Frontiers in G-Quadruplex therapeutics in cancer: Selection of small molecules, peptides and aptamers
Banerjee and Panda contributed equally to this article.
Abstract
G-Quadruplex, a unique secondary structure in nucleic acids found throughout human genome, elicited widespread interest in the field of therapeutic research. Being present in key regulatory regions of oncogenes, RNAs and telomere, G-Quadruplex structure regulates transcription, translation, splicing, etc. Changes in its structure and stability leads to differential expression of oncogenes causing cancer. Thus, targeting G-Quadruplex structures with small molecules/other biologics has shown elevated research interest. Covering previous reports, in this review, we try to enlighten the facts on the structural diversity in G-Quadruplex ligands aiming to provide newer insights to design first-in-class drugs for the next-generation cancer treatment.
1 INTRODUCTION
G-Quadruplex (G4) are one of the several non-canonical structures of nucleic acid. These are formed in stretches of DNA which are rich in guanine bases and is located throughout the entire genome. Computational estimates propose that sequence motifs of the type G≥3N1–7G≥3N1–7G≥3N1–7G≥3 in the human genome have the potential to form a G-Quadruplex structure (Bhattacharyya et al., 2016; Bochman et al., 2012; Hansel-Hertsch et al., 2017). Generally, four guanines arrange in a square-planar fashion bonded through cyclic Hoogsteen hydrogen bond to form a G-quartet. Typically, three or four such quartet stack upon one another separated by loops of varying length using π–π base stacking interaction to form a G4 structure (Figure 1). They are further driven by the presence of cations like K+, Na+, Pb+2, etc (Bhattacharyya et al., 2016; Li et al., 2009).
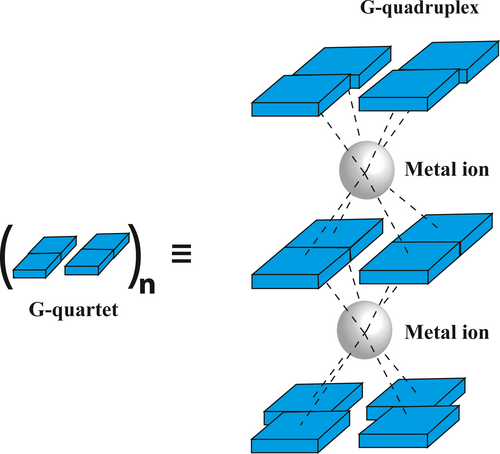
There are several factors that stabilizes a G-Quadruplex structures namely, base stacking, hydrogen bonding, hydration structure, and electrostatic interactions. Apart from this, the central cavity accommodates a cation which negates the incoming repulsive forces due to charges from the guanine O6 carbonyl groups. The position of cation in the G-quartet varies depending upon the ion and structure of the G-Quadruplex. Bivalent cation generally positions itself on alternate layers of G-quartet due to the electrostatic repulsion between the ions. The position of monovalent cation can be along the G-quartet plane or in between the quartet plane (Bhattacharyya et al., 2016; Li et al., 2020). Calculations exhibits that the energy for an average hydrogen bond increases from 0.22 to 0.42 eV, when involved in the G-quartet establishment (Rhee et al., 1999). This substantial surge in binding energies was credited to resonance-assisted hydrogen bonding. The N2, N3, and O4 of the guanine tetrad associated with water molecule further stabilizes G-Quadruplex giving rise to hydration ordered structures. The bases of DNA are non-polar in nature and thus they stack upon one another to reduce the area exposed to polar solvent. They are then acted upon by hydrophobic, electrostatic and Van der walls force to provide stability. Further both telomeric and non-telomeric quadruplexes are bound by a wide array of proteins which either stabilizes or destabilizes them.
Still all these interactions are not enough to stabilize the G-Quadruplex structures. Since in most of the cancer the G-Quadruplex gets destabilized (Tateishi-Karimata et al., 2018), developing ligands that specifically stabilizes the G-Quadruplex structures is a frontier research area in biology and chemistry. For example, the ability of small molecules to stabilize G-Quadruplex DNA and interfere with telomere extension in cancer cells by inhibiting the enzyme telomerase have highlighted the potential role of quadruplexes as anti-cancer drug targets (Neidle & Parkinson, 2002). There are several reviews that discuss various aspects and domains of G-Quadruplex including its importance as drug target (Kosiol et al., 2021; McKenzie et al., 2021; Parrotta et al., 2014; Stefan & Monchaud, 2019). In this review, we tried to summarize and discuss important ligands that are currently used to target different G-Quadruplex structures.
2 DISTRIBUTION AND FUNCTION OF THE G-QUADRUPLEX STRUCTURES IN-VITRO & IN-VIVO
Before we discuss about ligands targeting Quadruplexes, we must shed some light on why quadruplexes are important target. Thirty years ago, Aaron Klug made a statement about the in-vivo existence of G-Quadruplex scaffolds: “If G-Quadruplexes form so readily in vitro, nature will have found a way of using them in vivo.” Investigation in Disease biology have found a strong connection between G-Quadruplexes and various diseases (Kharel et al., 2020). Bioinformatics analysis has predicted that human genome has ~700,000 putative G-Quadruplex forming sequences (PGQS) (Ding et al., 2018). The interesting fact is these structures are not at all randomly located rather they are significantly associated with some key regulatory areas like telomere, replication origin, promoter, 5′-UTR and 3′-UTR, Ribosome binding sites (RBS) and in long non-coding RNA where they might modulate gene expression; suggesting that G4 structures may play a pivotal role in the control of a variety of cellular processes (Figure 2). G-Quadruplex located in promoter generally have an inhibitory effect to the gene expression, whereas those in 5′-UTR and 3′-UTR generally modulates translation, miRNA binding, premRNA splicing, alternate polyadenylation and mRNA targeting (Beaudoin & Perreault, 2013; Rouleau et al., 2017; Song et al., 2016; Weldon et al., 2018). Strikingly, these structures are often present in proto-oncogenes and apparently absent in tumor suppressor genes, which suggests evolutionary selection for G-Quadruplex structures based on their function (Eddy & Maizels, 2006; Huppert & Balasubramanian, 2007).
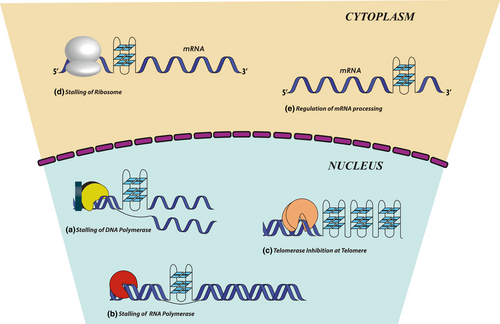
G-Quadruplexes were first identified within human telomeric DNA. The structure and stability of telomeres are closely related to cancer (Arthanari & Bolton, 2001), aging (Shammas, 2011), and genetic stability. Telomeric DNA has the propensity to form intramolecular G-Quadruplexes spontaneously which acts as tumor suppressor element by inhibiting telomerase thereby maintaining the telomere. Treatment with G-Quadruplex stabilizers induces a gradual reduction in the length of the G-rich 3′ end of the telomere without a reduction of the total telomere length, suggesting that telomerase activity is inhibited (Luu et al., 2006; Takahama et al., 2013). Telomeric quadruplexes are highly conserved and are associated with numerous telomeric proteins (Lin & Yang, 2017).
Studies led by Picard et al. (2014). found that almost 80% of replication origins overlaps with a PGQS. G4 structures are also formed during replication acting as “roadblocks” and impede replication fork progression (Kruisselbrink et al., 2008) (Figure 2). Evidence came from the loss-of-function mutation of DOG1 gene (FNACJ helicase) known to resolve quadruplex structures ensuing in hoarding of ~50–300 nucleotide upstream of potential G-Quadruplex motifs (Picard et al., 2014). Certain G4 structures also play a positive role in replication initiation. Research by Valton et al. (2014). pointed to the fact that deletion of G4 motifs in med14 origin led to the dramatic decrease in replication origin activity. Along with it point mutation in consensus G4 motif βA led to much lower replication origin activity compared to wild type cell. These studies combined with several other notable research points to the fact that G-Quadruplexes are involved with DNA replication (Teng et al., 2017).
In-silico studies exposed the fact that G4 motifs are mostly clustered in promoter regions of almost 20,000 genes (Huppert & Balasubramanian, 2007). Several oncogenes like c-MYC, BCL-2, c-KIT, K-RAS, VEGF, RB, PDGF-A, hTERT, etc. harbors, a putative stable G4 structure 1 kb of each side of transcription start site (TSS) (Brooks & Hurley, 2010; Cogoi & Xodo, 2006; Dexheimer et al., 2006; Rankin et al., 2005; Sun et al., 2005; Xu & Sugiyama, 2006). In most of these cases the PGQS acts as a transcription repressor thereby maintaining a threshold level of transcript of the gene (Figure 2). Studies have shown that stabilization of G4 structure in NHEIII1 results in decreased expression of the c-MYC transcription. Similarly, the guanine-rich strand on the proximal promoter region of the VEGF gene regulates its expression. Stabilization of this G4, downregulates the VEGF expression (Sun et al., 2008). Ribosome biogenesis, occurring in nucleolus, is meticulously controlled by multiple cell signaling pathways and the quadruplex in non-template strand. Synthesis of rRNA is the rate limiting step of ribosome biogenesis. There are two G4 – NUC 19P and NUC 23P – present in the ribosomal DNA (rDNA) (Cho, 2016), which controls the rate of synthesis of rRNA. Formation and stabilization of these quadruplex by nucleolin protein results in overexpression of rRNA. Here quadruplex acts as a transcriptional activator. There are several other examples which corroborates to the fact that G-Quadruplexes are key regulatory checkpoints inside the cell. Genome-wide-survey of G4 motifs across several species has concluded that they are evolutionary conserved ratifying their important functional role in biology (Capra et al., 2010; Huppert & Balasubramanian, 2007).
G-Quadruplex structures have also been identified in RNA, which controls several biological functions, in prokaryotes, eukaryotes and even in viruses. RNA G4 was first identified in viral genomes viz. HIV-1 and Herpes Simplex Virus. Later it was proved that RNA G4 promotes strand transfer in HIV-1 genome (Shen et al., 2009) and its role in Epstein-Barr virus nuclear antigen function was deduced (Norseen et al., 2009). Putative RNA G4 are widely distributed in coding and non-coding regions of premRNAs and mRNAs such as introns and 5′- and 3′-UTRs. Thus, they have a huge role in the regulation of RNA splicing, turnover, localization, and stability. In the last 10 years, many roles of four stranded RNA G-Quadruplex has been identified in higher organisms like post-transcriptional regulation of IGF-II expression in rats and humans (Christiansen et al., 1994), translational regulation of FGF-2 expression in humans (Bonnal et al., 2003) etc. In the past few years this area draws deserving attention by the discovery of G-rich telomeric repeat RNAs (TERRA) and the presence of G-rich stretch within the 5′ UTR regions of mammalian NRAS mRNA sequences (Azzalin et al., 2007; Schoeftner & Blasco, 2008). Both these RNA G4s are highly connected with cancer formation thus making them considerable future therapeutic target. But till date though no specific TERRA G4s binding proteins have been discovered, work by Rocca et al. (2017). marked an important step in discovering TERRA specific ligands that can stabilize TERRA G4s. They conclude that selectivity of carboxy pyridostatin toward TERRA G4 is propelled by solvation energy and ligand conformation. Later it was also observed that RNA G4s can also endow with RNase resistance. Kumari et al. (2007) reported that 5′ UTR G-Quadruplex forming sequence of NRAS proto-oncogene downregulates protein expression upon stabilization of the G-Quadruplex and blocking the progress of the ribosome complex. Presence of GQ at 3′ UTR of mRNA in LRP5 and FXR1 increase alternative polyadenylation and mRNA shortening. Recently involvement of RNA G4s has also been discovered in mitochondrial transcription termination (Wanrooij et al., 2010). RNA G4 structures are also found to be linked with Fragile X mental retardation syndrome (FXS) and FRAXE-associated mental retardation (FRAXE), due to the abnormal expression of two specific RNA G-Quadruplex binding proteins, FMRP and FMRP2, respectively. As RNA G-Quadruplex structures have been found in a variety of biological processes and in a diverse range of organisms thus indicating that such structures can play a pivotal role in cellular machinery.
After the evidence of the in-vivo existence of G-Quadruplexes was established using specific antibodies against parallel and antiparallel G-Quadruplexes formed in telomeric DNA of the ciliate Stylonychia lemnae, directive and quantitative visualization of DNA G-Quadruplex structures in mammalian cells was recently reported (Biffi et al., 2013). With structure-specific antibody BG4, the G-Quadruplex was visualized in telomeres and non-telomeric regions. G4 was also seen to be formed in a replication-dependent manner in the cell cycle. More importantly, the increased BG4 foci number was observed after treatment with the G-Quadruplex binding ligand, indicating that the small molecule could trap and stabilize a G-Quadruplex in human cells (Biffi et al., 2013). In addition, the practical high-throughput DNA sequencing showed the significant co-localization of G4s with antibody 1H6 (Artusi et al., 2016), BG4 (Biffi et al., 2013), hf2 (Kudlicki, 2016), γH2AX (Murat & Balasubramanian, 2014) and DEAH/RHA family of helicases. Increased prevalence of G4 in cancer cells has been proven by ChIP-seq (Hänsel-Hertsch et al., 2016) and immunohistochemistry (Biffi et al., 2014). All these, substantial evidence not only provide for the existence and location of G4s in the genome but also implicate the profound function of G-Quadruplexes. The fact that a specific small molecule could modulate these structures in cells, strongly supports the therapeutic potential of targeting a G-Quadruplex. In the following section we will illuminate the diverse classes of molecule which targets G-Quadruplex structures.
3 DIVERSITY OF G-QUADRUPLEX-INTERACTIVE LIGANDS
3.1 Classification based on backbone
3.1.1 Anthraquinone derivatives
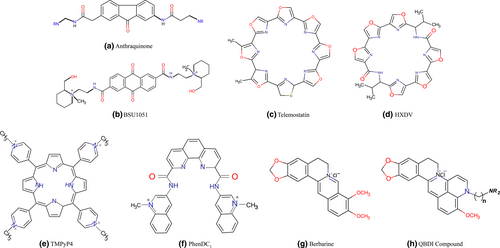
In 1997, Neidle and Hurley group reported the first quadruplex-interactive ligands. They developed 2,6-Diaminoalkylamido-anthraquinone, BSU-1051 (Figure 3), a telomerase inhibitor molecule with a telomerase IC50 of 23 µM (Sun et al., 1997). From there on, wide range of the disubstituted amido-anthraquinone family such as 1,4-, 1,5-, 2,6-, and 2,7- isomers were developed with substituents at various positions on the chromophore by changing side chain length and size. The nature of the side chain terminal groups were further modified by systemic structure-function studies. Molecular modelling studies showed that anthraquinone compounds bind to G4 by a “threading intercalation” mode with π–π overlapping, where any flexible side chains can protrude into wider grooves of G4. But since the same binding mode is also observed for dsDNA, these compounds are not that much selective and specific for quadruplex DNA moreover their cytotoxicity can be high.
To reduce the cytotoxicity, a carbonyl group was removed from the anthraquinone moiety and a series of disubstituted 2,7-amido-fluorenones(2,7-FO) were designed and synthesized thus preventing redox cycling (Perry et al., 1999). These 2,7-FO derivatives have low telomerase IC50 values of 8–12 µM with up to 10-fold reduced cytotoxicity in different human cancer cell lines, compared with that of their parent anthraquinone derivatives. This slightly less telomerase activity of 2,7-FO derivatives with that of parent molecule is due to decreased electron deficiency in the chromophore and the curved scaffold which is not favorable for π-stacking interaction with G4 structures (Perry et al., 1999). A more recent study suggests that introduction of morpholino substituent and two or more different side groups onto the fluorenone scaffold results in higher activity and selectivity to G-Quadruplex. They further deciphered that switching the side group from position 4 to 2 and establishing a positive charge increase the specificity of fluorenones to hybrid G-Quadruplex form (Alcaro et al., 2010). Tetracyclic anthraquinone analogues with another heterocycle attached to the anthraquinone core in place of side chains were reported. These G-Quadruplex interacting compounds also show anti-telomerase activity. Benzonaphtho-furan compound like tricyclic anthraquinones are modified to tetracyclic derivative. Molecular models showed that tetracyclic compounds provide more surface area suitable for stacking interaction with G-quartet.
3.1.2 Acridine derivatives
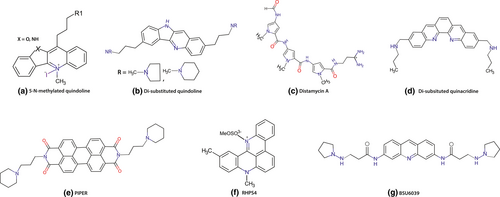
Chromophore containing positively charged central ring has been extensively used for designing G4 binding ligands as the positively charged ring is likely to be complementary to the negative electrostatic potential channel of G-Quadruplexes. Thus, to overcome the problem of the specificity and selectivity of anthraquinones for quadruplex DNA and duplex DNA, Neidle and his groups modified the acridine core and its side chains by introducing a pyridine ring in the ligand core in place of quinine which increase the positive charge of the ligand core thus enhancing the electrostatic interaction with the negatively charged G-quartet channel (Harrison et al., 1999). They synthesized a series of 3,6-disubstituted acridines including BSU 6039 (Figure 4) (Haider et al., 2003). But these compounds failed to increase telomerase inhibition probably because of the insufficient basicity of the pyridine ring nitrogen. Based on X-ray crystal structures and molecular modelling study of telomeric G4 and BSU 6039 complex, disubstituted acridines were further modified by replacing the core with acridine, changing the substitution pattern using polyamine. Using this strategy a variety of 3,6,9-trisubstituted acridines were synthesized (Harrison et al., 2003). The basicity of the pyridine ring nitrogen is increased by the incorporation of the anilino group at the 9-position, allowing the ligand core to be protonated at physiological pH. The 3,6,9-scaffold brings the 9-position substituents significantly deeper into the cavity of this groove as compared to disubstituted acridines providing an additional interaction with the third groove. Due to incorporation of the anilino group at the 9-position, the 3,6,9 acridine scaffold can interact better with the surface of this groove without creating steric clashes, especially when they carry a positive charge (Harrison et al., 2003). Two side chains at 3- and 6-positions were subjected to the two widest grooves. 3,6,9-Trisubstituted acridines analogues have much stronger binding affinity and specificity for quadruplex DNA over duplex DNA (10–70-fold greater binding constants), whereas 3,6- disubstituted acridines have similar binding constants for duplex and quadruplex DNA. BRACO-19, being a member of 3,6,9-trisubstituted acridines, showed very high binding affinity for quadruplex DNA over duplex DNA and high anti-telomerase activity in range of 10–20 nM (Moore et al., 2006a). Further study identified the antitumor activity of BRACO-19 in human xenografts (Burger et al., 2005). Stevens et al. reported that the N-methylated pentacyclic acridinium salts are another class of pentacyclic acridine derivatives. RHPS4, a pentacyclic acridine 3,11-difluoro-6,8,13-trimethyl(8H)-quino[4,3,2-kl] acridinium methylsulfate (Figure 4), is one such compound under this class which exhibits telomerase inhibitory activity (telIC50 0.33 µM by TRAP assay) and inhibit cell proliferation within 2–3 weeks at non-cytotoxic concentrations (Bejugam et al., 2007). This high activity is due to the strong electrostatic interaction between the positively charged ligand core, brought by methylated quaternary nitrogen and negatively charged quadruplex center. However, this small-sized aromatic compound can also intercalate into duplex DNA with modest selectivity. Based on the finding that oxidized riboflavin binds to an intramolecular G-quartet with moderate affinity, trisubstituted isoalloxazines were also synthesized. These compounds lacks duplex selectivity but some trisubstituted isoalloxazines were found to be approximately 15-fold more selective for c-kit promoter than that of telomeric G4 (Bejugam et al., 2007). This result indicates that coplanar tricyclic small molecules can selectively bind to G4 over duplex DNA. Recent studies have shown that simple anthracenes with tethered side chains at appropriate positions can also act as selective G-Quadruplex ligands.
3.1.3 Bis-aryl compounds
Bis-aryl compounds are one of the most extensively used G4 stabilizing ligand. These compounds contain two symmetrical aromatic ring connected to the core, thus giving a “butterfly” like appearance. A variety of aromatic rings have been reported including, quinolinium, triazole, benzimidazole, and substituted phenyl rings. Linkers like urea, benzene, diketene, pyridine, benzene are generally attached to the core. Due to the flexible rotation of these linkers, these molecules can adapt different structural conformation so that they can fit into groove and loops of G4, showing higher binding affinity.
First reported bis-aryl compounds were bis-quinolinium triazines (compound 12459) (Gomez et al., 2003). These compounds show low cytotoxicity and acts as a potent human telomerase inhibitor (telIC50 = 0.13 µM) (Riou et al., 2002). Later derivatives of bisquinolinium compounds with a pyridodicarboxamide core (e.g., Compounds 360A) were synthesized and these compounds were found to be 100 times more selective to G4 than duplex DNA (Lemarteleur et al., 2004). Internal syn-syn hydrogen bond favors these ligands to interact G4 with high selectivity. Later Phen-DC derivatives (Figure 3) were synthesized with an expanded aromatic core, by replacing pyridine with bipyridine or phenanthroline thereby showing higher selectivity and specificity toward G4 than duplex DNA (Monchaud et al., 2008). PhenDC derivatives also exhibit anti-telomerase activity with telIC50 value of 0.3 µM.
Ligands with diarylurea skeleton are extensively studied due to their drug like characteristics. These ligands contains 1,2,3-triazole rings attached to a diphenyl urea moiety (Drewe & Neidle, 2008). These ligands can orient themselves by positioning urea oxygen atom over the ion channel and allowing deep penetration of the side chains into G4 grooves by triazole–phenyl bond rotation thus stabilizing G-quartet more efficiently by π-stacking interactions. Further modelling studies suggested that the ortho- and meta-substituted analogues are optimal for G4-DNA affinity as they can adopt a “square-planar” conformation, than that of the para-substituted ligands which are sterically too large for optimal interaction with the G-quartet. Also, by the formation of an intramolecular H-bond, ortho-substituted analogues are conformationally constrained by the urea NH and the triazole-N3 atom, which enforces the desired “square-planar” conformation (Drewe & Neidle, 2008).
Due to the presence of three rotatable bonds in the scaffold, triphenylpyridine has one of the most rigid and diverse skeletons as G4 ligand. The side chains positioned by central core help to discriminate different G4 loops. Compounds with two amine chains linked directly to the aryl rings, with piperazine(N-Ppz) substituents confers specificity and the highest affinity to human telomeric and c-KIT G4-DNA, but fails to discriminate between them. Data of different substitution reaction, suggests that the protonation of the amine is critical. According to FRET study, compounds with a para-thiomethylphenyl or para-bromophenyl substituent on the central pyridine ring and four carbon atom length side chains, confers the higher stability and specificity toward all G4-DNA sequences over dsDNA. Compounds containing thiophene, furan and piperonal units exhibit much higher specificity for G4 over dsDNA but with moderate stabilization and ligands with alkyl amine phenyl ether, bulky benzyl ether and para-dimethylaminophenyl 4-substituents on the central pyridine present the poorest G4 stabilization (Table 1).
| No | Ligand/chemical skeleton | Binding mode | Protonation type | telIC50 (µM) |
|---|---|---|---|---|
| 1. | TMPyP4, Cationic porphyrin | Stacking Interaction | N-methylated aromatic | 8.9 |
| 2. | Telomestatin, HXDV-Cyclic polyamines | Stacking Interaction | Natural Macrocycles | 0.005 |
| 3. | Acridine, RHPS4, Polycyclic Methyl acridinium | Stacking Interaction | N-methylated aromatic | 0.33 |
| 4. | BSU-1051, Anthraquinones | Threading intercalation | N-methylated aromatic | 23 |
| 5. | BSU6039, BRACO-19, Acridines. | Interaction with grooves, Stacking interaction | Protonated in situ | 0.01–0.02 |
| 6. | MOQ(monomer), BOQ1(dimer), Quinacridines | groove/loop interactions, Stacking interaction | Protonated in situ | 0.028, 0.137 |
| 7. | PIPER, Perylene tetracarboxylic diimide | π–π interaction | Protonated in situ | 20 |
| 8. | Naphthalene diimide | Stacking interaction | Protonated in situ | 0.05 |
| 9. | Se2SAP, di-selenasapphyrin | Stacking Interaction | N-methylated aromatic | — |
| 10. | Pentacationic Mn(III) porphyrin | π–π stacking | N-methylated aromatic, metal center | 0.58 |
3.1.4 Oxazole derivatives
Telomestatin (SOT-095; Figure 3), a macrocyclic natural product that was isolated from Streptomyces annulatus 3533- SV4 in 2001 by Shin-ya et al. (2001), consists of seven oxazole rings and one thiazoline ring and is currently the most effective in-vitro telomerase inhibitor, with an telIC50 value of 5 nM obtained from TRAP assay. This compound selectively binds to intramolecular G4 rather than intermolecular quadruplex and specific to G4 than duplex DNA with more than 70-fold preference. Docking and Molecular dynamics study confirmed that both enantiomer (R and S) of telomestatin binds with four guanine residues by the help of stacking interaction as well as interaction free energy thereby stabilizing the parallel G4 at a greater extent (Stefano et al., 2012). Anti-proliferative role of telomestatin has been reported from wide range of cancer cell lines like breast cancer (Tahara et al., 2006), myeloid leukaemia (Sumi et al., 2004), human pancreatic carcinoma (Liu et al., 2005) etc. Later, telomestatin has also been used to stabilizes and to throw light on the biological roles of other G4s formed at the promoter regions of various oncogenes like, BCL-2 (Dexheimer et al., 2006), c-MYC (Seenisamy et al., 2005), VEGF (Sun et al., 2005) etc. But certain disadvantages like low solubility in water makes intracellular delivery of the drug difficult. Furthermore, difficulty in large scale synthesis of telomestatin restricts its wider use.
Recently hexaoxazole macrocyles (HXDV), composed of two symmetric trioxazoles linked by amino acids, such as, valine and bistrioxazole acetate have been synthesized by Rice et al. (Barbieri et al., 2007; Minhas et al., 2006) Due to its telomestatin-like concave shape, HXDV (Figure 3) has been reported to show high specificity toward G4 by entropically driven “terminal capping” binding mode. To increase the water solubility of hexaoxazole macrocycles, amino acid linkers are modified by changing a valine residue to lysine and HXDL has been synthesized. HXDL interacts with G4 with higher affinity because of positively charged amine groups of lysine at physiological pH (Rzuczek et al., 2008). Hexaoxazole with an arginine linker has been found to have telomestatin-like activity. On the other hand, bistrioxazole acetates, having a macrocyclic bisamide structure, were synthesized with a telIC50 ⁼ 2 µM (Tera et al., 2007).
3.1.5 Porphyrin derivatives
Porphyrins are one of the most well studied binding agents for duplex DNA. They show intercalating interaction while binding to dsDNA. The aromatic rings of porphyrin provide a planar arrangement which allows them to bind to the G4s by stacking interaction with the Guanine tetrads. Porphyrins have four N-methylated pyridines. Water solubility and π–π stacking interactions are increased by the polycationic moiety due to ionic configuration and decreased electron density of the core aromatic system. Hurley et al. reported TMPyP4 as the type molecule of this class (Figure 3). CD melting analysis and NMR experiments, divulge that TMPyP4 (tetra-(N-methyl-4-pyridyl)porphyrin) binds both dsDNA and G-Quadruplex structures (parallel and antiparallel) with only two fold greater affinity for G4 than duplex DNA (Dexheimer et al., 2006; Liu et al., 2005; Wheelhouse et al., 1998). Thus, TMPyP4 showed poor selectivity for G4 over duplex DNA. Later, it was found that TMPyP4 binds and stabilizes different G4 in oncogenic promoters like K-RAS, c-MYC, BCl-2, etc. CD melting and ITC experiments suggested that, TMPyP4 binds to c-MYC Pu27 G4 at a stoichiometry of 4:1 (TMPyP4:Pu27). But, its isomer TMPyP2 (tetra-(N-methyl-2-pyridyl) porphyrin showed very weak affinity to G4s. From the NMR Chemical shift, it was clearly observed that TMPyP4 binds to G4 by external stacking interaction. Recently, from the crystal structure of TMPyP4 and human bimolecular telomeric G4 complex, it was found that TMPyP4 stacks upon TTA nucleotides either at the external loop or at the 5′ region of the G4, involving hydrogen bonding between the base pairs which are not taking part in the G4 formation (Phan et al., 2005). As TMPyP4 is a standardized molecular skeleton to probe G4, this porphyrin skeleton is modified to increase G-Quadruplex selectivity and specificity. By replacing the cationic pyridinium ring of TMPyP4 with a phenol ring which possess a quaternary ammonium moiety, TQMP, a non-pyridinium cationic porphyrin has been synthesized, showing 30-fold increase in G4 selectivity than TMPyP4 through specific groove and loop binding (Wang et al., 2006).
Later to increase specificity, expanded porphyrin derivative, 5,10,15,20-[tetra(N-methyl-3-pyridyl)]-26,28-diselenasapphyrin chloride (Se2SAP) was synthesized and reported to bind selectively to G4-DNA with a 50-fold increase over duplex DNA (Seenisamy et al., 2005). Se2SAP was found to bind selectively to c-MYC G-Quadruplex even in the presence of other quadruplexes and duplex DNA and convert parallel c-MYC quadruplex into mixed parallel and anti-parallel hybrid conformation (Freyer et al., 2007). Se2SAP can even bind and stabilize bcl-2 promoter quadruplex into mixed anti-parallel and parallel hybrid structures (Dexheimer et al., 2006). But due to the low synthetic yields this ligand failed to draw further attention. At present, pentacationic manganese (III) porphyrin, containing four flexible cationic arms attached to the central aromatic core, is the most specific ligand that can discriminate between G4 and dsDNA, increasing the selectivity by 10,000 folds (Dixon et al., 2007). Other TMPyP4 variants, such as, cationic N-confused porphyrin (Zorzan et al., 2016), pyridyl-substituted corrole (Ma et al., 2009), porphyrazines (Gonçalves et al., 2006) and phthalocyanines have been recently reported. Among them, Tetramethylpyridinium porphyrazines (TMPyPz), nonsymmetrical phthalocyanine azo derivatives, was found to show higher binding affinity (by 100 fold) to human telomeric G4 than duplex DNA by 100-fold compared to TMPyP4 (Gonçalves et al., 2006). Zinc complex (Zn-TMPyPz) derivatives was found to show similar affinity and selectivity (Ren et al., 2007). Later water soluble octa-cationic zinc phthalocyanine (ZnPc) was also found to be an effective G-Quadruplex binding ligand with anti-telomerase activity (telIC50 = 0.23 µM) (Ren et al., 2007).
3.1.6 Quinacridine analogues
In 2001, Mergny et al. (2001b) reported a new class of crescent shaped dibenzophenanthroline (quinacridine) pentacyclic ligands containing five fused aromatic rings in a linear arrangement and extended amino side chains that increase the propensity of overlapping with the G-quartet and electrostatic interaction with grooves. Many quinacridine derivatives, including mono ortho quinacridine (MOQ) and mono meta quinacridine (MMQ) were already synthesized and CD melting studies suggest that these ligands bind to the human intramolecular quadruplex and have anti-telomerase activity (Hounsou et al., 2007). The two active compounds like disubstituted quinacridines (Figure 4) and cyclic-bis-quinacridine BOQ have ΔTm values of 19.7 and 12.5°C, and IC50 values of 0.028 and 0.5 µm, respectively (Mergny et al., 2001). Cyclic bis-quinacridine (BOQ1), scaffold is mainly composed of two quinacridines connected by polyamine linkers, having greater quadruplex selectivity than mono-quinacridine compounds. BOQ1 binds to G4 preferentially (ΔTm = +28°C) over duplex DNA, due to the steric hindrance effect of the macro-cyclic scaffold which makes it more favorable for groove/loop interactions. Thus, BOQ1 appears as one of the most potential scaffold targeting G4. Recently, a novel platinum–quinacridine hybrid (PtMPQ) composed of single functional Pt moiety and mono-para-quinacridine, was reported to interact with quadruplex DNA by a dual covalent- noncovalent binding mode (Bertrand et al., 2007).
3.1.7 Berberine derivatives
Berberine, a plant alkaloid (Figure 3), was first isolated from Chinese herbs and reported to have anti-microbial activity but later it was established as a G4 stabilizing agent. In 1999, berberine was reported to have anti-telomerase activity (Naasani et al., 1999) and many 1,3-substituted berberine derivatives are synthesized by Neidle et al. These derivatives bind to G4 with very high affinity (Binding constants Ki = 2.05 × 10−6 M−1 to 3.06 × 10−6 M−1) (Franceschin et al., 2006; Zhang et al., 2007). They also found that piperidino-berberine has better stabilizing effect on G-Quadruplex and increased anti-telomerase activity. Investigation into the mechanism of binding of berberine to human telomeric G4-DNA using different DNA topologies by Moraca et al. revealed that in case of antiparallel folding, berberine seems to preferentially bind to G4-DNA loops and grooves, whereas in the hybrid-type conformations the binding probably occurs at the terminal G-tetrads. Using funnel-metadynamics simulations they also found that in case of biologically relevant parallel G4-DNA [PDB ID: 3r6r] two berberine molecules stacked at the 5′-end assuming an antiparallel orientation, whereas the other two are bound to the 3′-end in a parallel orientation (Moraca et al., 2017). This marked an important step in finding out the mode of interaction of molecules bearing similar chemical structure. Huang et al. synthesized 9-subsituted berberines having an alkyl side chain with a terminal amino group, which can efficiently interact G4 with greater binding affinity than berberine. 9-substituted berberines were again modified by the incorporation of one extra pyridine ring on berberine scaffold and thus, quinolino-benzo dihydroisoquindolium (QBDI) compounds (Figure 3) were synthesized, which have enhanced G4 interacting potential due to the increased aromaticity of the QBDI backbone. Later, it was reported that these 9-substituted berberine and QBDI derivatives possess higher selectivity for G-Quadruplex DNA in c-MYC oncogene than the others.
3.1.8 Quindoline derivatives
Molecular modelling study of anthraquinones and acridines derivatives revealed that a tricyclic chromophore is not specific for stacking interaction to G-quartet terminal. The disubstituted derivatives (Figure 4) of the natural product quindoline with two alkyl amino groups at the 2,7-positions or at the 2,10-positions, showed telIC50 values in the range of 6–16 µM (Caprio et al., 2000) and another class of tetracyclic chromophore ligands containing four aromatic rings, benzo[b]naphtho [2,3-d]furan have been reported to have telIC50 value of 7.0 µM. Another report suggests that 11-subsitituted quindolines show stronger anti-telomerase activity than the disubstituted quindolines, with telIC50 values of 0.4–12 µM (Zhou et al., 2006) due to the presence of the nitrogen atom in the pyridine ring of quindoline. Substituted amino groups at the 11-position act as electron-donating groups which can enhance the basicity of the pyridine nitrogen atom (Zhou et al., 2006). This nitrogen atom in the pyridine ring can be protonated at physiological pH, thus increasing the electrostatic interaction between the positively charged quindoline scaffold and the negatively charged center of the G4. This 11-substituted quindoline also triggers G-rich sequence in c-MYC promoter to fold into a G4 and downregulate their cellular expressions (Ou et al., 2007).
3.1.9 Polyamides
Distamycin A (Dist-A; Figure 4) is a highly studied antibiotic which binds to the narrow, deep A/T sequence of the minor groove of B-DNA because of its curved helical polyamide structure. It binds to duplex DNA in a drug/DNA stoichiometry of 2:1 (Asagi et al., 2010). Later, it has been reported that Dist-A interacts with diverse array of parallel G-Quadruplex sequences. In 2007, Randazzo et al. reported the NMR structure of Dist-A-[d(TGGGGT)]4 complex. They found that two dimers formed by four Dist-A molecules binds to two opposite grooves of the quadruplex through the interaction with G-quartets and sugar-phosphate backbone by the help of π–π stacking interaction. It also achieves a dipole-dipole interaction between G4 and the pyrrole ring and the carbonyl group of Dist-A (Martino et al., 2007). Different polyamides have been synthesized by using distamycin scaffold. Amide-linked oligopyrroles, 2,4 and 2,5-pyrrole polyamides show selectivity toward duplex DNA than G4 but selectivity toward G4 can be increased in these molecules by changing the heterocyclic skeleton (Moore et al., 2006b). These ligands show anti cell proliferative activity in human lung carcinoma (A549), human colorectal carcinoma (HT-29) and against breast cancer with IC50 values in micromolar range (Rahman et al., 2009).
3.1.10 Perylene, coronene and napthalene diimide
Perylene is one of the most suitable G4 binding ligands due to its electron-rich polyaromacity and flat geometry. By the help of molecular docking studies, Fedoroff et al. synthesized PIPER (Figure 4) based on the perylene scaffold with telIC50 values in the low-µM range (Fedoroff et al., 1998). NMR study indicates that PIPER binds to many G4 structures in 1:1, 1:2, or 2:1 stoichiometric ratios (Han et al., 1999). Later, PIPER derivatives were synthesized by the modification of the aromatic core and side chain lengths. Protonated or alkylated amino groups of the side chains increases the binding affinity with G4. Further incorporation of cationic side chains [DAPER4C(1,6)] increased the water solubility of hydrophobic perylene system (Michelia et al., 2009).
Coronene is another polyaromatic system used as a ligand. Many coronene derivatives were synthesized, which can stabilize G4-DNA. They also show anti-telomerase activity (Franceschin et al., 2007). Later, many naphthalene diimides substitutes were synthesized by incorporating DNA alkylating side chains varying groups by flexible spacers and/or spermidine- and spermine-like side chains with altering length between inner nitrogen atoms to increase G4 selectively (Hampel et al., 2010; Marchetti et al., 2015). These compounds have been shown to increase stabilization of telomeric and cKIT2 quadruplex sequence. The hydrophobic planar core of these molecules stacks on to G4 and the additional ionic interactions with the loops are increased due to the alkylated amino side chains.
3.2 Oncogene and telomere sequence specific ligand
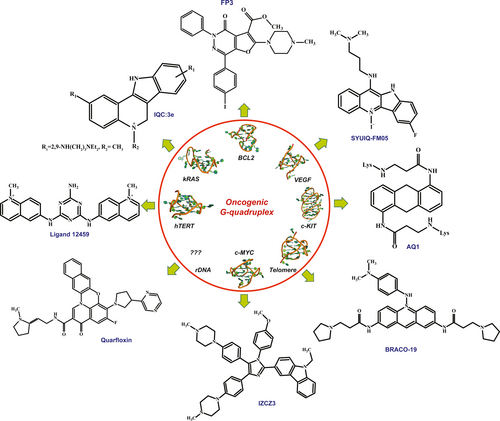
Supressing the expression of an oncogene or developing an inhibitor for a particular protein is an effective way to treat cancer. But it is not an efficient method as the inhibitors are not selective and might bind to other homologous proteins. At this juncture quadruplex comes to rescue as they are a perfect target for drug/ligands, as certain vital oncogenes harbor a quadruplex in them which regulates their expression. Thus, developing molecule with high drug-likeness and G4 specificity is the key to generate next-gen anti-cancer drug. In this section we discuss on some crucial oncogenes that are found to be most over-expressed across all types of tumor and cancer and the ligands that are specific to those oncogenic quadruplexes.
3.2.1 Telomere
Telomere in general protects the chromosome end from shortening and regulates senescence and apoptosis. Telomerase, a telomeric G4 binding protein, plays a key role in tumorigenesis (Calabrese et al., 2018; Chen & Yang, 2012). This, together with the fact that telomerase is over-expressed in >85% of all tumors and across all tumor types, suggest that embarrassment of telomerase would be an operative anticancer therapeutic tactic (Burger et al., 2005). Since telomerase binds to telomeric G4, the later becomes a fine druggable target for cancer treatment. The most well studied ligand that was first developed against telomere G4 to inhibit telomerase was the N,N′-(9-((4-(dimethylamino)phenyl)amino)acridine-3,6-diyl)bis(3-(pyrrolidine-1-yl)propanamide), or BRACO-19 (B19; Figure 5) in short. The specification of B19 to G4 is due to an acridine core and a nitrogen atom in the heterocyclic frame that could be protonated at physiological environments (Ruggiero & Richter, 2018). As a result, the electron deficiency in the chromophore was amplified, with subsequent boost of the G4 interaction. These classes of compounds muddle to G4 via π–π interaction and additionally requests tertiary amine groups, since it is protonated at physiological pH, in their side chain to interact with the grooves. Telomestatin is another naturally occurring compound that binds most tightly to the telomeric G4 (Table 2). Though it has an EC50 value of 5 nM, research by De Cian et al. (2007) show that it might not be that potent as it was claimed. Based on the idea of alkylating hybrid ligands that could combine the aptophore properties of flat aromatic moieties with reactive chemical groups Doria et al. developed the model of a new ligand where the naphthalendiimide core (NDI) was trussed to (NDI–QMPs) quinone methides. They synthesized a new class of tri- and tetra-substituted NDIs in order to conquer poor water solubility of the ensuing alkylated oligomers and investigated the G4 reversible recognition and triggerable covalent damage–stabilization of telomeric G4 structures (Doria et al., 2012).
| Ligand | Targeting G-Quadruplex | Cell line | Tumor type | Biological activities |
|---|---|---|---|---|
| TmPyP4 | Telomere | PC-3, K562, Y79, and B78-H1 | Human prostate carcinomas, leukemia, retinoblastoma, melanoma | Shortening of telomere length, telomerase inhibition,inhibiting transcription by stabilizing both G-Quadruplex and iMotif |
| Ni-P | Telomere | MCF-7, MDA-MB-231 | Breast cancer (Adenocarcinoma) | Induction of apoptosis in Cancer cells including cancer stem cells, suppression of tumor growth in MDA-MB-231 xenograft model in vivo |
| Telomestatin | Telomere | K-562,OM9;22 | Leukemia | Induction of telomeric DNA damage, apoptosis,shortening of telomeres, enhanced chemosensitivity toward daunorubicin |
| BRACO-19 | Telomere | A431, DU145, HCT-116 | Human epidermal carcinoma, prostate cancer, Colorectal cancer | Inhibition of telomerase,induction of telomeric DNA damage,uncapping of 3′telomeric ends |
| Pyridostatin | Telomere | BRCA1 | Breast cancer | Inhibition of telomerase activity, induction of DNA double strand breaks, displacement of POT1 from the telomeric G-overhang |
| GTC365 | hTERT | MCF-7 | Breast cancer (Adenocarcinoma) | Induction of apoptosis, decreased telomerase activity and shortening of telomeres |
| TH-3 | c-MYC | HeLa, A549 | Cervical cancer, Lung cancer | Induction of apoptosis, proliferation becomes lower, minimal cytotoxicity to normal cells |
| Tz1 | c-MYC | HCT-116 | Colorectal carcinoma | Inhibition of transcription of c-MYC and dependent genes, induction of apoptosis |
| IZCZ-3 | c-MYC | HeLa, Huh7, A375 | Cervical cancer, liver cancer, malignant melanoma | Induction of apoptosis with minimal effect on normal cells, Inhibition of transcription of c-MYC and dependent genes, suppression of tumor growth in SiHa xenograft model in vivo |
| FP3 | BCL-2 | HeLa,MCF-7, Jurkat cells | Cervical cancer, breast cancer | Down-regulation of BCL-2 transcription with high selectivity, induction of anti-inflammatory function, increased cytotoxicity |
| ISb | cKIT | HT-29, GIST882 | Colorectal cancer, gastrointestinal stromal tumor | Induction of anti-proliferative effect, down-regulation of cKIT transcription |
| SYUIQ-05 | VEGF | MCF-7 | Breast cancer | Suppression of VEGF transcription, reduction of angiogenesis potential |
| IQc:3e | KRAS | HCT-116, MiaPaCa2 | Colon cancer, pancreatic cancer | Down-regulation of KRAS transcription in KRAS dependent cancer cells |
| CX5461 | rDNA | BRCA deficient cancer cells | Breast cancer | Inhibition of rRNA synthesis and ribosome biogenesis,inducing single-stranded breaks, induction of autophagy. Now clinical trial(advanced Phase 2) is going on for patients with BRCA1/2 deficient tumors |
| CX-3543 | rDNA | MDA-MB-231, MIA PaCa-2 | Breast cancer, pancreatic cancer | Disruption of Nucleolin-rDNA G-Quadruplex complexes in the nucleolus,induction of apoptosis,induction of anti-tumor activity in murine xenograft models of different human cancers |
3.2.2 hTERT
As mentioned earlier, the transcriptional instigation of this telomerase reverse transcriptase (hTERT) gene is crucial during tumorigenesis, its expression remains repressed in adult somatic cells (Saha et al., 2017). Thus, the expression of hTERT gene is highly regulated by several transcription factors as well as epigenetic state. It also harbors a G4 structure in its core promoter which regulates the expression of the catalytic subunit of the telomerase. Mutations in the hTERT promoter has been reported in >75% of glioblastomas, melanomas and in aggressive thyroid cancer and most importantly all these mutations are found to be in the G-rich region which forms quadruplex (Chaires et al., 2014; Huang et al., 2013; Vogelstein et al., 2013; Xing et al., 2014). Metastasis suppressor non-metastatic 2 (NME2) and RE1-silencing transcription factor (REST)–lysine-specific histone demethylase 1 (LSD1) co-repressor complex associates with the G4 in hTERT promoter to repress the expression of telomerase (Saha et al., 2017). In most of the cancer the hTERT G4 gets resolved to duplex DNA and in absence of G4 structure the NME2 and REST co-repressor complex fails to bind to the promoter resulting in the high level of telomerase expression leading to cancer and tumorogenesis. So technically stabilizing the hTERT G4 in cancer cell seemed to be novel therapeutic avenue. On treating with eleven G4 specific ligands ∼50%–85% downregulation of hTERT is seen in HT1080 cells (Figure 2) (Saha et al., 2017). But the problem is these ligands are not hTERT G4 specific ligand rather they bind to any G4 structures. Gomez et al. (2004) developed a 2,4,6-triamino-1,3,5-triazine derivative, ligand 12459, a potent G4 stabilizer present in hTERT intron 6 (Figure 5). It downregulates the expression of telomerase by altering the hTERT splicing pattern, thereby reducing the expression of active (+α+β) transcript and over-express inactive (−β) transcript. 12459 induces the guanine-rich region present in the 5′ end of intron 6 of hTERT to form quadruplex (Gomez et al., 2004). It is also more specific to hTERT G4 than telomeric quadruplex. Compared to telomestatin and B19, 12459 was found to be more potent in achieving 50% inhibition in annealing the guanine-rich strand with its complementary strand. The IC50 value of the reaction was found to be 1.75 ± 0.37 µM for G4TERT1 sequence, which is way less than telomestatin and B19 which had IC50 value of 5.0 ± 1.7 and 5.7 ± 1.1, respectively (Gomez et al., 2004). It also induces massive telomeric dysfunction along with DNA damage at 0.5–1 µM concentration (Douarre et al., 2013). 12459 reforms the hTERT alternative RNA splicing in A549 cells, thus suggesting that 12459 affects hTERT mRNA processing rather than hTERT DNA transcription leading to the downregulation of telomerase activity previously observed in A549 cells. The IC50 values of 12459 were found to be 1.75, 2 and 6.25 mM for G4TERT1, G4TERT2 and VNTR6-1, respectively. Two other potent G-Quadruplex ligands, telomestatin and BRACO19, did not amend splicing and transcript levels of hTERT, suggesting that the change in the RNA splicing is specific for triazine derivative (Gomez et al., 2004). Hence to conclude we can assume that 12459 induces the formation of G4 structure causing alternative splicing to reduce active telomerase formation. Stabilizing the G4 also allows the NME2 and REST co-repressor complex to bind to it and repress telomerase expression. Many structurally related Ru-polypyridyl complexes has already been reported to have higher affinity toward G-Quadruplex. Using Time-Resolved Infrared Spectroscopy, Devereux et al. (2020) showed the binding of the light-switch compound, [Ru(phen)2(dppz)]2 +, to quadruplex DNA in solution. Recently the first X-ray crystal structure of a quadruplex, d(TAGGGTT) bound RuIIdppz derivative has been reported. Besides organic G4-ligands, metal complexes based G4-ligands have recently attracted a lot of interest because of their selectivity. A series of TMPyP4 complexes with various metal centers, including main-group metals and transition metals (Ni(II), Mn(III), Mg(II), Cu(II), Zn(II), Pd(II), Pt(II), Fe(III), Co(II) etc.) have been synthesized. All these TMPyP4-metal complexes efficiently found to stabilize the hTelomere quadruplex and inhibit telomerase activity in vitro, due to the π-stacking abilities and cationic charge. Gold(III)–TMPyP4 complex has also been reported as an hTelomere G4 stabilizer and a potent telomerase inhibitor (Cao et al., 2017). Another novel platinum-quinacridine hybrid, composed of a monofunctional Pt moiety and a G-Quadruplex ligand (mono-para-quinacridine or MPQ), has been synthesized and reported to interact with quadruplex DNA (22AG, oligonucleotide that mimics the human telomeric repeat) via a dual noncovalent/covalent binding mode (Bertrand et al., 2007).
3.2.3 BCL-2
Certo et al. (2006) found that being the central protein to maintain a balance between apoptosis and survival, tumor cells depend upon the BCL-2 for their survival. Aberrant expression of BCL-2 is also responsible for neurodegenerative disease like Parkinson, Alzheimer's and multiple sclerosis (Kuhlmann et al., 1999; Marshall et al., 1997; Satou et al., 1995). There are G-Quadruplex located upstream of major promoter P1 of BCl-2 (Dexheimer et al., 2006). Several organic molecules are reported to bind to BCL-2 G4. But they fail to discriminate between BCL-2 G4 and other G4 in cell, thereby hampering their advancement as clinical drugs. A derivatives of furo[2,3-d]pyridazin-4(5H)-one (FP3; Figure 5) could selectively bind to the BCL-2 quadruplex and stabilize it; thereby down-regulating the expression and transcription of BCL-2 gene (Amato et al., 2018). The pyridazinone moiety was selected because of its anti-cancerous, anti-inflammatory and anti-thrombotic property. FP3 also was able to discriminate and selectively binds to BCL-2 G4. It showed substantial cytotoxicity in Jurkat cells with 30% inhibition at 10 µM concentration and 40% reduction in Bcl-2 protein on treatment with 25 µM for 24 hr (Table 2). Jana et al. recently confirmed that another quindoline derivative, chelerythrine, interacts with BCL-2 G4. Even though it shows higher quadruplex-to-duplex selectivity and induce selective cytotoxicity to varied cancer cells, the molecule is found to interact with telomeric and other oncogenic quadruplexes (Jana et al., 2017).
3.2.4 c-MYC
A triaryl-substituted imidazole/carbazole conjugate, with two 1-methylpiperazine side chains and a phenyl moiety in the imidazole ring, IZCZ-3 (Figure 5), can selectively bind c-MYC G-Quadruplex. It was seen that on removing the 1-methylpiperazine side chain the selectivity to c-MYC quadruplex was reduced. The 1-methylpiperazine helps to increase electrostatic interaction and the phenyl moiety reduce its binding affinities with antiparallel or hybrid G-Quadruplexes thus focusing it to bind to parallel c-MYC G-Quadruplexes (Hu et al., 2018). This ligand has the four-clover leaf like structure which as shown in Figure 5, stacked perfectly on the terminal G-quartet plane of c-MYC via π–π interaction, and the positively charged central imidazole moiety was located in the ion channel of the c-MYC G-Quadruplex, leading to a relatively low binding energy of −8.7 kcal/mol (Hu et al., 2018), thereby showing significant anti-cancer activity by arresting cancer cells at G0/G1 cell cycle. Another molecule 6-methyl-3-(naphthalen-2-yl)-8a-(4-methylpyridin-2-yl)-indolizinone(indolizinone) also selectively recognize c-MYC G-Quadruplex in 1:1 binding model (Yao et al., 2017). Thus, it was seen that molecule with aromatic group can stack onto the quadruplex and interact strongly (Yao et al., 2017) (Table 2). A series of Zn(II) complexes with amido-armed phthalocyanines has also been reported with excellent affinities and specificities for hTelomere G4. These molecules also showed the strongest binding (KD = 2 nM) when interacting with c-MYC G-Quadruplex DNA (Alzeer et al., 2009). A penta-coordinated Au(III) pyrazolylpyridine complex has been reported to interact strongly and selectively with c-MYC quadruplex DNA, through π–π stacking (Suntharalingam et al., 2010).
3.2.5 KRAS
KRAS is another most frequently mutated yet undruggable oncogene in human cancer. To target KRAS at gene level and develop new therapeutic strategy, one regioisomers of 5-methyl-indolo[3,2-c]quinoline derivatives (IQc:3e; Figure 5) with a range of alkyl diamine side chains was designed to target DNA promoter of the KRAS gene. IQc:3e has flat extended heteroaromatic surface that can stack onto G-quartets through π–π stacking and the basic side chains can hydrogen bond and establish electrostatic interactions with the phosphate backbone and loops/grooves of G4-DNA, either directly or mediated via water molecules. It was seen that molecules lacking alkyldiamines as their side chain produced lower affinity toward KRAS gene. The IQc:3e has IC50 value of 1.8 and 1.98 µM against KRAS dependent colon cancer cell line HCT-116 and pancreatic cancer cell line MiaPaCa2, respectively (Table 2). Whereas it has higher IC50 value for KRAS independent breast cancer cell line (IC50 = 11.40 µM) and lung fibroblasts WI-38 cell line (IC50 = 10.08 µM), respectively (Lavrado et al., 2015). Later, a series of novel multicarbazole derivatives, tricarbazolebenzene (TCB), has been synthesized to enhance G4 selectivity and stabilization. Further it was shown that C3-symmetry and a crescent shaped architecture incorporated by heteropolyaromatic multicarbazole scaffold impart both G4 interaction and selectivity. Ou et al. reported that multicarbazoles can bind selectively to G-Quadruplex over duplex DNA and show a higher stabilization for KRAS G4 compared to telomeric and other promoter G-Quadruplexes. They also reported the first high-resolution (1.6 Å) crystal structure of KRAS G-Quadruplex as a 5′-head-to-head dimer with extensive poly-A π-stacking interactions (Ou et al., 2020). Zinc complex of an isopropylguanidinium- modified phthalocyanine has also been reported as potent stabilizer KRAS promoter G-Quadruplex DNA (Membrino et al., 2010).
3.2.6 cKIT
Another gene c-KIT is generally involved in diverse biological function namely, maintenance of hematopoietic stem cell, melanogenesis, erythropoiesis, spermatogenesis, etc including cancer (Ray et al., 2008). Its dysregulation gives rise to plethora of diseases including cancer. Its gain-of-function mutation is linked to gastrointestinal stromal tumors (>70%), mast cell tumors (>70%) and some acute myeloid leukemia (>68%) (Zorzan et al., 2016). At first tyrosine kinase inhibitor (TKI) was used to treat cancer overexpressing c-kit. But soon cancer cells developed drug resistant against TKI leading toward the failure of those drugs. Just upstream c-KIT transcription start site, there are two G4 forming element, KIT1 and KIT2, regulating the c-KIT expression and hence they offer an excellent target for drug. Two anthraquinone derivative with (AQ1) and without (AQ7) phenylalanine moiety in its side chain was found to selectively bind to the c-KIT G4. The binding affinity drastically reduced on removing the phenylalanine moiety. Based on Alamar Blue cytotoxicity test, AQ1 (Figure 5) was found to have an IC50 value of 1.65 and 3.00 µM for HCG27 and MCF-7 cells, respectively (Zorzan et al., 2016). Whereas AQ7, being a better c-KIT quadruplex binder, showed meagre to no cytotoxicity. This is could be due to the inability of AQ7 in reaching intracellular target due to its large side chain. This proves that affinity does not corresponds to cytotoxicity every time. Another derivative of indenoisoquinolines (ISb) also selectively targeted c-KIT G4. Previously indenoisoquinoline derivatives have revealed antiproliferative properties in several human cancer cell lines (Morrell et al., 2007a, 2007b). ISb particularly stabilized c-KIT1 G4 having IC50 value of 4.1 ± 0.43 µM and 0.3 ± 0.034 in c-KIT overexpressed Gastrointestinal stromal tumor GIST882 cell line and colorectal cancer cell line HT-29, respectively (Table 2) (Bejugam et al., 2010). Recently an indolo-naphthyridine derivative discovered through structure-based pharmacophore models has shown strong affinity for c-KIT1 G4 as the result of the π–π stacking and two hydrogen bond interactions betrothed by morpholinopropyl side chain with the backbone residues of c-KIT1 G4. But being a non-optimized compound, this molecule could only impart very low cytotoxicity on HT-29 and A2780 cell lines (Catalano et al., 2019).
3.2.7 rDNA
In genome perhaps, the densest population of G4 after telomere is present in rDNA having 12% more G4 than rest of the genome. There are approximately 1,500 G4 motifs in rDNA non-transcribed strand that act as transcription activator (Sabouri et al., 2014). RNA Polymerase I complex (Pol I) is responsible for transcription of rRNA genes and as a result control ribosome biogenesis. Studies by Derenzini et al. have found that in cancer the Pol I activity and rRNA synthesis is remarkably high (Derenzini et al., 2000). Nucleolin stabilizes these G4 structure and enhances the activity of Pol I, to synthesize more rRNA (Hanakahi et al., 1999; Rickards et al., 2007). Thus, this nucleolin/G4 complex offers a potential therapeutic target to treat cancer. A fluoroquinolone derivative called quarfloxin (Figure 5) have been developed which targets and disrupts nucleolin/G4 complex inducing apoptosis in cancer cells. This is the first molecule to go to Phase II clinical trial (Drygin et al., 2009). It is selective in disrupting rRNA synthesis and does not inhibit DNA or protein synthesis. It has an IC50 value of 3.3 µmol/L (Table 2) (Hanakahi et al., 1999). This molecule is highly selective and kills cancer cells associated with over-expressive ribosome biogenesis. It does not hamper cellular effects associated with other G4 structures of other oncogenes. Quorfloxin is also resistant to multi drug resistant and hence has been developed as a key therapeutic molecule.
3.2.8 VEGF
Vascular endothelial growth factor (VEGF) plays a pivotal role for tumor angiogenesis by stirring the growth, migration, survival and permeability of endothelial cells thereby causing metastases. There are several VEGF targeting drugs available in market such as bevacizumab (Hurwitz et al., 2004; Sandler et al., 2006) sorafenib (Escudier et al., 2007) etc. The VEGF promoter contains a guanine-rich region which can fold to G4 structure and provides a platform for binding of multiple transcription factor such as sp1, Ap2 and Egr1 to regulate VEGF expression at transcriptional level (Sun et al., 2008). This calls for a novel methodology for rational drug design targeting VEGF quadruplex. Wu et al. (1840) synthesized quindoline derivatives and introduced 5-N-methyl and 7-fluoro groups to augment its interaction with the VEGF G-Quadruplex DNA. The IC50 of SYUIQ-FM05 (Figure 5) was found to be 1.0 ± 0.4 μM which is much lower compared to its parent molecule SYUIQ-05 (IC50 = 16.6 ± 0.7 μM; Table 2). Electron donating alkyl group, Fluorine and N,N-dimethylpropane-1,3-diamine side chain thus made it more potent and VEGF specific drug. The positive charge in the 5-N-position contributes to electrostatic interaction and improves the π–π stacking to quadruplexes. The alkyl amino group at the 11-position increases basicity of the quinolone nitrogen, which allows in situ protonation of the 5-N-atom at physiological pH and the cationic ligand core to contact the negatively polarized quartet center. Further VEGF transcription was suppressed dramatically at 0.5 µM concentration. But it also targets c-KIT and BCL-2 G4, thus lacks selectivity (Cammas et al., 2015; Wu et al., 1840).
3.2.9 c-Myb
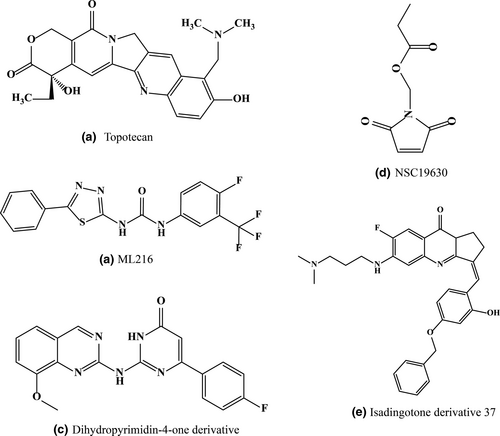
c-Myb gene was first associated with leukemia, later it was found to be linked with lymphomas, colonic tumors and several other solid tumors. The role of c-Myb gene in regulating cellular proliferation, differentiation and apoptosis is well established thus is an important switch in cancer therapy. The mammalian c-myb promoter contains three highly conserved GGA motif (Q3, Q2, Q1). Matsugami et al. (2001) solved the structure of one GGA G-Quadruplex motif and found that it forms a intramolecular or intermolecular quadruplex with a tetrad:heptad:heptad:tetrad (T:H:H:T) structure (Palumbo et al., 2008). Cui and Yuan (2011) demonstrated using ESI-MS that dehydrocorydaline, a natural compound can distinguish duplex from the cmyb quadruplex and bind to it specifically. This compound binds to the quadruplex with 1:2 binding stoichiometry to the cmyb quadruplex and increases the melting temperature by 18°C which is high enough for the molecule to be treated as a c-myb stabilizing agent (Cui & Yuan, 2011). Furthermore, it prefers the end-stacking mode with one molecule at each end site through π–π interaction with the G-Quadruplex. Another ligand dehydroevodiamine isolated from Evodia rutaecarpa is found to bind selectively with this promoter (Li et al., 2017). This ligand having hypotensive (Yang et al., 1990), antiamnesic and antiarrythmic (Loh et al., 2014) properties, binds to all three quadruplex structures. One interesting fact is that both compounds are natural alkaloid extracted from a Chinese herbal medicine thus the ligands have far fewer side effects. Li et al. after screening several molecules found topotecan (Figure 6), a small molecule with aromatic system having more affinities toward c-myb quadruplex. The binding is further strengthened by its heteroatoms which augments the electrostatic interaction with G-Quadruplex (Li et al., 2018). But this molecule stabilizes the quadruplex structure and enhances the transcriptional as well as translational activity of the c-myb gene. Hence topotecan acts as an enhancer of mRNA and protein expression (Li et al., 2018).
3.3 Ligands conjugated with peptides, oligonucleotides and glycosides
Since small molecules lack the G4 to dsDNA distinguishing property, G4 specificity and selectivity can be increased by incorporating additional structural motifs such as peptides, oligonucleotides and glycosides. Not only does these conjugates provide G4 to dsDNA specificity but even helps to specifically discriminate among different conformations of G4. Among them synthetic peptide-ligand conjugates are extensively studied. Specific tetrapeptides like RKKV, KRSR, and FRHR were tethered to the side chains of acridine ligands which shows higher affinity and specificity toward G4 over duplex DNA by interacting with the quadruplex loops (Ladame et al., 2004; Redman et al., 2009; Schouten et al., 2003).
NCQ, a neomycin (aminogylcoside) capped macrocycles was reported to bind G4 by interacting with the G-quartet and the loop by the formation of hydrogen bonds between base pairs and the amino and hydroxyl groups of neomycin (Kaiser et al., 2006). Later it was found that NCQ specifically binds to loop containing G4 than non-loop containing G4s. An open neomycin-perylene conjugate has also been reported recently with both base stacking and groove binding intaerction (Xue et al., 2011).
Acridine-oligonucleotide conjugates were also designed and found to interact with both single-stranded telomeric sequences and G4 structure (Casals et al., 2006). Several peptide nucleic acids (PNA) were also synthesized (Paul et al., 2008). Acridone scaffold was modified by conjugating peptide containing guanine repeats and found to interact with single-stranded fragment of (1 + 3) hybrid quadruplex forming telomeric DNA (Paul et al., 2008). Oligonucleotide conjugates interact with both external surface of G4 and its central scaffold. Thus, such ligand conjugates serve as an efficient G4 binding scaffold for better recognition, higher specificity, and improved solubility. Above all, it is necessary to choose the right biomolecules for designing future ligand conjugates with higher bioavailability, biocompatibility, and lower cytotoxic effects.
Bonnat et al. showed the application of cyclic peptides-based conjugates to stabilize folded topology of G-rich DNA oligonucleotides of sequence d(5′-TGGCCTGGGC-3′) and d(5′-GGACTGGG-3′). They increased the specificity by loading these oligonucleotides sequences onto a cyclic peptide scaffold. G-Quadruplex motif from the promoter region of the HIV-1 long terminal repeat (LTR) were conjugated onto a cyclopeptide skeleton through sequential oxime ligation, a thiol-iodoacetamide SN2 reaction, and copper-catalyzed azide–alkyne cyclo addition reactions. The resulting conjugate was shown to fold into a highly stable antiparallel G4 architecture (Bonnat et al., 2017).
Rotaxanes have recently been employed as triggerable cages by Kench et al. for the binding of Pt(II) complexes with G4-DNA and to enhance the selective cellular uptake, cellular localization, controlled release and high degree of spatiotemporal control of the cytotoxicity in cancer cells. They emerged as highly selective G4-DNA binder and probe, with Ka values in the range of 106 M−1 (Vilar et al., 2021).
3.4 Peptide selective to G-Quadruplex
Since developing complex conjugated ligand requires intricate complicated reactions, people tried developing simple yet strong and specific G4 binders. It is seen that naturally several proteins have evolved that specifically binds to G4s either to stabilize or destabilize it viz. NM23-H2, Nucleolin etc (Thakur et al., 2009; Tosoni et al., 2015). However, the inherent properties of protein like large Molecular weight, low permeability, immunogenicity, and enzymatic volatility generate impediments in developing protein as a drug candidate. On the other hand apart from other biologics (Protein and DNA) peptide offer an attractive therapeutic potential that could be used to target G4s. Peptides act as excellent protein mimetic, biologically mimicking the activity of proteins, interacting sturdily and selectively with its target quadruplex. Being smaller sized it is non-immunogenic, and its ease of synthesis permits it to be synthesized in industrial quantity. Present combinatorial chemistry allows the development of series of peptide having wealth of therapeutic potential. Though the research with developing anti-cancer peptide that targets G4 is at a premature stage, still some worthwhile work has been carried out.
Jana et al. (2013) reported that LL37 (Table 3), a human cathelicidin antimicrobial peptide, which has different immunological function, is a binder of G4. LL37 selectively binds and stabilizes the human telomeric G-Quadruplex structures and downregulate telomerase activity. The LL37 peptide binds to G-Quadruplex with 10-fold selectivity over duplex DNA. Four different telomeric sequences, P1 [TAGGG(TTAGGG)3TT], P2 [GGG(TTAGGG)3T], P3 [GGG- (TTAGGG)3TT], P4 [TAGGG(TTAGGG)3] are chosen and the binding affinity of the G-Quadruplex with LL37 was found in the order P4 > P3 > P1 > P2. Molecular Dynamics simulation studies showed that Arg7 and Arg23 are the two important residues of LL37 which mainly interacts the negatively charged phosphate backbone of G-Quadruplexes, thus stabilizing it and probably pushes proliferating cells toward apoptosis. But since LL37 is a large protein it is cell membrane impermeable also the binding affinity of LL37 toward G-Quadruplex is too weak for it to become a viable drug (Figure 7).
| No | Peptide name | Peptide sequence | Net charge at PH = 7 | Targeted quadruplex |
|---|---|---|---|---|
| 1. | 003 | EHKGKWK | +1.1 | WNT |
| 2. | 007 | EWKGKWK | +1 | WNT |
| 3. | 015 | EYKGKWK | +1 | WNT |
| 4. | 010 | KFEGKWK | +1 | c-MYC |
| 5. | KR12C | KRIVKLIKKWLR | +6 | c-MYC |
| 6. | LL37 | LLGDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPRTES | +6 | Telomeric |
To address these issues, our group had truncated the quadruplex binding domain of LL37 and made some selective mutations to increase the affinity toward c-MYC G4 developing a peptide molecule, KR12C (Table 3), which is highly specific to previously undruggable c-MYC G4 and stabilize it by 9°C as observed from Circular Dichroism melting experiment. We also observed binding affinity (KD) of the peptide to the c-MYC G4 is 901 nM (Sengupta et al., 2018) through Isothermal titration Calorimetry, in the presence of MCF-7 nuclear extract (which mimicked a molecular crowding condition). KR12C was involved in a multitude of electrostatic and weaker CH3–π interactions to impart selectivity and stability to MYC G4 structure. The guanidino group of R12 attaches to the sugar-phosphate backbone of by electrostatic interaction. Similarly, the penultimate R2 at N-terminus clings to the sugar-phosphate backbone of 5′ end of MYC G4 (Sengupta et al., 2018). These two connections were extremely steady as experimented from Molecular Dynamics Simulation. They protected the G-quartet proximal to 5′-terminus by gripping the backbone of the guanines delineating the quartet. Furthermore, L11 and I7 came into spatial proximity of the terminal quartet and stabilized the Hoogsteen hydrogen bonding between via CH3–π interactions (Sengupta et al., 2018). This peptide also downregulates c-MYC transcription by ~5 fold (Sengupta et al., 2018). The peptide achieved this feat by binding to the G4 and depleting the occupancy of both NM23-H2 and Nucleolin.
Kobayashi et al. also developed a peptide library to check interaction of the peptides with various quadruplexes by electrophoresis. He generated two series of peptides with the hope to find NRAS/c-MYC/WNT/FGF G4 specific peptide (Table 3). He added varied amino acids to the N-terminus of the KWK motif with the hope to vary aromatic character (H, W, F, or Y), flexibility of the main chain (G or P) and charge of the peptide (K or E) (Kobayashi et al., 2011). It was found that peptide 003, 007 and 015 binds specifically to WNT G4 binder (Kobayashi et al., 2011) and peptide 010 binds to c-MYC G4 (Kobayashi et al., 2011). They furthered their study with the peptide 010 and found that it increases Tm of MYC G4 by 8°C and that of FGF G4 by 4°C (Kobayashi et al., 2011). Circular Dichroism study reported that 010 is a parallel quadruplex specific binder. Though specific studies with these peptides are yet to be done work by Kobayashi gives an excellent start point.
So, to summarize the G4 interacting peptide must have an aromatic amino acid to stack to quadruplex and must be positively charged so that it can pass through the cell membrane easily and interact with the phosphate backbone of DNA. But one major drawback of peptide is that they are susceptible to peptidases/proteases in body (Thundimadathil, 2012). But it can be evaded by using several mechanisms like blocking the C- and N-termini, using D-amino acids, and chemical modifications improving their pharmacodynamic and pharmacokinetic profiles (Borghouts et al., 2005).
3.5 G-Quadruplex recognizing probes and sensors
As the research around G-Quadruplex gained momentum, so did the obligation of visualizing the G-Quadruplex in a duplex rich genome amplified. To cater this demand several versatile sensors and fluorescent probes were developed which can bind to different conformers of G-quartet or G-Quadruplex structures in-vivo and ex-vivo. The important aspect to remember is a good fluorescent probe must show both higher selectivity and high fluorescent intensity upon binding to the target quadruplex. Since discussing in detail about the probe is beyond the scope of this review, we summarized a few key probes that are used to target G4s.
3.5.1 Cyanines
Cyanines are a class of dye that have the tendency to bind to the G-quartet structures. The pentamethine analogs of cyanine dye unveiled an entropically driven (Nanjunda et al., 2012) initial 1:1 binding stoichiometry with an end-stacking binding mode with a preferential binding for parallel over hybrid G-Quadruplexes followed by a weaker binding. The pentathemine linker furnished the desired elasticity for efficient stacking at the terminal tetrads of the quadruplex. Further dimethyl substitutions on both indolenine rings substantially diminish their affinity for DNA duplex (Nanjunda et al., 2013) as the bulky methyl group causes steric hindrance and fails to bind to the duplex groove. The quadruplex affinity and solubility are further enhanced by introducing ammonium cations to the heterocyclic nitrogen. Halogenation at the 5-position and the meso-carbon of the polymethine-bridge helps increasing the pharmacological features (Marcelo Zaldini et al., 2010). The dimethyl indolenine cyanine scaffold was selected based on the concepts that it could not fit into the minor groove, as with planar cyanines, but could stack on the terminal tetrads of quadruplex structure with cationic chains in the quadruplex grooves. On the other hand, another DNA sensitive cyanine dye- meso-substituted trimethine “Cyan 2” interacts strongly with both triplex (through groove binding) and G-Quadruplex DNA (through DNA intercalation) (Kovalska et al., 2011). But since cyan 2 can bind to G4-DNA only in absence of K+ and other cations its use as in-vivo probe is limited (Kovalska et al., 2011). Research by Tang's group shows that the size of aromatic group also plays a very important role in the quadruplex selectivity. He showed that large aromatic substituents are required for aggregation and higher selectivity toward G-Quadruplex structures whereas smaller aromatic substituents lack quadruplex selectivity suggesting that large surface area are essential for proper π–π stacking of the dye with quadruplex (Yu et al., 2015). Cyanines are thus a major group which can bind to quadruplex using end-stacking mode and act as a probe for G-Quadruplex visualization.
3.5.2 Template-assembled synthetic G-Quartets (TASQs)
G-quartets are classically templated and stabilized by cations, whereas in the absence of cations guanines aggregates to form ribbon like structures (Sessler et al., 2000). Cation-free G-quartets are rare due to the repulsion of the coplanar carbonyl groups and the high stability of less-ordered polymeric ribbons. Nikan and Sherman (2008) developed a lipophilic guanine linked cavitands named template-assembled synthetic G-Quartets (TASQ) by click chemistry which are stable at low concentration in a cation independent environment. The chemical shift value of 1H NMR of this compound suggests that the guanine bases of TASQs are not assembled as the guanines of native quadruplex. They also found a strong intra-residue NOE among H8 and H1′ signifying a syn conformation along with glycosidic bond which thwarts the unstructured G-ribbon formation. A negligible shift (Δδ = 0.2 ppm) in the imino signal even at 50°C indicates a stable H-bond and hence a stable G-quartet structure at high temperature. These TASQs have high application as G-quartet aptamers or as G-quartet recognizing protein screens. Another water soluble TASQ named PNADOTASQ (DOTA-templated synthetic G-quartet), acts as both DNA and RNA G-Quadruplex Ligand (Haudecoeur et al., 2013). The DOTASQ also can catalyse pseudo-enzymatic oxidation reactions in a DNAzyme-inspired manner. Previous reports show that quadruplex/hemin complex in presence of H2O2 can perform peroxidase like oxidation reaction (Josephy et al., 1982). This TASQ can in fact replace quadruplex and mimic peroxidase and perform the same function albeit with a decreased affinity (Haudecoeur et al., 2013). They also have several other bionanotechnological applications that are yet to be fathomed. It is of no doubt that though research with synthetic Quadruplex (SQ) has just begun but the application and rich diversity that the field present is astounding, and its true potential is yet to be realized in nanobiotechnology.
3.5.3 Distyryl dyes
The distyryl skeletons are synthesized by condensation between pyridinium salts and aldehydes. Distyryl dyes has been used in designing of sensors for different types of analytes (Li et al., 2004). Spectroscopic studies showed that cationic dyes like 2,4-bis(4-dimethylaminostyryl)-1-methylpyridinium and its quaternary aminoalkyl derivative not only selectively binds to G-Quadruplex in micromolar concentration with far greater affinity compared to dsDNA but also can discriminate between different G-Quadruplex (Xie et al., 2013). Recent report suggested that these dyes interact with human telomeric G-Quadruplex with a very high affinity than that of oncogenic promoter G-Quadruplex. One sees an increased fluorescence intensity and melting temperature by the binding of c-MYC and c-KIT1 G-Quadruplex with these dyes. They even can selectively detect G-Quadruplex structure from dsDNA crowded condition. For this specificity, they can be used for selective staining of G-Quadruplex DNA in polyacrylamide gel. Due to its high fluorescence enhancement and luminescence responses, this distyryl dye can be used as a promising G-Quadruplex probe.
3.5.4 BODIPY dyes
Due to its high molar absorption coefficients, sharp absorption bands, high fluorescence quantum yields and photochemical stability, BODIPY dyes has been used as probe (Ulrich et al., 2008). Recently few BODIPY based molecules has been synthesized by screening a library of molecules, which can specifically bind to parallel G-Quadruplex DNA with exposed ends and four medium grooves with higher affinity than that of anti-parallel G-Quadruplexes, dsDNA, or ssDNA. Molecular modeling study showed that these compounds prefers groove binding upon end stacking (Zhang et al., 2014). Hence, BODIPY dyes can be used as a potential groove targeted, structure sensitive G-Quadruplex probe. The importance of disaggregation and carboxymide group in these sensors for fluorescent probing has also been demonstrated. The hydrogen binding capacity of the carboxymide group with guanine and adenine is essential for high affinity docking to the quadruplex (Zhang et al., 2014).
3.5.5 Tetraphenylethene dyes
There are some non-emissive dyes whose fluorescence intensity can be enhanced by aggregate formation. Tetraphenylethene (TPE) dyes has this unique aggregation-induced emission properties. Two tetraphenylethene derivative with positively charged side arms, that is, DATPE (1,2-Bis-1,2-tetraphenylethene dibromide; (Zhang et al., 2015)) and QATPE (1,1,2,2-Tetrakistetraphenylethene tetrabromide), two and four 4- (N,N,N,-trimethylammonium)butoxy substituents, respectively, has been synthesized (Zhang et al., 2015). DATPE binds only with multimeric G-Quadruplexes, having a low in-vivo cytotoxicity and little effect on the stability of multimeric G-Quadruplexes. On the other hand, QATPE not only has a high binding affinity toward multimeric G-Quadruplex but also has a higher stabilizing activity on them. But the lower G-Quadruplex recognizing activity compared with that of DATPE, makes QATPE a promising multimeric G-Quadruplex targeting drug candidate. Due to the low cytoxicity, tetraphenylethene dye emerges as a promising probe for detection of multimeric G-Quadruplex in cells.
4 LIGANDS THAT INDUCE CHANGES IN G4 TOPOLOGY WHILE MAINTAINING QUADRUPLEX FORM
Though G-Quadruplex formation is determined by stacking of more than two planar G-quartets, the combination of the number of stacked G-tetrads, the polarity of the strands and the location and length of the loops results in the formation of varied G-Quadruplex topology. Generally, G4 structures are categorized into three topologies, that is, parallel, hybrid, and anti-parallel, reliant upon the alignment of the G-tracts.
In the parallel topology, all four G-main chains are parallel to one another. In the hybrid and anti-parallel topologies, one and two G-tracts are in anti-parallel orientation, respectively. The G-tracts in quadruplexes are connected by loops, and there are three types of loops: propeller, lateral, and diagonal. Propeller type of loops connect the bottom G tetrad to the top G tetrad between adjacent parallel strands. Lateral loops are formed when adjacent anti-parallel G-backbones are joined while diagonal loops are formed when an anti-parallel and parallel G-main chain on the diagonal of the G-quartet are connected.
The Balasubramaniam group was one of the first to develop a triamino-anthracene derivative 1 that could induce single-stranded DNA to fold into parallel G-Quadruplex in the absence of added cation. This molecule has two amine groups at optimum distance which mimic the distance between K+ ions in the native G4, permitting the protonated amine moieties to perform the role of channel cations at physiological pH.
There are rare examples of ligands that drive the transformation to antiparallel or hybrid structures. The Hurley group in 2005 reported that telomestatin can induce the conversion of hybrid G4 to the antiparallel form in K+ buffer (Rezler et al., 2005). Sanders et al. reported that the porphyrazine derivative reversed the activity of anthracene 2 to regenerate an antiparallel G4 structure in telomere DNA(telo24) (Gonçalves et al., 2006). Classical G4-ligands like 360A, PhenDC3, and pyridostatin can induce changes in G-Quadruplex folding topology in cation stoichiometry in telo22, telo23, telo24, telo26 DNA, observed as a shift from two to one K+ cation per G4, indicating the presence of only two contiguous G-tetrads. Spectral features from CD data also supported the emergence of an antiparallel folded structure upon ligand binding. Gray et al. (2009) reported that porphyrin derivative, TMPyP4, despite its poor selectivity for G4 over duplex DNA, could induce the switching of telomeric DNA from antiparallel basket to hybrid in Na+ buffer as monitored through CD.
5 G-QUADRUPLEX INTERACTING PROTEINS: AN ALTERNATIVE TARGET FOR LIGANDS
Many studies have suggested that the dynamic nature of G-Quadruplex is highly regulated by different types of proteins which interacts with it. The folding and unfolding of G-Quadruplex are critically maintained by these proteins. G-Quadruplex interacting proteins are divided into three groups based on their effects on G4s- stabilizing G-Quadruplex (Nucleolin, Topo1, thrombin), unwinding G-Quadruplex (Pif1, RHAU/DHX36, BLM, Fanconi Anemia Complementation Group J [FANCJ], WRN, hnRNP) and degrading G-Quadruplex (GQN1, Mre11).
The effects of TmPyP4 and telomestatin on G-Quadruplex are well studied but in recent years the effect of these molecules on G-Quadruplex targeting proteins are also extensively considered. In HeLa cells, Telomestatin competes with different telomere binding proteins by downregulating their expressions. Telomestatin leads to the dissociation of TRF2,inducing the dissociation of different proteins of shelterin complex, that is, TOP3, POT1, leading to telomere uncapping (Tahara et al., 2006; Temime-Smaali et al., 2009). Telomestatin also shows inhibitory activity on FANCJ thus increasing DNA damage levels, impaired proliferation, and apoptosis (Bharti et al., 2018). On the other hand, TmPyP4 inhibits RecQ helicase which unwinds the structure of G-Quadruplex DNA and arrests cells at S and G2/M phase by inhibiting Telomerase (Morris et al., 2012). N-methyl mesoporphyrin IX (NMM), a similar compound like TmPyP4 also inhibits different G-Quadruplex related helicases (Wu & Maizels, 2001). On the other hand, PIPER also inhibits different quadruplex specific helicase by unwinding the structure without any effect on double stranded DNA (Han et al., 2000). Competition experiments indicate that this inhibitory activity of PIPER is due to the interaction of PIPER with G-Quadruplex structures rather than the helicase itself. All the results showed a probable mode of action for these G-Quadruplex-interactive agents inside cells. They might induce G-Quadruplex formation in G-rich regions on genomic DNA, stabilize these structures, and prevent them from being cleared by enzymes such as helicases.
5.1 Designer ligands blocking protein-G-Quadruplex interactions
Earlier it was reported that, NDPK/NME-NM23, a metastasis suppressor gene has many roles in transcriptional regulations (Steeg et al., 1988). NM23-H2 is a G-Quadruplex binding protein, regulating PDGF, c-MYC from various cancer cell lines like human cervical, Burkitt lymphoma etc (Thakur et al., 2009). Thus, selective small molecules targeting NM23-H2 can be a promising way to block the interaction between NM23-H2 and G-Quadruplex. SYSU-ID-01, an isaindigotone derivative has been synthesized to inhibit NM23-H2–G-Quadruplex interaction. But due to its low binding affinity, the skeleton was modified (Compound 37; Figure 6) to develop selective binding ability to stabilize NM23-H2, thus inducing anti-tumor activity. Molecular dynamics study showed that Compound 37 is suitably adjusted into the narrow, curved pocket of the dinucleotide and interact with Gly113 and Asp 121 of the active site of the protein by means of hydrogen bonding. It also interacts with His118 and Lys66 by hydrophobic interaction and Phe60 and Tyr67 via π–π interaction. Other isaindigotone derivatives were also developed to bind to both NM23-H2 and G-Quadruplex, thus distorting the G-Quadruplex-NM23-H2 interactions and inhibiting c-Myc dependent cellular effects in SiHa cells by impeding transcription, translation and inducing apoptosis.
In 2013, a large high-throughput screening for BLM inhibitors was carried on using a virtual library of 350,000 compounds from Molecular Libraries Small Molecule Repository library. One compound MLS000559245 was selected. The modified version of this molecules, that is, ML216 (Figure 6) has been selected as lead compound. It is a BLM helicase inhibitor. BLM is found to interact with GQ in-vivo (Sun et al., 1998). ML216 induce anti-proliferating activity in BLM expressing cells, promoting exchange of sister chromatids and increased amphidicolin toxicity (Nguyen et al., 2013). Porphyrin skeleton is also another promising core for helicase inhibitors. But still there are no evidence related to G4. Interestingly another such virtual screening experiment in the same year led to the discovery of a ligand bearing phenyl-psoralen moiety having selective G4 binding property (Alcaro et al., 2013). Though there is no evidence that if this molecule could inhibit protein-Quadruplex interaction, but it was found that interaction of photoexcited psoralen derivative to the telomeric G4 is indulged by parallel/quasi-parallel orientation between the two (Paul & Samanta, 2018).
From National Cancer Institute Diversity Set, NSC 19630 (1-(propoxymethyl)-maleimide) (Figure 6), a small molecule was identified and synthesized. From in vitro helicase activity screening, it has been observed that NSC 19630 has an inhibitory effect on Werner syndrome helicase (WRN) activity with higher selectivity but did not have any effect on other DNA helicases like RECQ1, RecQ, DnaB, FANCJ etc. It has been previously shown that WRN interacts with quadruplex motif (Wu et al., 2018). NSC 19630 treated human cells shows decreased growth and proliferation, delayed S-Phase, accumulation of stalled replication forks, inducing apoptosis in a WRN-dependent fashion. Increased number of γH2AX and PCNA (proliferating cell nuclear antigen) foci have also been observed. NSC 19630 was also found to sensitize the cells to the telomestatin or PARP inhibitor. At higher concentrations (6 and 12 μM), NSC 19630 inhibits proliferation by 99% even after 1 day of treatment in HeLa cell line (Aggarwal et al., 2011).
6 MODES OF G-QUADRUPLEX–LIGAND INTERACTION
From the example shown above it is clear that presence of G-Quadruplex in and around key oncogenes, RNAs and telomere makes it a suitable site for ligand targeting. There are many ligands which binds to G-Quadruplex either to stabilize or destabilize it. G-Quadruplexes have several interaction sites. Since true intercalation (opening of the two quartet to incorporate a planar molecule) is not possible, end stacking is more preferred mode of binding, as it does not require the transient unstacking of G-quartet (Fedoroff et al., 1998). Several ligands rather than binding to preformed quadruplex facilitates the guanine-rich region to fold to quadruplex. Kinetic study has led to the better understanding of the mechanism of these events. A perylene derivative called PIPER (Figure 4) is an example of such compound which promotes the folding of telomeric sequence TTAGGG to dimeric and tetrameric G4s (Han et al., 1999). Generally, most of the ligands possess similar chemical structure namely planar aromatic ring and charged side chain. The G-Quadruplex forms an ideal platform where ligand can bind with π–π stacking. Some exploit the groove formed by the backbones and bind to the G4, which mainly include the terminal G-tetrads, the middle G-tetrads, the grooves/loops/backbones, and the central channel. Ligands with planar aromatic rings interact with the G-Quadruplexes mainly through π–π stacking on the terminal G-tetrads and less through intercalating into the G-tetrads (Tawani et al., 2017; Yao et al., 2017). Ligands containing amino groups and (or) side chains can interact with the grooves, loops, and the negatively charged phosphate backbone of the G4s. The amino groups become positively charged by protonation or methylation, which may result in better recognition and stronger binding to the grooves as well as to the negatively charged phosphate backbone through electrostatic interactions (Calabrese et al., 2018; Murat et al., 2011). The side chains can recognize the loop bases and grooves by forming hydrogen bonds, which also improves the water solubility of the ligands. More interesting is that small-sized ligands with long positively charged side chains can lie at the central channel of a G-Quadruplex, which in turn leads to an increase in the stabilization of the G-Quadruplex. Research has indicated that although planar ligands can insert into the G-tetrad layers, charged molecules are more inclined to interact with the grooves, loops or backbones of G –quadruplex structures (Calabrese et al., 2018; Chen & Yang, 2012).
7 CONCLUSION
Playing a major role in regulating gene expression, G-Quadruplex can become a striking anti-cancer target. As quadruplex offer a varied biological function, targeting them has become an attractive area of drug discovery research. A fresh perspective to these motifs has led to the development of ligands that interact with these structures. In this review, we have seen that there are several small molecules developed against G4 structures. After years of research, G-Quadruplex targeting molecules are being tested clinically. One of the most promising therapeutic candidate against several tumors is Quarfloxin (CX-3543) (Drygin et al., 2009), a G4 ligand which has already reached Phase II trials and more recently, CX-5461 (Xu et al., 2017), which is currently in the advanced Phase I clinical trials for patients with BRCA1/2-deficient tumors. Another important ligand triarylpyridine 20A showed high binding affinity toward telomere repeats in-vitro and affecting many biological activities (Kerkour et al., 2017). Transcriptome analysis of triarylpyridine 20A-treated HeLa cells showed the growth arrest of cancer cells in culture and in a HeLa cell xenografted mouse model. This response is associated with the induction of senescence and apoptosis. It has been reported to have more G4 motifs in all those down regulated genes compared with unchanged and upregulated genes, suggesting multiple G4-dependent inhibitory effects of 20A (Beauvarlet et al., 2019). Over the last few years, many G4 aptamers came to light for their anticancer activity. One of the most famous example of a G4 aptamer is AS1411, which has already reached Phase II clinical trials and showed promising activity against metastatic renal cell carcinoma and acute myeloid leukemia with minimal toxicity (Rosenberg et al., 2014). AS1411 is a 26 base-pair guanine-rich aptamer that specifically targets nucleolin, a protein that is overexpressed on the surface of cancer cells, making it a tumor-selective target (Bates et al., 2017). But still the numbers are too small to excite us. In-fact most of the small molecules fails to distinguish between different quadruplexes and show selectivity to more than one G4. Since G-Quadruplex have high polymorphic and flexible conformation it is difficult to design ligands that can detect and interact with these structures. Artese et al. (2013) addressed and discussed this issue in their research with telomeric quadruplex. More focus and in-depth investigations are required to address the folding mechanism of the G4 structures as it would not only help to integrate the knowledge of G4 stabilization but one would also be able to exploit those information to design more selective G4 binders. With this in mind and to get further insight into the intermediate states of the folding of parallel h-telo G4s Rocca et al. (2020) used Well-Tempered Metadynamics simulation, to uncover detailed atomistic sketch of previously unknown significant metastable folded states and retrieved an ensemble of six G4s having two/G-tetrad conformations drawn by the G-triplets vertical slippage.
The major drawback of small molecule as a quadruplex targeting drug is its off-target effect, toxicity and high hydrophobicity. Sometimes these molecules degrade to produce toxic metabolite and show low body clearance. All these drawbacks must be addressed to develop a molecule as a drug. To be a G4 targeting drug any molecule must adhere to the following condition- (a) It must distinguish quadruplex from duplex and bind to it. (b) It must discriminate between different G-Quadruplexes. (c) It should have low off-target effect. (d) It must be cell-penetrable and (e) It must not degrade to toxic metabolite in cell. A molecule which checks all these criteria is yet to be discovered. Therefore, drug discovery calls for an alternative treatment and hence conjugative molecules and peptide as a drug is flourishing. Slowly researchers are focusing more to derive G4 stabilizing peptide. Present combinatorial chemistry and phage display library technique allows one to evaluate multiple peptides against a target and select best from the lot in one go. Since peptide can fold back and forth on their own, they are able to modify themselves according to changing G4 landscape while binding to them, providing more selectivity. Moreover since peptides are composed of amino acids, metabolism of peptides doesn't give rise to toxic metabolites. But the major drawback of peptide drug is their sensitivity to peptidases and proteases which reduces their plasma half-life. This enzymatic degradation of peptide can be evaded by finding out potential cleavage site followed by relevant amino acid substitution. Alternatively, one can also introduce stapling, cyclization, or lactam bridge to prevent enzymatic cleavage (Ali et al., 2019; Gang et al., 2018).
Studies by structural biologists suggest that G4 structures are highly polymorphic and display discrete variances between each other. These countless topologies arise from the combination of numerous well-defined structural elements such as nucleic acid type (DNA or RNA), molecularity (monomer, dimer or tetramer), strand orientation (parallel, antiparallel or hybrid), loops (orientation, sequence and length), grooves type (narrow, wide and/or medium), end-cap morphology and number of G-tetrads. Taking all these elements to account provides opportunities for discovery and study of new G4 specific ligands. One also need to apply descriptors-based approach like 3D-QSAR, CoMFA (Alignment depended method), CoMMA (utilizes moments of the molecular mass and charge distributions) for successful ligand discovery and rationalize molecular activity profile. The role of water molecules in the G4 structures are neglected a lot while designing ligand and thus investigation by Alcaro et al. (2011) using Averaged Solvent Accessible Surface Area (ASASA) descriptor as a tool has provided much needed insights into the role of the solvent in the binding event of known G-Quadruplex ligands demonstrating diverse conformational contours.
The ill-fated drawbacks that make G4 an elusive target can be handled by incorporating bioinformatics and computational approach which can lead to the discovery and development of more novel ligand that can distinguish varied topology of G4 with much more stringency. Adopting structure-based drug design and ligand screening from a virtual database is the next step to ensure that one gets a ligand that is selective to a quadruplex (Rocca et al., 2017b). NMR and X-ray crystal structure of G-Quadruplex-ligand complex has shed light onto the atomic interactions that take place between them and this information can be harnessed to develop more potent drug (Campbell et al., 2008).
Though there is still no G4 targeting ligand which can be used as a drug, G4 directed therapy is at a rise given its importance in cancer and other disease. Current research and generation of new techniques is mining out wealth of knowledge about the G4 structures and it is not long till we find next-generation G4-targeting ligands with higher therapeutics and lower cytotoxicity. There appears to be merit and rationale in selecting G-Quadruplexes as a potential drug target. But the research should now change its outlook to see what else the ligands can do other than stabilizing G-Quadruplex structures which might have direct impact in both clinical and non-clinical sectors.
Biographies
Nilanjan Banerjee is a PhD graduate student in the Department of Biophysics at Bose Institute. He received his Master’s degree in life sciences at Bose Institute with specialisation in biophysical chemistry. His research focuses on the structural biology of G-quadruplex scaffolds and their role in cellular processes and neoplastic transformation. His current research interests include de novo anti-cancer peptide design targeting the G-quadruplex structures embedded in the oncogene promoters.
Suman Panda is a PhD graduate student in the Department of Biophysics in Bose Institute. He received his Master’s degree in Botany from University of Calcutta with specialisation in Advanced cell biology. His research focuses on the intervention of G-quadruplex structures in Telomerase overexpressed and ALT (Alternative lengthening of telomere) positive cancers. His current research interests also include finding out lead molecules, peptides having high therapeutic potential and selectivity towards G-quadruplex.
Subhrangsu Chatterjee is an Associate Professor in Department of Biophysics at Bose Institute. He obtained his PhD in nucleic acid structures and dynamics from the Department of Organic Chemistry and Biochemistry at Uppsala University. He has co-authored articles in many publications including Journal of the American Chemical Society, Nucleic Acid Research, Proceedings of the National Academy of Sciences, Immunity and Oncotarget. He worked on prion protein misfolding in the Department of Biochemistry at the University of Alberta. His current research is focused on the structure and functions of G-quadruplexes in oncogenic promoters and noncoding RNAs and their implications in transcription and translation modulation to drive cancer progression.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




