involved in the study design, interpretation of the data and the writing of the manuscript.