A subset of walnut allergic adults is sensitized to walnut 11S globulin Jug r 4
Funding information
This research was funded by The Netherlands Organization for Applied Scientific Research TNO, Zeist, The Netherlands. Line blots and reagents were provided by EUROIMMUN, Lübeck, Germany.
Summary
Background
The role of sensitization to commercially available allergens of English walnut (Juglans regia) Jug r 1, 2 and 3 in walnut allergy has been previously investigated in walnut allergic adults and was unable to explain all cases of walnut allergy.
Objectives
Identify recognized walnut allergens, other than the ones previously investigated (Jug r 1-3), in walnut allergic adults and determine the sensitization frequency and diagnostic value.
Methods
Three different in-house walnut extracts were prepared and analysed on SDS-PAGE blots to identify allergenic walnut proteins. Immunoblots and immunoprecipitation, followed by LC-MS analysis, were performed to screen for, and confirm, IgE binding to walnut allergens in selected walnut allergic adults. In a cohort of 55 walnut challenged adults, including 33 allergic and 22 tolerant, sensitization to native and recombinant walnut allergen Jug r 4 was assessed using immunoblotting and immuno-line blot (EUROLINE), respectively.
Results
Screening of sera of 8 walnut allergic adults identified Jug r 4 as an allergen in our population. In the total cohort of 55 subjects, 5 were positive for Jug r 4 on immunoblot and 10 on EUROLINE. All but one EUROLINE positive subject had a positive food challenge (sensitivity 27%, specificity 95%, PPV 90%, NPV 47%). All 5 subjects positive on immunoblot were also positive on EUROLINE. LC-MS analysis showed a lack of Jug r 4 in the ImmunoCAP extract. Co-sensitization to other 11S albumins (eg hazelnut Cor a 9) was common in Jug r 4 sensitized subjects, potentially due to cross-reactivity.
Conclusions
Walnut 11S globulin Jug r 4 is a relevant minor allergen, recognized by 27% of walnut allergic adults. It has a high positive predictive value of 90% for walnut allergy. Specific IgE against Jug r 4 occurred mostly with concomitant sensitization to other walnut components, mainly Jug r 1.
1 INTRODUCTION
The nut of the English or common walnut tree (Juglans regia) is a frequently consumed tree nut. Ingestion of walnut is associated with potentially severe allergic symptoms in walnut allergic individuals.1 Threshold analyses of walnut allergic adults indicated that walnut is a potent allergen, similar to hazelnut.2 Walnut allergy appears to be one of the most reported tree nut allergies in the USA, where hazelnut allergy is most prevalent in Continental Europe.3
Currently, 5 proteins from English walnut have been recognized by the WHO/IUIS Allergen Nomenclature Sub-committee as allergens. These include a 2S albumin (Jug r 1), a vicilin-like 7S globulin (Jug r 2), a lipid transfer protein (Jug r 3), a legumin-like 11S globulin (Jug r 4) and a PR-10 protein (Jug r 5). Additionally, a vicilin-like cupin (Jug r 6) has recently been accepted as a new walnut allergen,4 but at the time of writing, details were not available yet. Jug r 1 to 3 are currently commercially available for specific IgE (sIgE) testing, and we reported previously on their role in diagnosing walnut allergy.1 While sensitization to Jug r 1 appears to be most prevalent in walnut allergic patients (61%), 11 cases could not be explained by sensitization to the currently available allergens, including 4 with moderate to severe walnut allergy.1 At the same time, 11S globulins (like Jug r 4) are known to be major allergens in hazelnut and cashew allergy.5, 6 Additionally, other unidentified allergens could play a role. Therefore, we aimed to identify relevant walnut allergens, other than the previously investigated Jug r 1, 2 and 3 using immunoblot, immunoprecipitation and LC-MS and determine their diagnostic value in establishing walnut allergy in a cohort of 55 adult outpatients with a suspected walnut allergy.
2 METHODS
2.1 Preparation of defatted walnut powder
The outer skin of the walnuts (Juglans regia) was removed using compressed air. The deskinned walnuts were frozen using liquid nitrogen and grinded using a mortar. The walnut particles were first freeze-dried to remove remaining water. After freeze-drying, the particles were defatted using a Soxhlet with petroleum ether. After the Soxhlet, the remaining petroleum ether was removed using a vacuum stove at 40°C. The defatted particles were further grinded using a Grindomix GM200 (Retsch, Aartselaar, Belgium) to obtain a fine powder.
2.2 Extract preparation
Proteins were sequentially extracted from this defatted walnut powder (1 gr) using in succession 3 different buffers: 2 times 10 mL PBS (0.1 mol/L Sodium phosphate pH 7.2 + 0.85% NaCl), 2 times 10 mL of 8 mol/L Urea in PBS and 2 times 10 mL of 2% SDS + 1% DTT in PBS. After extracting the proteins for 60 minutes at a tube roller, the mixture was centrifuged for 10 minutes at 4500 g. The 2 supernatant fractions were pooled and immediately stored at −20°. In this way, 3 walnut extracts were obtained: PBS, Urea and SDS/DTT.
2.3 Jug r 4 purification
Four grams of defatted walnut powder was extracted with 35 mL 0.1 mol/L sodium phosphate buffer + 0.85% NaCl pH 7.2. The extraction was performed in a 50-mL Greiner tube on a rotating disc. After 60-minute rotating, the suspension was centrifuged (30 minutes, 13300 g). The supernatant was discarded, and a second extraction with 35 mL 0.1 m sodium phosphate buffer + 0.85% NaCl pH 7.2 was performed for 15 minutes. The supernatant after the second centrifugation (30 minutes, 13300 g) was again discarded, and the pellet was extracted with 35 mL 50 mmol/L TRIS pH 8.1 + 2 mol/L NaCl. The pellet was dispersed in the buffer using a Potter-Elvehjem PTFE pestle and glass tube. The suspension was centrifuged (30 minutes, 47808 g), and the supernatant filtered over a 0.2-μ filter. The filter extract was chromatographed using a Superdex 200 column (XK50/100 1800 mL) at 2 mL/min with 50 mmol/L TRIS pH 8.1 + 0.5 mol/L NaCl as elution buffer. The fractions containing Jug r 4 were pooled and frozen in liquid nitrogen and stored at −80°C. The purified Jug r 4 fraction contained a very small amount of Jug r 2, which could not be removed.
2.4 Patient selection
Previously, a prospective diagnostic study into walnut allergy was conducted. The study protocol was described elsewhere.1 In short, adult outpatients with a suspected walnut allergy based on patient history were included, regardless of any previous skin prick test (SPT) or sIgE results. A double blind placebo controlled food challenge (DBPCFC) with walnut was performed to confirm or rule out walnut allergy. For the SDS–PAGE and the first immunoblotting experiments, 8 sera from allergic subjects were selected based on sensitization results for walnut extract and walnut components Jug r 1, 2 and 3 (Table S1). By selecting walnut allergic subjects with high, low and absence of sensitization to these 3 allergens, we aimed to identify all relevant allergenic proteins. For the assessment of the diagnostic value of identified allergens, sera from all 55 walnut challenged subjects were tested on immunoblot and line blot analysis. Of these 55 subjects, 33 were walnut allergic and 22 tolerant (ie had a positive and negative challenge, respectively).1, 2 Sensitization to walnut extract as well as recombinant (r) walnut components, rJug r 1 and rJug r 3, on ImmunoCAP and native (n) walnut component, nJug r 2, on ImmunoCAP ISAC (both TheromoFisher, Uppsala, Sweden) was assessed previously.1 The tests were performed according to manufacturer's instructions. For walnut extract and rJug r 1 on ImmunoCAP, the optimal cut-off values 0.46 and 0.1 kU/L, respectively, were used, as previously established.1 For nJug r 2 on ImmunoCAP ISAC and rJug r 3 on ImmunoCAP, a result of 0.3 ISU and 0.35 kU/L, respectively, was considered positive. Subjects with sIgE against nJug r 2 were considered truly sensitized to Jug r 2 in case of no detectable sIgE against CCD marker nMUXF3 on ISAC, excluding reactivity to known carbohydrate epitopes on nJug r 2 on ISAC.7
2.5 SDS–PAGE and immunoblotting
The 3 walnut protein extracts (5 μg protein each) were diluted with sample buffer (50 mmol/L Tris pH 6.8, 2% SDS, 10% glycerol, 2% b-mercaptoethanol) and analysed using Any kD precast polyacrylamide gel (Bio-rad, München, Germany). A Precision plus protein dual colour standard (Bio-Rad) was run on each gel. Proteins were visualized using Coomassie gel staining (Expedion, Cambridge, UK) or used for immunoblotting. For immunoblotting, proteins were transferred to a PVDF membrane (Bio-Rad). Membranes were blocked with 4% (w/v) low-fat milk powder (Elk, Campina, Amersfoort, The Netherlands) in PBS/0.1% Tween-20 for 60 minutes after which they were incubated overnight with diluted patient sera (1:50) at 4°C. Bound IgE was detected with 1:50 000 diluted peroxidase-conjugated goat anti-human IgE (KPL/SeraCare, Milford, MA, USA). For the blots with purified nJug r 4 (approximately 0.75 ug), nitrocellulose membranes were used (Bio-Rad), together with a 1:25 diluted patient serum and 1:25 000 diluted peroxidase-conjugated goat anti-human IgE (KPL, USA). Control blots were performed to exclude a-specific and IgG binding. Visualization was performed using a chemiluminescent peroxidase substrate kit, and blots were scanned using a Chemidoc XRS+ image scanner with Imagelab software (Bio-Rad). PVDF blots were scanned for 600 seconds, and 50 scans were taken. The scoring was performed with the 50-second scan. Nitrocellulose blots were scanned for 500 seconds, and 50 scans were taken. The scoring was performed with the 52-second scan.
2.6 Identification of IgE binding proteins
Bands of interest were excised from SDS-PAGE gel for identification of IgE binding proteins. Gel bands for mass spectrometric analysis were processed according to Shevchenko et al8 Gel pieces were washed with 100 mmol/L NH4HCO3 and HPLC-grade acetonitrile (1:1, v/v) (buffer A). Proteins were in-gel reduced by 10 mmol/L DTT in buffer A, and subsequently alkylated with 55 mmol/L iodoacetamide in buffer A. Proteins were digested overnight at 37°C in digestion buffer (40 mmol/L NH4HCO3, 10% acetonitrile) containing 12.5 ng/μL proteomics-grade trypsin. Peptides were extracted with 100 μL 2:1 (v/v) ACN: 5% FA. Extracts were dried in a vacuum centrifuge and reconstituted in 10 μL of mobile phase A.
For immunoprecipitation, Dynabeads M-280 Tosylactivated (10 mg, Invitrogen, Carlsbad, CA, USA) were used according to the manufacturer's instructions. After coating with 0.2 mg Goat anti-Hu IgE (AP175 Upstate, Milipore/Merck, Burlington, MA, USA), the separate beads were incubated for 1 hour at 37°C with 1 mL serum from 2 walnut allergic subjects. Conjugated beads were cross-linked with 5 mmol/L BS³ (Pierce, Thermo Scientific, Waltham, MA, USA) according to the manufacturer's instructions to ensure reusability of the beads. The beads were washed 3 times with 0.1% Tween-20 in PBS pH 7.4, followed by overnight incubation at 37°C with 100 μL Tris/urea mealworm mixture diluted with 900 μL PBS. After washing 3 times, proteins were eluted with 2 times 100 μL 0.1 mol/L glycine and the pH of the solution was neutralized using 30 μL of 1 mol/L Tris-HCL pH 8.5. Incubation with walnut extract was repeated 3 times, and all eluates (800 μL) were pooled. Before analysis, samples were freeze-dried, reconstituted in 250 μL 0.05% SDS, reduced with 10 mmol/L DTT (1 hour, 37°C), alkylated with 24 mmol/L iodoacetamide (1 hour, 37°C) and digested with 600 ng proteomics-grade trypsin after quenching with 2 mmol/L DTT (20 minutes, 37°C). Peptides were purified by strong cation exchange stage tips and subsequently injected for mass spectrometric analysis.
2.7 Identification of proteins in ImmunoCAP extract
Captured proteins from the ImmunoCAP walnut extract (f256; TheromoFisher, Uppsala, Sweden) were released from the filters by a step of on-filter tryptic digestion. Briefly, on-filter digestion was performed in parallel on 2 filters, by applying 50 μL of digestion buffer (100 mmol/L Tris/HCl pH 8.5, 0.1% Triton X-100, 200 ng trypsin) on the top of the filters. Gravity allowed the digestion buffer to pass slowly through the filters. After complete elution, the digestion buffer was collected and the procedure was repeated, using the same collected digestion buffer, for a total of 10 times (2 minutes intervals). The filters were washed with 50 μL of washing buffer (100 mmol/L Tris/HCl pH 8.5, 0.1% Triton X-100) to fully release peptides from the filters. The supernatants from on-filter digestion and the washing step from both filters were collected and combined. In order to ensure full tryptic digestion of the peptide mixture, an additional standard in-solution digestion protocol, including cysteine reduction and alkylation, was applied to the sample. Protein disulphide bonds were reduced by the addition of dithiothreitol (DTT, 10 mmol/L 1 hour at 37°C). Then, cysteines were alkylated by treatment with iodoacetamide (24 mmol/L, 1 hour at 37°C). Iodoacetamide was neutralized by a further addition of DTT to a final concentration of 2 mmol/L (30 minutes at 37°C). Subsequently, 200 ng of trypsin was added (37°C, overnight incubation).
Half of the resulting peptide mixture was purified by StageTip SCX (EmporeTM Cation 47 mm Extraction Disks), in accordance with Rappsilber et al9 The eluate from the SCX cleanup was evaporated to dryness and resuspended in 30 μL of mobile phase A (2% Acetonitrile/0.1% formic acid (v/v)).
2.8 NanoLC-MS/MS and database search
NanoLC-MS/MS analysis was performed on an Easy LC 1000 nanoscale liquid chromatography (nanoLC) system (Thermo Fisher Scientific, Odense, Denmark) coupled to a Q-Exactive mass spectrometer (Thermo Scientific, Bremen, Germany).
The analytical column was a 10-cm pulled fused silica capillary (75 μm i.d.) self-packed with 3 μm C18 silica particles (Entringen, Germany) (Mobile phase A: 2% Acetonitrile/0.1% formic acid (v/v); mobile phase B: 80% Acetonitrile/0.1% formic acid (v/v)). Four microlitres of the peptide mixtures (corresponding to about the 6% of the starting sample) was loaded at a 500 nL/min flow rate onto the analytical column. Peptides were eluted via a 70-minute linear gradient of increasing mobile phase B at a flow rate of 300 nL/min. Mobile phase B ramped from 2% to 8% in 1 seconds, then ramped from 8% to 35% over 55 minutes, then from 35% to 100% in 8 minutes, and finally kept for 5 minutes at 100%. The analytical column was re-equilibrated at 2% B for 2 minutes before the subsequent injection. The column effluent was continuously directed into a Q-Exactive mass spectrometer operating in positive mode by the application of a potential of 1900 V to the front-end of the analytical column through a tee piece.
A top-12 method was used for data-dependent acquisition. Full MS scans at a resolution of 70 000 were followed by the HCD fragmentation (normalized collision energy of 25%) of the 12 most abundant precursors from the Full MS events. MS/MS spectra were acquired at a resolution of 17 500. AGC target and maximum injection time for full MS and MS/MS were 1e6/1e5 and 50 ms/60 ms, respectively. The intensity threshold for triggering MS/MS events was set at 1.7e4. Precursors were excluded from any further MS/MS fragmentation for 30s.
The MS data were processed using Proteome Discoverer v.1.4 (Thermo Scientific) and SEQUEST as search engine. Proteins were identified by searching the mass spectrometric data against a decoy version of the Uniprot Plantae database (3 090 757 entries) accessed on November 2015 as sequence database. Carbamidomethylation of cysteines (+57.021 Da) and oxidation of methionines were (+15.995 Da) set as fixed and variable modifications, respectively. The following search parameters were used: MS tolerance 15 ppm; MS/MS tolerance 0.02 Da; enzyme trypsin; max. missed cleavages 2; high confidence peptides (confidence >99%) were filtered out, using Percolator, integrated in Proteome Discoverer. Protein hits based on 2 successful peptide identifications were considered valid.
2.9 EUROLINE
In the 55 walnut challenged subjects, sensitization to recombinant Jug r 4.0101 was assessed using a line blot (EUROLINE, EUROIMMUN, Lübeck, Germany) according to manufacturer's instructions. EUROIMMUN kindly provided the line blots and reagents. Briefly, the test strips were incubated at room temperature on a rocking shaker overnight patient sera diluted 1:11 in working strength universal buffer (WSUB). Bound antibodies were visualized with an enzyme-labelled anti-human IgE antibody in combination with the substrate nitro-blue tetrazolium/5-bromo-4-chloro-3′-indolyphosphate. The reaction was evaluated using the software “EUROLineScan”. The intensity of the bands was reported as an intensity level and a class, corresponding to the Enzyme-Allergo-Sorbent Test classification (class 0-6).10 A class of 1 or higher, corresponding to intensity level of 3 or higher, was considered positive.
2.10 Data analysis and statistics
The immunoblot results were independently scored by 3 researchers, blinded to challenge and sensitization results. Discrepancies in scores were discussed in a panel meeting until agreement was reached. To assess the performance of the diagnostic tests, sensitivity, specificity, positive and negative predictive value were calculated. The diagnostic value was determined by calculating the area under the curve (AUC) of the receiver operating characteristic (ROC) curve. All analyses were performed using SPSS Statistics 21 (IBM Corporation, Armonk, NY, USA).
3 RESULTS
3.1 IgE binding proteins identified as Jug r 1, Jug r 2 and Jug r 4
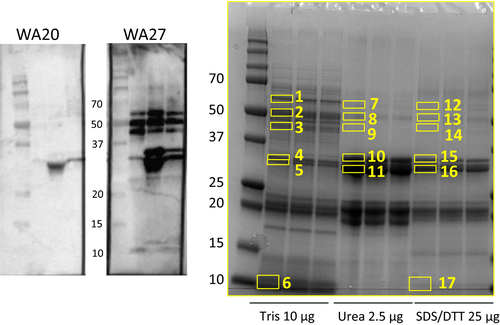
As described earlier, sera from 8 allergic subjects were selected, based on varying sensitization profiles of Jug r 1, Jug r 2 and Jug r 3 (Table S1), to explore walnut protein recognition by IgE. Figure 1 shows two representative immunoblots using the 3 reduced walnut extracts (PBS, Urea and SDS/DTT). The right blot demonstrated IgE binding to several distinct protein bands around 50, 35 and 10 kD. Most subjects showed the same IgE binding profile except for 3, who only showed binding to the 35 kDa band in the urea and SDS/DTT extract (left blot). The bands with IgE binding proteins in the 8 immunoblots were exercised from an SDS-PAGE gel (Figure 1) and analysed using LC-MS. The bands were identified as fragments of Jug r 1 and 3 (band 6) around 10-15 kD, Jug r 2 for the bands between 40-60 kD (band 1-3, 7-9, 12-14) and Jug r 4 around 30 kD (mainly band 11) (Table S2). The bands representing Jug r 4 had the strongest presentation in the Urea extract. For the immunoprecipitation experiment using magnetic beads, 2 sera with high sIgE levels against walnut and clear IgE binding bands on the immunoblot (subject 8 and 27) were selected. This experiment confirmed the presence of specific IgE against Jug r 4, as well as Jug r 1 and 2 (Table S3).
3.2 Jug r 4 detected in subgroup of walnut challenged subjects
As sensitization to Jug r 4 had not yet been investigated in our previously characterized cohort of 55 walnut challenged subjects,1 we investigated Jug r 4 sensitization by 2 methods: immunoblotting and line blotting (EUROLINE). The immunoblots were performed with the Urea extract, due to its strong representation of Jug r 4, as well as purified nJug r 4 to confirm our findings from the extract. For the urea extract, scoring was focused on Jug r 4 bands while the purified Jug r 4 was used to confirm the finding of the extract. EUROLINE line blots were performed with rJug r 4. Four immunoblots could not be interpreted and were deemed inconclusive. Five subjects scored positive for nJug r 4 on immunoblot and 10 were positive for rJug r 4 on EUROLINE (Table 1, Figure S1 and Table S4). All 5 subjects positive on immunoblot were positive on EUROLINE as well. Of the 10 Jug r 4 sensitized subjects on EUROLINE, all but one had a positive food challenge. This results in a sensitivity and specificity of 27% and 95%, respectively, and a positive and negative predictive value of 90% and 47%, respectively. ROC curve analysis returned an AUC value for rJug r 4 of 0.61.
| Subject | Age | Sex | Walnut challenge (symptoms) | Immunoblot nJug r 4 (+/−) | EUROLINE rJug r 4 (class) | Walnut extract (kU/L) | CAP rJug r 1 (kU/L) | ISAC nJug r 2 (ISU) | CAP rJug r 3 (kU/L) |
|---|---|---|---|---|---|---|---|---|---|
| 8 | 20 | M | U, AE, R, C, Dy | + | 6 | 11 | 6.0 | 0.7 | 0.1 |
| 20 | 21 | F | OAS, U, AE, A | + | 1 | 9.7 | 6.0 | <0.3 | <0.1 |
| 25 | 25 | F | OAS, U, A, Dy | + | 4 | 51 | 45 | 0.5 | <0.1 |
| 26 | 23 | F | OAS, A | + | 1 | 11 | 6.6 | <0.3 | 0.2 |
| 27 | 20 | F | OAS, U, A, Dz | + | 2 | 51 | 58 | 0.4 | 0.2 |
| 40 | 27 | F | OAS, A | − | 1 | 8.3 | <0.1 | <0.3 | 1.0 |
| 46 | 32 | M | OAS, A, AE, P | − | 1 | 7.2 | 6.2 | 1.3 | <0.1 |
| 56 | 30 | M | OAS, A, V | − | 1 | 3.4 | 0.3 | <0.3 | <0.1 |
| 57 | 35 | F | OAS, A, D | − | 1 | 6.3 | 5.0 | <0.3 | <0.1 |
| 64 | 37 | F | Negative | − | 2 | 2.0 | 1.1 | <0.3 | <0.1 |
- OAS, oral allergy symptoms; U, urticaria; AE, angioedema; R, rhinitis; C, conjunctivitis; Dy, dyspnoea; A, abdominal pain/nausea; Dz, dizziness; P, pruritus; V, vomiting; CAP, ImmunoCAP; ISAC, ImmunoCAP ISAC.
3.3 Jug r 4 not detected in walnut extract on ImmunoCAP
No Jug r 4 (and Jug r 3) could be detected in the ImmunoCAP walnut extract, using LC-MS analyses (Table S5). Other allergens including Jug r 1 and jug r 2 could be distinctly identified.
3.4 Jug r 4 co-sensitizes with Jug r 1 and other 11S globulins
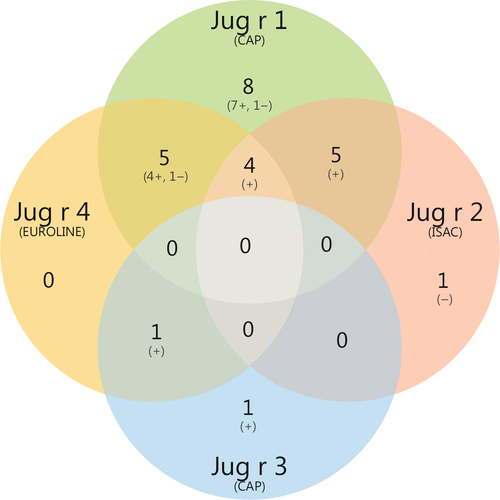
Co-sensitization to other walnut components was present in all subjects sensitized to rJug r 4 (Table 1), mostly to Jug r 1. One subject (no. 40) did not demonstrate co-sensitization to Jug r 1, but was sensitized to Jug r 3. In this subject, there was no sensitization to other non-specific lipid transfer proteins (nsLTPs) on ImmunoCAP ISAC (data not shown). Twenty-five, of 55 challenged subjects, were sensitized to at least one of the walnut components Jug r 1, 2, 3 or 4 (Figure 2). Eighty-eight per cent of these subjects were sensitized to Jug r 1. Mono-sensitization occurred mostly to Jug r 1 (n = 8) and none were mono-sensitized to Jug r 4. Fifteen (60%) were sensitized to more than one walnut component, of which 14 had a positive challenge. Four (16%) were sensitized to 3 components, namely Jug r 1, 2 and 4, and all had a positive challenge. Of the 33 walnut allergic subjects, 11 were not sensitized to any of the 4 components.
To assess potential cross-reactivity, sensitization to other 11S globulins on ImmunoCAP ISAC was assessed in the 10 subjects sensitized to rJug r 4 (Table 2). Co-sensitization to hazelnut Cor a 9 was most frequent (6 of 10), followed by cashew Ana o 2, soy Gly m 6 and peanut Ara h 3.
| Subject | Cashew rAna o 2 (ISU) | Hazelnut nCor a 9 (ISU) | Peanut rAra h 3 (ISU) | Soy nGly m 6 (ISU) |
|---|---|---|---|---|
| 8 | 1.0 | 0.6 | 5.6 | 6.9 |
| 20 | 0 | 0 | 0 | 0 |
| 25 | 18.7 | 2.6 | 0.5 | 1.1 |
| 26 | 1.5 | 0.7 | 0 | 0.4 |
| 27 | 0.8 | 1.4 | 0 | 0.4 |
| 40 | 0 | 0 | 0 | 0 |
| 46 | 0 | 0.3 | 0 | 0 |
| 56 | 0 | 0 | 0 | 0 |
| 57 | 0 | 0 | 0.5 | 0 |
| 64 | 0 | 0.4 | 0 | 0 |
4 DISCUSSION
In this study, we screened walnut allergic adults, using both immunoblotting and immunoprecipitation combined with LC-MS analyses, to identify and confirm IgE binding to walnut proteins other than the previously tested Jug r 1, 2 and 3. This way we demonstrated that Jug r 4 is a commonly recognized walnut allergen, in addition to allergens currently commercially available for specific IgE testing (Jug r 1, 2 and 3). Sensitization to Jug r 4 occurred in 27% of the walnut allergic subjects, compared to 61% for Jug r 1. In all but one Jug r 4 positive subject, co-sensitization to Jug r 1 was found. This makes Jug r 4 a relatively minor, but relevant allergen.
To our knowledge, this is the first study investigating the diagnostic value of specific IgE against Jug r 4 in a cohort of walnut challenged adults. Upon first characterization, Wallowitz et al demonstrated sensitization to rJug r 4 in 21 of 37 walnut allergic subjects (57%) from the United States. These patients were not challenged, but selected based on a history of severe systemic reactions to walnut.11 This could explain the difference in Jug r 4 sensitization, as our study also included subjects with only very mild, oral allergy symptoms, which could be the result of Bet v 1-related walnut allergy. Recently, Zhang et al12 isolated and characterized an 11S globulin from black walnut (Juglans nigra; Jug n 4), which had a 91% sequence identity with Jug r 4. Extensive cross-reactivity between English walnut cultivars and other Juglans species, including black walnut, has been demonstrated before.13 Zhang et al12 showed, using immunoblotting experiments, that 9 of 27 (33%) sera of walnut allergic patients from the United States were positive for nJug n 4, which is similar to the 27% rJug r 4 we found in our cohort. Asero et al investigated sensitization to walnut extract using immunoblot analysis in 7 walnut allergic subjects not sensitized to cross-reacting allergens (ie PR-10/LTP). They found a heterogeneous response without distinct bands around the molecular weight of Jug r 4 under non-reduced conditions (58 kD).14
Sensitization to Jug r 4 has a high positive predictive value (PPV) for walnut allergy (90%). This is similar to what we previously established for Jug r 1 sensitization (PPV 91% on CAP ≥0.1 kU/L).1 However, as it was recognized by a minority of subjects, it only has a low discriminative ability (AUC 0.61) compared to 0.78 and 0.79 for Jug r 1 and walnut extract on ImmunoCAP, respectively.1 In our cohort, 9 out of 10 Jug r 4 sensitized subjects were co-sensitized to Jug r 1. Due to the lack of mono-sensitization to Jug r 4, it remains unclear what the role and contribution of sensitization to Jug r 4 is in the clinical presentation of walnut allergy. Clinically irrelevant Jug r 4 sensitization occurred in our cohort in only one subject (Table 1) with a negative challenge, notably also with concomitant (irrelevant) sensitization to Jug r 1. In our 11 walnut allergic adults not sensitized to any of the available component, as discussed in the introduction, no sensitization to Jug r 4 was found. In these subjects, other walnut allergens are expected to play a role.
In the total cohort, co-sensitization to multiple walnut allergens was very common. Interestingly, co-sensitization to 2 or more walnut components (Jug r 1, 2, 3 and/or 4) was strongly associated with a positive challenge (14 of 15; 93%). Additionally, all 4 subjects co-sensitized to 3 components (Jug r 1, 2 and 4) had a positive challenge. Additionally, there appeared to be a trend of more severe symptoms (Mueller 2-3 vs 0-1) in subjects co-sensitized to more than one walnut component, but this was not statistically significant (data not shown).
We also found that the walnut extract on ImmunoCAP appears to contain Jug r 1 and Jug r 2, but lacks Jug r 4 (and Jug r 3). All our Jug r 4 sensitized subjects had sIgE against the walnut extract on ImmunoCAP, which can be explained by co-sensitization to other walnut allergens. Subject 40, however, sensitized to Jug r 3 and 4, also was sensitized to the walnut extract (Table 1). It therefore remains unknown which allergen in the walnut extract was recognized by this subject.
Overall, we found a decent concordance between nJug r 4 on the immunoblots and the EUROLINE blots with rJug r 4. The 2 subjects with high intensity levels (class 3 or higher) on EUROLINE were also positive on the immunoblot. The discrepancies found between the 2 platforms can have several causes. Firstly, the immunoblot might be less sensitive than the EUROLINE method. This could explain multiple class 1 sensitizations on EUROLINE, which were not detected on the immunoblots. Secondly, borderline-positive results (EUROLINE class 1) could be false-positives. However, 2 subjects with a positive EUROLINE (class 1) were confirmed by a positive immunoblot result. Additionally, one subject was negative on immunoblot and class 2 positive on EUROLINE. Ultimately, different results from 2 different test platforms can be expected, as in the immunoblot experiment was performed under reducing conditions with native Jug r 4 and the EUROLINE line blot used a recombinant form of Jug r 4 under non-reduced conditions. These differences could influence IgE binding due to protein folding/unfolding, presence of different isoforms and post-translational modifications and thus disappearance or modification of IgE epitopes.
Jug r 4 is known to consist of an acidic and a basic subunit. Under reducing conditions, these subunits can be analysed as separate bands on the immunoblot.11 In our study we found, in the 5 positive immunoblots with reduced extracts, only IgE binding to the acidic subunit around 30 kD. In contrast, Wallowitz et al and Zhang et al reported also sIgE binding to the basic subunit of Jug r 4 and Jug n 4, respectively, alone or to both subunits, using sera from walnut allergic subjects.11, 12
Jug r 4 has sequence identity with 11S globulins from other tree nuts, such as hazelnut Cor a 9 (63%) and cashew Ana o 2 (53%).11 Previous epitope mapping and 3D structural analysis of Jug r 4 also revealed similar IgE binding sites on 11S globulins from cashew, hazelnut, peanut and soya bean,15, 16 potentially leading to cross-reactivity. Also for sesame 11S globulin Ses i 6, in vitro cross-reactivity with Jug r 4 was previously established by inhibition immunoblotting using sera of 37 patients with a history of severe reactions to peanut, walnut and/or sesame.17 In our study, co-sensitization to one or more 11S globulins from these 4 foods on ImmunoCAP ISAC was observed in 7 subjects, further supporting the potential role of cross-reactivity between 11S albumins from these foods. Overall, co-sensitization to hazelnut Cor a 9 occurred most frequently. Previously, we established cross-reactivity between Cor a 9 and peanut Ara h 3 using ImmunoCAP inhibition, although only in few cases.18 Notably, the 2 subjects with the strongest sensitization (ie highest intensity values) to Jug r 4 on EUROLINE (subject 8 and 25) both demonstrated co-sensitization to all 4 other 11S globulins on ISAC. In the EuroPrevall study, a strong correlation was found between Cor a 9 and walnut extract in subjects from Prague as well as from our centre in Utrecht, but not in other centres.19 This can be explained by the apparent absence of Jug r 4 from the ImmunoCAP extract as established in this study. The lack of Jug r 4 in the ImmunoCAP extract could be the result of poor solubility of Jug r 4. Jug r 4 requires a high salt buffer (at least 2 mol/L NaCl) for complete solubility.
In conclusion, we established that walnut 11S globulin Jug r 4 is a relevant allergen in walnut allergy and is not present in the commercially available ImmunoCAP extract. Specific IgE against Jug r 4 occurred in a subset of subjects, mostly with concomitant sensitization to other walnut components, and has a high positive predictive value of 90% for walnut allergy. In Jug r 4 sensitized subjects, co-sensitization to other 11S globulins is common, potentially due to cross-reactivity.
ACKNOWLEDGEMENTS
The authors would like to thank Henrike Broekman (UMC Utrecht) for performing part of the immunoblot experiments.
CONFLICT OF INTEREST
W.M. Blom, G.A.H. de Jong, M. Gaspari, G.F. Houben, A.C. Knulst and K.C. Verhoeckx are members of the COST Action ImpARAS FA1402.