Current insights and molecular docking studies of HIV-1 reverse transcriptase inhibitors
Abstract
Human immunodeficiency virus (HIV) causes acquired immunodeficiency syndrome (AIDS), a lethal disease that is prevalent worldwide. According to the Joint United Nations Programme on HIV/AIDS (UNAIDS) data, 38.4 million people worldwide were living with HIV in 2021. Viral reverse transcriptase (RT) is an excellent target for drug intervention. Nucleoside reverse transcriptase inhibitors (NRTIs) were the first class of approved antiretroviral drugs. Later, a new type of non-nucleoside reverse transcriptase inhibitors (NNRTIs) were approved as anti-HIV drugs. Zidovudine, didanosine, and stavudine are FDA-approved NRTIs, while nevirapine, efavirenz, and delavirdine are FDA-approved NNRTIs. Several agents are in clinical trials, including apricitabine, racivir, elvucitabine, doravirine, dapivirine, and elsulfavirine. This review addresses HIV-1 structure, replication cycle, reverse transcription, and HIV drug targets. This study focuses on NRTIs and NNRTIs, their binding sites, mechanisms of action, FDA-approved drugs and drugs in clinical trials, their resistance and adverse effects, their molecular docking studies, and highly active antiretroviral therapy (HAART).
Abbreviations
-
- ABCMP
-
- Abacavir monophosphate
-
- AIDS
-
- Acquired immune deficiency syndrome
-
- ART
-
- Antiretroviral therapy
-
- ARV
-
- Antiretroviral
-
- CBVMP
-
- Carbovir monophosphate
-
- CBVTP
-
- Carbovir triphosphate
-
- DAPYs
-
- Diarylpyrimidines
-
- DNA
-
- Deoxyribonucleic acid
-
- dNTPs
-
- Deoxyribonucleotide triphosphate
-
- FDC
-
- Fixed-dose combinations
-
- HAART
-
- Highly active antiretroviral therapy
-
- HIV
-
- Human immunodeficiency virus
-
- INIs
-
- Integrase inhibitors
-
- INSTI
-
- Integrase strand transfer inhibitor
-
- LTRs
-
- Long terminal repeats
-
- NC
-
- Nucleocapsid
-
- NDP
-
- Nucleoside diphosphate
-
- NRTIs
-
- Nucleoside reverse transcriptase inhibitors
-
- NNRTIs
-
- Non-nucleoside reverse transcriptase inhibitors
-
- NNIBP
-
- Non-nucleoside reverse transcriptase inhibitor binding pocket
-
- NtRTIs
-
- Nucleotide RT inhibitors
-
- PBS
-
- Primer binding site
-
- PDR
-
- Pretreatment drug resistance
-
- PPT
-
- Poly-purine tracts
-
- RT
-
- Reverse Transcriptase
-
- RNaseH
-
- Ribonuclease H
-
- RNA
-
- Ribonucleic acid
-
- TDRTI
-
- Translocation- defective reverse transcriptase inhibitors
-
- UNAIDS
-
- The Joint United Nations Programme on HIV/AIDS
1 INTRODUCTION
Human immunodeficiency virus (HIV) causes acquired immunodeficiency syndrome (AIDS), a dangerous global disease (Bhatti et al., 2016). HIV belongs to the Retroviridae family, subfamily Orthoretrovirinae, and genus Lentivirus. It infects immune system cells, including T cells, lymphocytes, and macrophages, diminishing the immunity of infected persons. HIV has two subtypes: HIV-1 and HIV-2. HIV-1 is more virulent and pathogenic than HIV-2 and responsible for most human infections (Zulfiqar et al., 2017).
HIV infection is difficult to detect in the initial asymptomatic stages (Jones et al., 2019). Antiretroviral therapy is required for individuals with CD4+ T-cell counts <200 cells/μL or a viral load greater than 100,000 copies/mL (Downs et al., 2017). HIV-1 has a reverse transcriptase (RT) enzyme that synthesizes double-stranded DNA from single-stranded viral RNA (Ding et al., 2018). Antiretroviral agents terminate the growing DNA (Morse & Nanzigu, 2016). Viral RT is an important target for HIV drugs (Corona et al., 2016).
Nucleoside reverse transcriptase inhibitors (NRTIs) were the first class of approved antiretroviral drugs. Protease inhibitors (PIs) were the second. Subsequently, new non-nucleoside reverse transcriptase inhibitors (NNRTIs) were approved as anti-HIV drugs. Both NRTIs and NNRTIs are reverse transcriptase inhibitors (de Béthune, 2010). According to the Joint United Nations Programme on HIV/AIDS (UNAIDS) data, in 2021, 6.5 million people died with HIV worldwide. In 2021, 38.4 million people were living with HIV. 36.7 million were adults, and 1.7 million were children under 15 years of age (Organization, 2017).
This review addresses HIV-1 structure, replication cycle, reverse transcription, and HIV drug targets. This study focuses on NRTIs and NNRTIs, their binding sites, mechanisms of action, FDA-approved drugs and drugs in clinical trials, their resistance, adverse effects, and highly active antiretroviral therapy (HAART). In addition, we have performed molecular docking studies of NRTIs and NNRTIs against HIV RT to understand their binding patterns in the protein active site.
1.1 HIV-1 structure
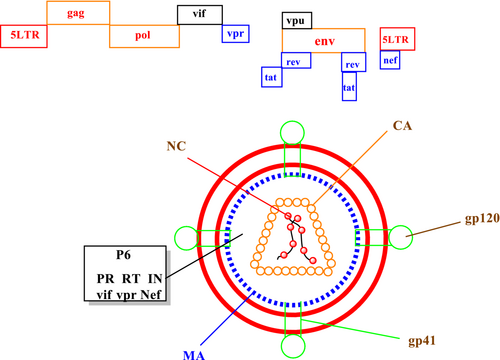
The HIV-1 virion is spherical, with a 100–130 nm diameter, surrounded by a lipoprotein-rich membrane. HIV virion contains two main components: two identical molecules of viral RNA (genome) and capsid—enzymes RT, ribonuclease H (RNaseH), and IN bound with the nucleic acid. The HIV genome contains the typical retroviral genes env, gag, and pol. HIV virion contain an outer lipid bilayer membrane called an envelope. Envelope is a trimer of the Env protein, has 72 knobs, and encodes external surface protein gp120 and the transmembrane protein gp41 (Fanales-Belasio et al., 2010).
The gp120 is the co-receptor-binding site, while gp41 contains the fusion machinery. The cone-shaped capsid includes an 80 amino acid C-terminal domain and a 150 amino acid N-terminal domain. It has two single strands of viral RNA with positive polarity and a length close to 9749 nucleotides. The ≈10 kb of RNA is associated with nucleocapsid proteins and the RT and integrase (Kirchhoff, 2013). The HIV genome contains the typical retroviral genes gag, pol, and env. The gag gene is transcribed and translated into the p55 and p160 gag precursor polyprotein. These proteins are essential for viral assembly, budding, virion structure, and early entry support maturation of the HIV particle. Upon development, it is cleaved into the p6gag, nucleocapsid (NC/p7gag), matrix (MA, p17gag), and capsid (CA/p24gag). A single gene, pol, encodes the three HIV enzymes: reverse transcriptase, integrase, and protease. Reverse transcriptase transcribes the RNA genome. Integrase integrates the viral cDNA into the host genome, and protease cleaves the Gag and Gag-Pol polyproteins during maturation (Swanson & Malim, 2008).
HIV also has two regulatory proteins, Tat and Rev. Tat enhances proviral transcription and accelerates the virus production rate approximately 100-fold. Rev is responsible for exporting incompletely or unspliced viral mRNAs into the cytoplasm and newly formed HIV RNA splicing length. HIV virion has four accessory proteins, negative factor (Nef), viral protein U (Vpu), viral protein R (Vpr), and viral infectivity factor (Vif). Nef causes 10 CD4 deficient. Vpu, Vpr, and Vif influence and accelerate virus particle production rate. HIV-1 virus capsid structure and genome are depicted in Figure 1 (Kirchhoff, 2010; Zhao et al., 2011).
1.2 The HIV replication cycle
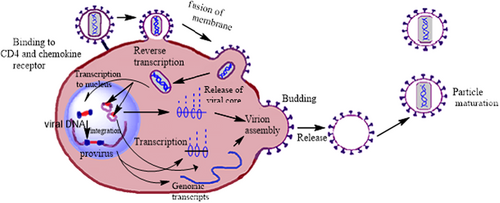
The HIV life cycle was known at the end of the 20th century. The HIV-1 life cycle consists of different events, such as viral entry into the host cell and fusion, virus replication, integration, assembly, budding, and maturation. All seven steps are arbitrarily divided into two distinct phases: the early and late phases (Nisole & Saïb, 2004). The replication cycle consists of multiple steps detailed in the following sections.
1.2.1 Binding and fusion
The HIV-1 life cycle begins with the virus binding to the host cell and its entry (Malik et al., 2017). In this step, the viral envelope glycoproteins attach to the T lymphocyte CD4 receptors and bind to chemokine co-receptors CCR5 or CXCR4. After binding two viral envelope glycoproteins, gp120 and gp41, fusion into host cell membranes leads to the viral genome transformation into the host cell (Boyd & Pett, 2008).
1.2.2 Reverse transcription
The reverse transcription process converts the HIV-1 RNA genome into double-stranded DNA by viral reverse transcriptase (Jonckheere et al., 2000). Reverse transcriptase contains three sequential enzymatic biochemical activities: (1) RNA-dependent DNA polymerase activity: RNA-dependent DNA polymerase enzyme synthesizes DNA using viral RNA as a template hybridization of the protein binding site. (2) RNaseH: RNaseH causes the removal or cleavage of the genomic RNA strand from the RNA–DNA hybrid double helix by a hydrolytic mechanism. (3) DNA-dependent DNA polymerase activity: DNA-dependent DNA polymerase is essential for DNA replication and completes double-stranded DNA synthesis (Munk et al., 2017).
1.2.3 Integration
Integration reaction consists of the viral DNA insertion into the host cell genome. The first step of this process is the translocation in which newly generated viral dsDNA is bound to various viral and cellular factors. Transcription and translation are the last steps in this process. From transcription, only short viral messengers are produced. Full-length viral messengers are made only after successfully translating the viral trans-activating factor. In the integration process, ultimately, the formation of proviruses (Colin & Van Lint, 2009).
1.2.4 Assembly, budding, and maturation
These are the last three phases of the HIV-1 life cycle that leads to the release of infectious viral progeny. The protease enzyme is the crucial element in this process (Kapila et al., 2016). New virions assemble and bud from the plasma membrane. Budding virions are released immaturely, and proteolysis is required to achieve infectivity. The viral protease enzyme domain of pol and sequential proteolysis of gag cleave HIV polyproteins into individual subunits, producing infectious, mature virions that can enter and replicate in a new host cell (Marsden & Zack, 2013). The HIV-1 life cycle is depicted in Figure 2.
1.3 HIV reverse transcription
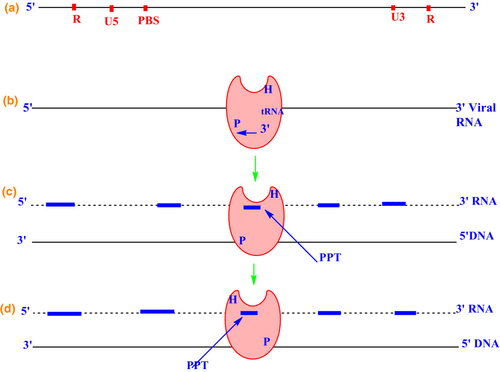
Reverse transcription begins when the viral particle enters the cytoplasm of a target cell (Coffin et al., 1997). The HIV RT contains a ribonuclease enzyme that catalyzes the degradation of the viral RNA. DNA-dependent DNA polymerase is essential for DNA replication that copies the antisense cDNA strand synthesized from mRNA into a sense DNA strand to form a double-stranded viral DNA intermediate. This newly formed DNA integrates into the host cell genome and becomes a transmissible element. Subsequent transcription into RNA completes the cycle (Schinazi et al., 2013).
The process of reverse transcription in HIV-1 begins with synthesizing the minus strand at the 5′ end of the plus strand by tRNA Lys3 to the primary binding site on the 5′ ends (Arts et al., 1996). RT performs its DNA polymerase function using viral RNA as a template. After the hybridization of the protein binding site, the tRNA Lys3 begins the reverse transcription process for the synthesis of DNA. As the DNA synthesis reaches the 5′ end of the single-stranded RNA, it forms the first minus-strand strong-stop DNA (Soni et al., 2013). Transfer of this nascent cDNA strand from the 5′ end of the genome to the 3′ end is required to continue the synthesis of complete minus-strand cDNA (Galvis, 2014). Then a stop codon is generated, and RNA is cleaved by RNaseH (Esposito et al., 2012). Figure 3 depicts the reverse transcriptase process in HIV-1.
1.3.1 Accessory proteins in the reverse transcription process
HIV-1 nucleocapsid protein
The proteolytic activity of the HIV-1 protease from the Gag and Gag-Pol polyprotein precursor generates the nucleocapsid protein (NC) of HIV-1.
Other viral proteins
Besides the nucleocapsid protein, several other viral proteins are present in the nucleoprotein complex, which can influence the reverse transcription process. The viral Vpr interacts with the cellular Lys-tRNA synthetase, consequently inhibiting its enzymatic activity. The accessory proteins Nef and Vif have also been shown to affect reverse transcription. Tat may facilitate interactions between TAR RNA and other RNA elements involved in reverse transcription (Jonckheere et al., 2000).
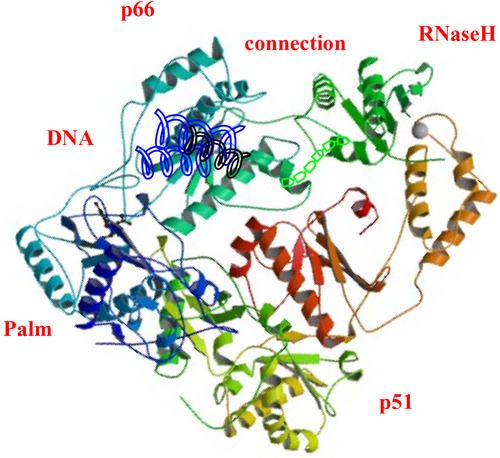
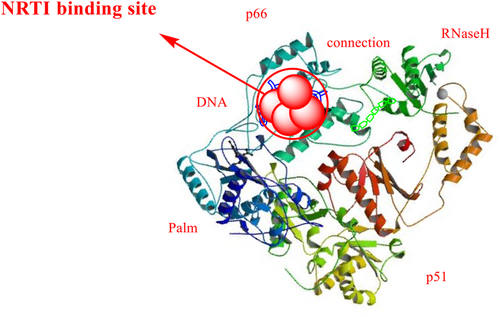
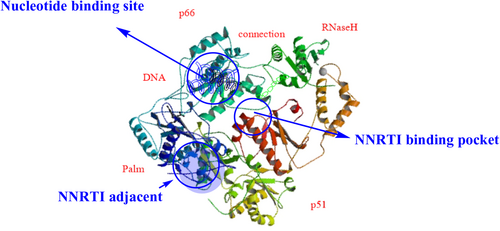
Reverse transcriptase is a heterodimer consisting of a polypeptide chain of two subunits, p51 and p66. These subunits have different tertiary structures but the same coding sequence. The p66 subunit contains (66 kDa, 560 amino acid residues), and the p51 subunit contains (51 kDa, 440 amino acid residues; Slack et al., 2019). The p66 chain contains two domains: (1) the N-terminal polymerase domain and (2) the C-terminal RNaseH domain (15 kDa). It has four catalytic acidic residues Asp443, Glu478, Asp498, and Asp549. The polymerase domain of the p66 subunit contains a “right hand” shape with a finger (1–85 and 118–155), palm (residues 86–117 and 156–236), thumb (237–318), and connection (319–426) subdomains. The polymerase active site is 60 Å away from the RNaseH at the other end. The DNA duplex passes by the RNaseH active site at a distance of at least 5.5 Å (Ilina et al., 2012). It has been proposed that the p51 subunit's relatively rigid structure is formed by the cleavage of viral protease from the p66 subunit. The p51 subunit lacks the C-terminal RNaseH domain. The subdomains of p51 provide structural support to p66. The p66 subdomains are flexible and responsible for the functional activity of RT (Das et al., 2012; Das & Arnold, 2013; Hughes, 2001). The structure of the HIV-1 RT is depicted in Figure 4.
2 HIV DRUG TARGETS
There are many drug targets in HIV, including reverse transcriptase. Food and Drug Administration (FDA) approved anti-HIV drugs can be divided into many groups, including co-receptor inhibitors (CRIs), fusion inhibitors (FIs), protease inhibitors (PIs), capsid Inhibitors, and integrase inhibitors (INIs), and RT inhibitors, including NRTIs, nucleotide RT inhibitors (NtRTIs), and NNRTIs. This review focuses on RT inhibitors as anti-HIV agents.
3 NUCLEOSIDE REVERSE TRANSCRIPTASE INHIBITORS
Both NRTIs and NtRTIs bind at the nucleotide-binding site with the same catalytic site substrate-binding site of the enzyme. NRTIs and NtRTIs are active in phosphorylated form, the NRTIs triphosphate and NtRTIs diphosphate forms (De Clercq, 2009). The NRTI binding pocket is shown in the Figure 5.
Nucleoside reverse transcriptase inhibitors were the first class of HIV treatment to be approved. They are preferred as first-line drugs because of the favorable pharmacokinetic profile, high oral bioavailability, and availability as fixed-dose combinations (FDC) with convenient once- or twice-daily dosage schedules. They also have a low risk of drug–drug interactions and an incredibly long intracellular half-life (Desai et al., 2012).
3.1 Mechanisms of nucleoside reverse transcriptase inhibitor activity
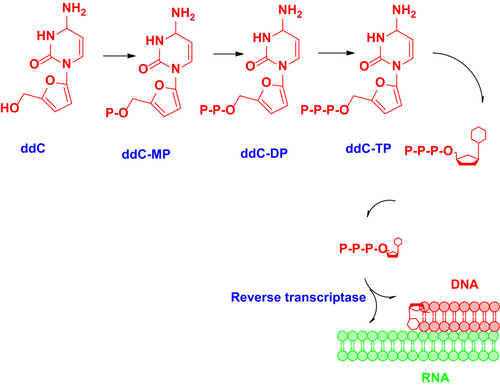
Nucleoside reverse transcriptase inhibitors inhibit the reverse transcription of viral RNA to DNA by inhibiting viral RT, resulting in DNA chain termination in the early stages of the viral life cycle. They are structurally similar to the naturally occurring building blocks of DNA and nonfunctional until they are metabolized into their active di- or triphosphate form before reverse transcriptase inhibition. Metabolism of NRTIs depends on the level of endogenous deoxyribonucleotide triphosphate (dNTP) and activated NRTI derivatives (De Clercq, 1995). All the NRTIs are derivatives of 2′,3′-dideoxynucleoside (ddN) analogs. They undergo biotransformation to form a triphosphate active metabolite. They act as competitive inhibitors of the binding of activated drugs to the enzyme–template–primer complex (N Sluis-Cremer et al., 2000). The mechanism of action of zalcitabine is depicted in Figure 6.
3.2 Food and Drug Administration-approved nucleoside reverse transcriptase inhibitors
The FDA has approved 43 antiretroviral drugs during the last 30 years, including 14 FDCs (Zhang, 2018). FDA-approved NRTIs are depicted in Figure 7.
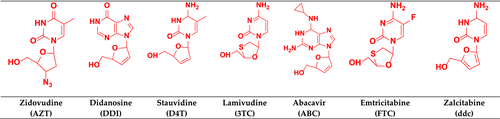
3.2.1 Zidovudine
Zidovudine (AZT), a thymidine analog, was FDA-approved in 1987 and was the first anti-HIV drug (Akanbi et al., 2012). Zidovudine acts by inhibiting viral replication through two mechanisms. Firstly, it inhibits the incorporation of thymidine into viral DNA by competitively inhibiting viral RT. Secondly, it prevents additional phosphodiester linkages to its unreactive 3′-azido group (Langtry & Campoli-Richards, 1989).
3.2.2 Didanosine
This purine analog was approved in 1991 and is degraded by gastric acid. It is available as enteric-coated tablets or powder formulations buffered with antacids (Chappuy et al., 2004). Pancreatitis and peripheral neuropathy are the significant toxicities associated with didanosine (DDI; Zapor et al., 2004). Recently, retinal degeneration has also been seen as didanosine toxicity (Haug et al., 2016).
3.2.3 Stauvidine
This thymidine analog was approved in 1994. It has good bioavailability and fewer interactions with food (Akanbi et al., 2012). Sensory neuropathy is the most frequent manifestation of stavudine (D4T; Huang et al., 2013). It is more neurotoxic than didanosine and zalcitabine (Bhatia & Chow, 2016).
3.2.4 Lamivudine
This thymidine analog was approved in 1995. Its negative enantiomer is active and has good oral bioavailability (Yuen et al., 1995). Lamivudine (3TC) is generally well tolerated and has side effects like those reported with other NRTIs [42].
3.2.5 Abacavir
This thymidine analog was approved in 2001 (Yuen et al., 2008). Cytochrome P450 enzymes do not metabolize abacavir and have no effects on these enzymes (Zapor et al., 2004). Abacavir (ABC) is firstly phosphorylated to abacavir monophosphate (ABCMP) by an adenine phosphotransferase, followed by deamination to carbovir monophosphate (CBVMP) and its active metabolite carbovir triphosphate (CBVTP; Foster & Faulds, 1998).
3.2.6 Emtricitabine
Emtricitabine (FTC) is converted intracellularly to emtricitabine 5′-triphosphate, which inhibits the activity of HIV-1 RT and hepatitis B disease (Bang & Scott, 2003). As a component of antiretroviral therapy (ART), emtricitabine effectively reduces or maintains viral load suppression (Frampton & Perry, 2005).
3.2.7 Zalcitabine
Zalcitabine (ddc) is also known as dideoxycytidine. Its triphosphate metabolite inhibits the replication of HIV (Whittington & Brogden, 1992). Peripheral neuropathy is its most frequent dose-limiting adverse effect but is generally reversible. Lactic acidosis and severe hepatomegaly with steatosis are also adverse effects of zalcitabine (Adkins et al., 1997).
3.3 Resistance against nucleoside reverse transcriptase inhibitors
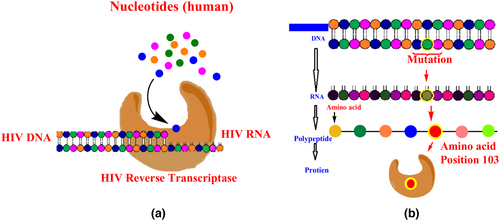
HIV resistance against NRTIs can develop via two distinct paths: discrimination and hydrolytic removal. The discriminatory mutations occur due to impaired NRTI binding and incorporation, allowing the RT enzyme to discriminate against NRTIs during polymerization, thereby preventing their addition to the growing DNA chain. The second mechanism is mediated by mutations that promote the hydrolytic removal of the chain-terminating NRTI and enable continued DNA synthesis by a process called “primer unblocking.” (Asahchop et al., 2012). This resistance mechanism is associated with pyrophosphorolysis, nucleotide excision, and primer unblocking. Pyrophosphorolysis is enhanced by critical mutations, primarily by zidovudine and stavudine. The RT enzyme lacks 3″ exonuclease activity (Holec et al., 2017).
Characteristic mutations that occur via the “primer unblocking” pathway include T215Y/F, D67N, L210W, K70R, K219Q/E, and M41L (Tang & Shafer, 2012). Point mutations such as K65R, T69D, Q151M, and M184V/I, non-thymidine analog-associated mutations such as K65R and L74V, and the multi-nucleoside resistance mutation Q151M act by decreasing NRTI incorporation, leading to altered affinity of RT for NRTIs. These mutations are in, or close to, the dNTP substrate-binding site and may therefore affect the initial binding. M184V is the most commonly occurring NRTI resistance mutation (Sluis-Cremer et al., 2000). HIV-1 NRTI resistance is depicted in Figure 8.
3.4 Adverse effects of nucleoside reverse transcriptase inhibitors
Common side effects of this class may be due to mitochondrial toxicity (Chen et al., 1991). Renal toxicity, hepatic steatosis, lactic acidosis, and bone toxicity are also significant adverse effects resulting from the prolonged used of NRTIs (Margolis et al., 2014).
3.5 Nucleoside reverse transcriptase inhibitors under clinical trial
Many NRTIs are in preclinical and clinical development. New NRTIs are being developed against HIV-1 to decrease adverse effects, toxicity, and viral resistance (Cory et al., 2015).
3.5.1 Nucleoside reverse transcription inhibitors (chain terminators)
Apricitabine
Apricitabine (ATC), Figure 9, also known as AVX754, SPD754, or BCH-10618, is a NRTI. It differs from lamivudine only in the substitution pattern on the 1,3-oxathiolane ring (Han et al., 2018). It causes DNA chain termination to take on an active triphosphate form by inhibiting HIV-1 RT. ATC showed potent activity against M184V, L74V, and TAMs mutations (Ramesh et al., 2021; Wainberg, 2012). ATC is a novel deoxycytidine NRTI and led to full viral suppression with good tolerability (Wainberg et al., 2007).
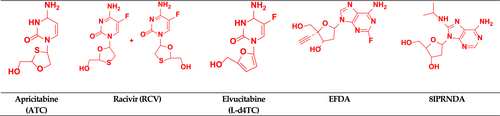
Racivir
Racivir (a cytidine analog) is an experimental drug known as RCV Figure 9. It is an oxothiolane nucleoside reverse transcriptase inhibitor and an enantiomer of emtricitabine. It is a 50:50 racemic mixture of the (−) and (+)-β-enantiomers of 2′-deoxy-3′-thia-5-fluorocytosine (FTC). Racivir was developed to treat HIV and hepatitis B virus (HBV). FTC's (−) enantiomer is approximately 10–20-fold more potent than its (+) enantiomer (Hurwitz et al., 2005; Ismail & Ayoup, 2022).
Elvucitabine
Elvucitabine (L-Fd4C, (−)-L-(1S,4R)-5-fluoro-2′, 3′-dihydro-2′,3′-dideoxycytidine) is an L-cytidine analog of stavudine Figure 9 (Bhattacharya & Osman, 2009; Kataev & Garifullin, 2021). It not only has no mitochondrial toxicity, but also has a protective effect against mitochondrial toxicity. It is active against M14V RT and K65R mutant strains (Esposito et al., 2012).
3.5.2 Nucleoside reverse transcription inhibitors with novel functional mechanisms
Some NRTIs also show innovative modes of action, such as translocation defective RT inhibition (TDRTI) and delayed chain terminator RT inhibition (DCTRTI; Esposito et al., 2012).
EFdA (islatravir) or MK-8591
EFdA (islatravir), 4′-ethynyl-2-fluoro-deoxyadenosine (also known as MK-8591), is a highly potent type 1 human immunodeficiency virus (HIV-1) NRTI Figure 9. Other approved NRTIs do not contain 3′-OH groups, but EFdA, in contrast, does have a 3′-OH group, a 4′-ethynyl, and a 2-fluoro group. EFdA works as a translocation-deficient RT inhibitor (TDRTI). Translocation of RT is blocked by incorporating EFdA-monophosphate in the template strand of the elongating DNA chain (Matthews et al., 2021; Singh et al., 2019). Phase 2b studies showed that islatravir with doravirine and lamivudine inhibits two stages of HIV replication. Islatravir has demonstrated high potency, long intracellular half-life, high tissue penetration, and a favorable resistance profile when taken orally or over extended intervals or via the subdermal implant (Coelho et al., 2019). Islatravir is currently phase 3 clinical trial (Gu et al., 2020).
8-Isopropyl-Amino-2 –Deoxy Adenosine
8-Isopropyl-Amino-2 –Deoxy Adenosine (8iPrNdA) Figure 9 is a natural deoxyribose analog with modification at the 8th position of the adenine base. A steric clash was produced by these modifications with helix αH in the thumb domain of the RT p66 subunit, causing delayed chain termination. Despite its low potency and selectivity, 8iPrNdA is an intriguing illustration of an NRTI that modifies the adenine base rather than the sugar moiety (Esposito et al., 2012; Vivet-Boudou et al., 2011).
3.6 Molecular docking studies
We performed molecular docking of the above-mentioned NRTIs against HIV RT (PDB IDs 1RTD and 3V4I) using Maestro 11.0 (Schrodinger 2016) software. The docking scores and significant interactions of NRTIs against proteins 1RTD and 3V4I are given in Tables 1 and 2, respectively. 3D interaction diagrams of the NRTIs with the best docking scores are shown in (Figures 10 and 11), respectively. In the case of 1RTD, stavudine, and didanosine, they had the highest docking scores (−14.737 and −14.703, respectively), whereas lamivudine and abacavir had the lowest docking scores (−10.711 and −10.264, respectively). The docking score of NRTIs against 1RTD protein was in the following order: stavudine > didanosine > EFDA > apricitabine > elvucitabine > zidovudine > zalcitabine > 8iPrNdA > racivir emtricitabine > lamivudine > abacavir. The phosphate group was essential in binding (H-bonding) with the receptor's active site. This H-bonding's primary amino acid residues were LYS 65, ARG 72, LEU 74, ASP 113, ALA 114, MG 600, and MG 601. The azide (N3) of zidovudine formed an H− bond with ASP 185, while the ring N of didanosine formed an H-bond with TYR 115. The carbonyl oxygen of the pyrimidine ring began an H-bond with MG 601 in the case of emtricitabine and lamivudine. GLU 40 was involved in H-bonding with the NH3+ of the pyrimidine ring with apricitabine and zalcitabine. In contrast, LYS 70 and ASP 113 were engaged in H-bonding with the carbonyl oxygen of the pyrimidine ring for apricitabine and zalcitabine. ARG 72 was involved in H-bonding with the oxathiolane ring of racivir. These H-bonding interactions (other than the phosphate group) are responsible for the high docking scores of these drugs. The furan ring of zalcitabine was involved in π–π interactions with LYS 65 and PHE 116, whereas in the case of elvucitabine, it was engaged in π–π interactions with ARG 72. The apricitabine, racivir, and EFDA furan rings were involved in π–π interactions with LYS 65, MG 601, and TYR 183, respectively. Due to these in π–π interactions, EFDA, apricitabine, elvucitabine, and zalcitabine showed better docking scores than the other NRTIs.
| Drug | Docking score (kcal/Mol) | H-bonding | π–π interactions |
|---|---|---|---|
| Zidovudine | −14.227 | LYS 65, ALA 114, LEU 74, MG 600, MG 601 (PO4−3), ASP 185 (N3) | – |
| Didanosine | −14.703 | ARG 72, LYS 65, MG 600, MG 601 (PO4−3), TYR 115 (Ring N) | – |
| Stavudine | −14.737 | ARG 72, LYS 65, ASP 113, MG 600, MG 601 (PO4−3) | – |
| Lamivudine | −10.711 | ARG 72, LYS 65, MG 600, MG 601, ASP 113, TYR 115(PO4−3), MG 601 (O of pyrimidine ring) | – |
| Abacavir | −10.264 | ARG 72, LYS 65, MG 600, MG 601, ASP 113, ALA 114 (PO4−3) | – |
| Emtricitabine | −12.955 | ARG 72, LYS 65, MG 600, MG 601 (PO4−3), MG 601 (O of pyrimidine ring) | – |
| Zalcitabine | −14.143 | ARG 72, LYS 65, ASP 113, MG 600, MG 601 (PO4−3), ASP 113, GLU 40 (O, NH3+) | LYS 65, PHE 116 (furan) |
| Apricitabine | −14.447 | LYS 65, ASP 113, MG 600, MG 601, ALA 114 (PO4−3), GLU 40, LYS 70 (NH3+, O of pyrimidine ring) | LYS 65 (pyrimidine) |
| Racivir | −12.987 | ARG 72, LYS 65, MG 600, MG 601 (PO4−3), ARG 72 (Oxathiolane) | MG 601 (pyrimidine) |
| Elvucitabine | −14.289 | ARG 72, LYS 65, MG 600, MG 601, ALA 114 (PO4−3) | ARG 72 (furan) |
| EFDA | −14.506 | ARG 72, LYS 65, MG 600, MG 601, ASP 113, ALA 114 (PO4−3) | TYR 183 (pyrimidine) |
| 8iPrNdA | −13.199 | ARG 72, LYS 65, MG 600, MG 601 (PO4−3) | – |
| Drug | Docking score (kcal/Mol) | H-bonding | π–π interactions |
|---|---|---|---|
| Zidovudine | −12.028 | ALA 114, ARG 72, LYS 65, LYS 70, LYS 219, ASP 113, MG 600 (PO4−3), ASP 185 (N3) | – |
| Didanosine | −13.475 | ARG 72, LYS 65, LYS 70, LYS 219, ASP 113, MG 600 (PO4−3), ARG 72 (Ring N) | – |
| Stavudine | −12.037 | ARG 72, LYS 65, LYS 70, LYS 219, ASP 113, MG 600 (PO4−3) | – |
| Lamivudine | −10.723 | ARG 72, LYS 65, LYS 70, LYS 219, ASP 113, MG 600, ALA 114 (PO4−3) | – |
| Abacavir | −11.516 | ARG 72, LYS 65, LYS 70, LYS 219, ASP 113, MG 600, (PO4−3), TRP153 (Amine of purine) | – |
| Emtricitabine | −11.011 | ARG 72, LYS 65, LYS 70, LYS 219, MG 600 (PO4−3) | – |
| Zalcitabine | −11.144 | ALA 114, ARG 72, LYS 65, LYS 70, LYS 219, ASP 113, MG 600 (PO4−3), TRP 153, TYR 115 (NH3+) | ARG 72 (furan) |
| Apricitabine | −11.343 | ARG 72, LYS 65, LYS 70, LYS 219, ASP 113, MG 600 (PO4−3), ASP 110, ASP 186 (NH3+ of pyrimidine ring) | MG 600 (pyrimidine) |
| Racivir | −11.553 | ALA 114, ARG 72, LYS 65, LYS 70, LYS 219, ASP 113, MG 600 (PO4−3) | – |
| Elvucitabine | −12.492 | ARG 72, LYS 65, LYS 70, LYS 219, MG 600, ASP 113 (PO4−3), GLY 152, TRP 153 (NH2 of pyrimidine ring) | ARG 72 (furan) |
| EFDA | −12.743 | ARG 72, LYS 65, LYS 70, LYS 219, MG 600, ASP 113, ALA 114 (PO4−3) | – |
| 8iPrNdA | −10.845 | ARG 72, LYS 65, LYS 70, LYS 219, MG 600, ASP 113 (PO4−3), TYR 115 (NH attached to purine) | – |
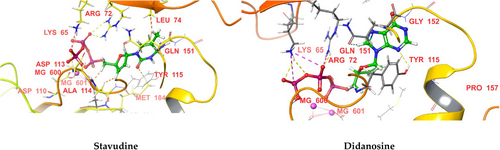
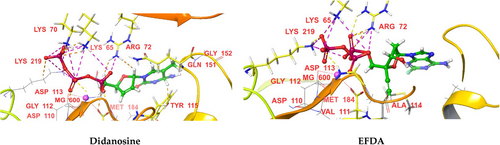
For protein 3V4I, didanosine and EFDA had the highest docking scores (−13.475 and −12.743, respectively), whereas 8iPrNdA and lamivudine had the lowest docking scores (−10.845 and −10.723, respectively). The docking scores of NRTIs against 1RTD protein were in the following order: didanosine > EFDA > elvucitabine > stavudine > zidovudine > racivir > abacavir > apricitabine > zalcitabine > emtricitabine > 8iPrNdA > lamivudine. The primary amino acid residues involved in H-bonding with phosphate groups were LYS 65, LYS 70, ARG 72, ASP 113, ALA 114, LYS 219, and MG 600. For protein 1RTD, the azide (N3) of zidovudine formed an H− bond with ASP 185, while the ring N of didanosine formed an H-bond with ARG 72. TYR 115 and TRP 153 were involved in H-bonding with the NH3+ of the pyrimidine ring of zalcitabine, whereas GLY 152 and TRP 153 formed H-bonding with the NH2 in the pyrimidine ring of elvucitabine. The NH attached to the purine moiety was involved in H-bonding with TYR 115 in the case of 8iPrNdA. The NH3+ attached to the pyrimidine moiety was involved in H-bonding with ASP 110 and ASP 186 for apricitabine. These H-bonding interactions (other than the phosphate group) are responsible for the high docking scores of these drugs. The amino group of purine was involved in H-bonding with TRP153 of abacavir, which was absent in the case of 1RTD protein and may be the reason for its better docking score against protein 3V4I. The furan ring of zalcitabine and elvucitabine was involved in π–π interactions with ARG 72, whereas the pyrimidine ring of apricitabine was involved in π–π interactions with MG 600. Due to these π–π interactions, these drugs showed better docking scores than other NRTIs. All the NRTIs had better docking scores against 1RTD than 3V4I, except lamivudine and abacavir.
4 NON-NUCLEOSIDE REVERSE TRANSCRIPTASE INHIBITORS
Non-nucleoside reverse transcriptase inhibitors are promising anti-HIV-1 reverse transcriptase inhibitors (De Clercq, 2004). The efficacy of NNRTIs is impaired by conformational flexibility and positional adaptability (Zhan et al., 2009). Due to their unique antiviral activity, high specificity, and low cytotoxicity, the NNRTIs have become an indispensable component of HAART (Chen et al., 2011).
4.1 Non-nucleoside reverse transcriptase inhibitors binding sites
Non-nucleoside reverse transcriptase inhibitors are targeted at a specific hydrophobic binding pocket about 10 Å from the polymerase catalytic site within the HIV-1 RT palm domain of the p66 subunit (Zhan et al., 2013). This binding site is located off the heterodimer protein, between the 6–10–9 and 12–13–14 sheets, and at the base of the 4–7–8 sheet, at a distance of approximately 10 Å from the catalytic site of the enzyme. This pocket is hydrophobic and is lined by five aromatic residues (Y181, Y188, F227, W229, and Y232), six hydrophobic residues (P59, L100, V106, V179, L234, and P236), and five hydrophilic residues (K101, K103, S105, D132, and E224) of the p66 subunit. It is also lined by two amino acids (I135 and E138) of the p51 subunit. The binding of an NNRTI induces a conformational change that rotates the side chains of the Y181 and Y188 amino acids up toward the catalytic site. This results in a 2 Å concomitant shift of the 4–7–8 sheet and the three catalytic aspartic acid residues that inhibit the enzymatic activity. The non-nucleoside reverse transcriptase inhibitor binding pocket (NNIBP) is flexible and elastic, and its conformation depends on the binding mode of the NNRTIs, their specific chemical composition, and their size (de Béthune, 2010). The NNRTI binding site is depicted in Figure 12. The flexibility of the binding pocket in RT results in structural and chemical diversity of NNRTIs and also provides a wide opportunity for lead discovery and optimization of new NNRTIs (Zhan et al., 2013).
4.2 Mechanism of action non-nucleoside reverse transcriptase inhibitors
Non-nucleoside reverse transcriptase inhibitors are HIV-1 specific and are inactive against HIV-2 infections (Wang et al., 2019). NNRTIs bind in a noncompetitive manner to a hydrophobic site in RT. This creates the NNIBP due to a conformational change in the RT structure (de Béthune, 2010). As a result of NNRTI binding, dNTP incorporation into DNA is blocked, preventing nascent copies from forming (Sharma et al., 2014). The mechanism of action NNRTIs is depicted in Figure 13.
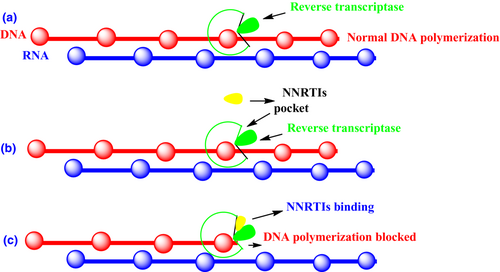
4.3 Food and Drug Administration-approved non-nucleoside reverse transcriptase inhibitors
Currently, there are six NNRTIs approved by the FDA, including the first-generation nevirapine, efavirenz, and delavirdine, and the second-generation rilpivirine, doravirine, and etravirine (Shirvani et al., 2019). Three NNRTIs used in the clinic (efavirenz, nevirapine, and delavirdine) can inhibit wild-type virus replication. Still, they are less effective against several commonly observed mutant viruses, such as Y181C, Y188C, K103N, and L100I. Although etravirine shows improved potency against many NNRTI-resistant viruses, its pharmacokinetic profile is still unsatisfactory. Therefore, there is a need for the design and development of new NNRTIs with improved drug resistance profiles (Zhan et al., 2009). FDA-approved NNRTIs drugs are depicted in Figure 14.
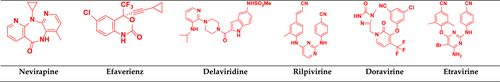
4.3.1 First-generation drugs
Nevirapine
Nevirapine was approved in 1996 by the FDA for the treatment of HIV-1 and by the European Medicines Agency in 1997–98 (Mbuagbaw et al., 2016). In combination with tenofovir and emtricitabine, nevirapine is more efficacious and safe for HIV-1-infected patients (Allavena et al., 2014). Long-term side effects of nevirapine include liver toxicity (de Requena et al., 2002), severe idiosyncratic skin rash, and hepatotoxicity (Dekker et al., 2016).
Efavirenz
Efavirenz is recommended for use in combination with two NRTIs. Efavirenz has a long plasma half-life, allowing it for once-daily dosing (Vrouenraets et al., 2007). The potency of efavirenz against HIV-1 variants expressing single reverse transcriptase substitutions at codons 48, 108, 179, 181, and 236 is similar to that seen against wild-type viruses (Lim et al., 2016).
Delavirdine
Delavirdine is a bis-heteroaryl piperazine derivative. Delavirdine binds to a hydrophobic pocket adjacent to the polymerase active site of HIV-1 reverse transcriptase, inhibiting RNA-dependent and DNA- dependent DNA polymerase (Scott & Perry, 2000). Delavirdine potently inhibits CYP450 3A4, thus inhibiting the metabolism of other drugs (Zapor et al., 2004).
4.3.2 Second-generation drugs
The second-generation NNRTIs are more flexible and always adopt “U” or “horseshoe” conformation to interact with the HIV-1 RT. Diarylpyrimidines (DAPYs) have conformational flexibility that makes them quickly adapt to the steric interference caused by different mutations at the allosteric binding site. DAPYs bind the NNRTI binding pocket (NNIBP) and the NNRTI adjacent binding site. DAPYs RTI complexes revealed that NNIBP has four typical pharmacophoric characteristics: (a) hydrophobic center, (b) hydrogen bond acceptor and donor, (c) solvent/protein interface, and (d) entrance channel. The pharmacophoric features of DAPY are depicted in Figure 15 (Das et al., 2008; Gu et al., 2018; Valuev-Elliston & Kochetkov, 2017).
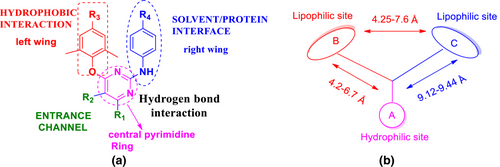
Etravirine
Etravirine (ETV) was approved in 2008 by the FDA for treating HIV-1 with unique antiviral activity, high specificity, and low toxicity (Feng et al., 2018). Etravirine is a promising drug for Friedreich's ataxia (Alfedi et al., 2019). It shows liver toxicity in HIV/ hepatitis C patients (Casado et al., 2016).
Rilpivirine
Rilpivirine (TMC278) is a DAPY approved by the FDA in 2011 (M. Sharma & Saravolatz, 2012). It is a noncompetitive HIV-1 RT inhibitor active against wild-type and mutant strains (M. Sharma & Saravolatz, 2012). Rilpivirine has shown promising safety and efficacy in phase I studies, but infection and resistance have been documented with lower dose (Orkin et al., 2019).
Doravirine
Doravirine is a diary pyrimidine derivative developed by Merck & Co against HIV-1 infection. This drug was approved by the US FDA on August 30, 2018, both as a single drug and as a combination product (BuRgos, 2019; Deeks, 2018). In vitro and clinical data suggest that doravirine has significant interactions with major drug-metabolizing enzymes or transporters (Khalilieh et al., 2017). Doravirine has shown excellent antiviral activity, a better safety profile, and fewer drug–drug interactions (Colombier & Molina, 2018).
4.4 Resistance against non-nucleoside reverse transcriptase inhibitors
Second-generation NNRTIs have a higher genetic barrier and are active against HIV-1 wild-type and clinically relevant mutant strains (Usach et al., 2013). Second-generation NNRTIs that are diaryl pyrimidine derivatives effectively inhibit wild-type HIV-1 due to binding with the NNRTI binding pocket (NNIBP) and the adjacent binding site, but they are less effective against familiar resistant mutants (Chen et al., 2011).
Resistance against NNRTIs can be caused by changes in the NNRTI binding pocket and blockage of entry/binding of the drug. It may also be caused by disrupting interactions inside the pocket (Sluis-Cremer, 2014). NNRTI binding in a hydrophobic pocket of RT causes functional changes in the enzyme and blocks it via noncompetitive inhibition (Schauer et al., 2013).
The NNRTI hydrophobic binding pocket region is predominantly lined by amino acids 98 to 108 and 179 to 190 and mutations occur most commonly here (Scheers et al., 2018). Leu 100, lys 101, lys 103, val 106, thr 107, val 108, val 179, tyr 181, tyr 188, val 189, gly 190, phe 227, trp 229, leu 234, tyr 318 (of p66), and glu 138 (of p51) are the key amino acids that line the binding pocket. K103N is the most frequently found mutation at or near the drug binding pocket (Deeks et al., 2001). NNRTI resistance is shown in Figure 16.
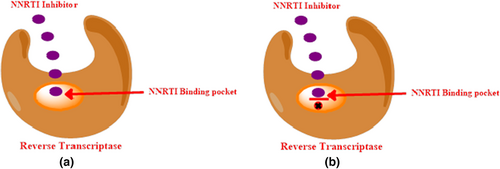
4.5 Non-nucleoside reverse transcriptase inhibitors undergoing clinical trials
4.5.1 Dapivirine
Dapivirine (TMC 120) Figure 17 is currently being investigated for use as a vaginal microbicide in two-dosage forms, a semi-solid gel, and a silicone elastomer vaginal ring (Akil et al., 2011). The phase III trial of dapivirine showed successful prevention of HIV-1 in African women (Palanee-Phillips et al., 2015). On July 24, 2020, The European Medicines Agency approved a dapivirine vaginal ring (25 mg) for the pre-exposure prophylaxis of HIV-1 infection (Li et al., 2022).
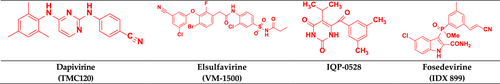
4.5.2 Elsulfavirine
Elsulfavirine (VM-1500) inhibits reverse transcription (Nayan et al., 2023) and is a prodrug of VM-1500A Figure 17. It received approval in Russia in 2017, but it is still to be approved in the United States and Europe. It shows potent antiviral activity against several resistant viruses. Elsulfavirine has in vitro antiviral efficacy in the nM range (EC50 = 1.2 nM; Singh et al., 2019). It has antiviral activity similar to efavirenz in phase IIb clinical studies (Rai et al., 2018).
4.5.3 IQP-0528
IQP-0528 (clinical trial NCT03082690) Figure 17 is a pyrimidinedione derivative under clinical trials. It binds to the p66 subunit in a hydrophobic pocket noncompetitively and causes the active site to shift conformationally, limiting its activity and slowing the rate of viral replication (Mahalingam et al., 2011; Ramesh et al., 2020). IQP-0528 vaginal film formulations exhibit promising safety and pharmacokinetics (Srinivasan et al., 2016). IQP-0528 is a novel and ideal clinical antiviral drug that showed high potency, stability, low toxicity, and favorable local absorption (Buckheit Jr et al., 2014).
4.5.4 Fosedevirine
Fosdevirine or GSK2248761A (IDX-899, discovered by Idenix) Figure 17 is in phase II clinical trial for the treatment of HIV-infected patients (Bellingham et al., 2018). IDX 899 has a 3-phosphoindole moiety (Richman et al., 2008). Its anti-HIV activity was determined by phenotypic assay against wild-type HIV-1, where it exhibited better antiviral activity against wild-type and mutant viruses than nevirapine (Li et al., 2016).
4.6 Molecular docking studies
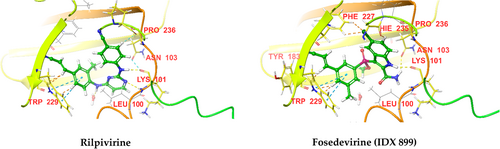
Molecular docking of the NNRTIs mentioned above against HIV RT (PDB IDs 3BGR and 4G1Q) was performed using Schrodinger software. The docking scores and significant interactions of NRTIs against proteins 3BGR and 4G1Q are given in Tables 3 and 4, respectively. 3D interaction diagrams of NRTIs with the best docking scores are shown in (Figures 18 and 19), respectively. In the case of 3BGR, rilpivirine, and fosedevirine (IDX 899), they had the highest docking score (−13.663 and −13.039, respectively), whereas doravirine and delavirdine had the lowest docking score (−7.184 and −5.776 respectively). The docking score of NNRTIs against 3BGR protein was in the following order: rilpivirine > fosedevirine (IDX 899) > etravirine > dapivirine > efavirenz > IQP-0528 > elsulfavirine > nevirapine > doravirine > delavirdine. NNRTIs formed H−bonds with LYS 101, ASN 103, and ILE 180 amino acid residues in the protein's active site, except nevirapine and elsulfavirine, which may be the reason for the low docking scores of these drugs. In the case of dapivirine, NH forms an H− bond with water in the receptor's active site. Except for efavirenz and delavirdine, all NNRTIs were involved in π–π interactions with TRP 229, which may be the reason for the low docking scores of these drugs, especially delavirdine. Elsulfavirine was also engaged in π–π interactions with TYR 338, but this interaction probably affected the binding affinity of the drug negatively.
| Drug | Docking score (kcal/Mol) | H-bonding | π–π interaction |
|---|---|---|---|
| Nevirapine | −7.431 | – | TRP 229 |
| Efavirenz | −11.612 | LYS 101 (NH) | – |
| Delavirdine | −5.776 | LYS 101, ASN 103 | – |
| Rilpivirine | −13.663 | LYS 101, ILE 180 | TRP 229 |
| Doravirine | −7.184 | LYS 101 | TRP 229 |
| Etravirine | −12.469 | LYS 101 | TRP 229 |
| Dapivirine | −12.624 | LYS 101, NH with water | TRP 229 |
| Elsulfavirine | −8.302 | – | TRP 229, TYR 338 |
| IQP-0528 | −9.924 | LYS 101 | TRP 229 |
| Fosedevirine (IDX 899) | −13.039 | LYS 101, ASN 103 | TRP 229 |
| Drug | Docking score (kcal/Mol) | H-bonding | π–π interaction |
|---|---|---|---|
| Nevirapine | −7.918 | – | TRP 229 |
| Efavirenz | −12.44 | LYS 101 (NH) | – |
| Delavirdine | −7.897 | ILE 180, TYR 181, TRP 229 | TRP 229 |
| Rilpivirine | −13.869 | LYS 101, TYR 181 | TRP 229 |
| Doravirine | −9.781 | – | TRP 229 |
| Etravirine | −13.127 | LYS 101 | – |
| Dapivirine | −13.096 | LYS 101 | – |
| Elsulfavirine | −10.999 | LYS 101, LEU 234, C=N with water molecule | TRP 229 |
| IQP-0528 | −11.005 | LYS 101 | – |
| Fosedevirine (IDX 899) | −13.671 | LYS 101, PRO 236, water with CN and LEU 228 | TRP 229 |
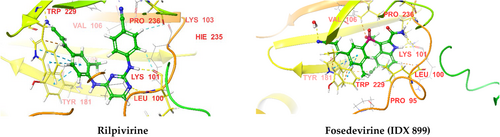
In the case of protein 4G1Q, rilpivirine and fosedevirine (IDX 899), we had the highest docking score (−13.869 and −13.671, respectively), whereas nevirapine and delavirdine had the lowest scores (−7.918 and −7.897, respectively). The order was: rilpivirine > fosedevirine (IDX 899) > etravirine > dapivirine > efavirenz > IQP-0528 > elsulfavirine > doravirine > nevirapine > delavirdine. NNRTIs formed H−bonding with LYS 101, ILE 180, TYR 181, TRP 229, LEU 234, and PRO 236 amino acid residues in the active site of the protein, except nevirapine and doravirine which may be the reason for the low docking score of these drugs. In the case of esulfavirine, CN forms an H− bond with a water molecule in the receptor's active site. In the case of IDX 899, CN forms an H− bond with water in the receptor's active site, and that water molecule forms H− bonds with LEU 228, which may be the reason for its high docking score. Nevirapine, delavirdine, rilpivirine, doravirine, elsulfavirine, and fosedevirine were all involved in π–π interactions with TRP 229. All NNRTIs had better docking scores against protein 4G1Q than 3BGR. Nevirapine had a better docking score than doravirine against protein 3BGR but had a lower score than doravirine against protein 4G1Q.
4.7 Future perspectives
There are various factors that need to be considered when developing next-generation NNRTIs. From a medicinal chemistry point of view, it is essential to improve conformational flexibility, avoid resistance to mutations, and improve physiochemical properties by introducing solubilizing groups into the entrance of the NNIBP or utilizing prodrug approaches. Although NNRTIs are structurally different, they fit within the NNIBP and NNRTI adjacent binding sites. This feature may be valuable for discovering potent NNRTIs and lead optimization in the future.
5 THE ROLE OF REVERSE TRANSCRIPTASE INHIBITORS WITH OTHER ANTI-HIV DRUGS FOR THE TREATMENT IN HIV-1
Anti-retroviral therapy is a critical determinant of HIV treatment that limits HIV replication. Recommended antiretroviral therapy regimens have fewer side effects, are more potent and effective, have a lower medication burden, and are less frequently dosed. Antiretroviral (ARV) therapy consisting of two nucleoside or nucleotide reverse transcriptase inhibitors (NRTIs) plus anti-HIV drugs like protease inhibitors is commonly prescribed for treating HIV. ART is highly active and cost-effective in India. HAART includes the following NRTI/ NNRTI combinations: A. NRTIs (2) + P (1) B. NRTIs (2) + NNRTIs (1) C. NRTIs (2) + Abacavir D. NRTIs (2) + P (1) + Ritonavir E. NRTIs(2) + NNRTIs(1) + PI (1).
Combination HAART drug therapy in India.
Under first-line treatment: 1. NRTIs 2. NRTIs (2) + NNRTIs (1).
Second-line regimen in HAART therapy: 3. NRTIs (2) + P (1), 4. NRTIs (2) + NNRTIs (1) + P (1), 5. INSTI.
These combinations are summarized in Table 5 (Cihlar & Fordyce, 2016; Venkatesh et al., 2013).
| S. No. | HAART | Regimen |
|---|---|---|
| 1 | NRTIs | Lamivudine and Zidovudine (Combivir) Abacavir and Lamivudine (Epzicom) Abacavir, Zidovudine, and Lamivudine (Trizivir) Tenofovir disoproxil fumarate and Emtricitabine (Truvada) tenofovir alafenamide and emtricitabine (Descovy) |
| 2 | NRTIs (2) + NNRTIs (1) | Zidovudine + Lamivudine + Nevirapine Stauvidine + Lamivudine + Nevirapine Stauvidine + Lamivudine + Efavirenz |
| 3 | NRTIs (2) + P (1) | Zidovudine + Lamivudine + Ritonavir Stauvidine + Lamivudine + Indinavir |
| 4 | NRTIs (2) + NNRTIs (1) + P (1) | Zidovudine + Lamivudine + Nevirapine + Ritonavir Stauvidine + Lamivudine + Efavirenz + Indinavir |
| 5 | INSTI | Tenofovir + Emtricitabine + Elvitegravir Abacavir + Lamivudine + Dolutegravir Lamivudine + Raltegravir |
6 CONCLUSION
HIV is a lethal disease, against which the HIV-1 RT is an excellent target for drug intervention. Various NNRTIs have been developed and approved by the FDA. Several drugs belonging to these categories are additionally undergoing clinical trials. RT inhibitors are used for the treatment of HIV either alone or as part of combination therapy. We performed molecular docking of NRTIs against the HIV RT proteins 1RTD and 3V4I and found that the phosphate group was essential for binding. Furthermore, NRTIS showed better docking scores against resistant protein 1RTD and 3V4I. NNRTIs were docked into the active site of HIV RT proteins 3BGR and 4G1Q. NNRTIs had better docking scores against protein 4G1Q than resistant protein 3BGR. However, specific issues related to resistance and adverse effects need to be addressed to make them a more effective and safer tool against HIV.
ACKNOWLEDGEMENTS
The author is thankful to DST-FIST and Central University of Punjab for providing infrastructural support for the successful completion of this study.
FUNDING INFORMATION
None.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.




