Sofosbuvir as treatment against dengue?
Abstract
Dengvaxia® (CTD-TDV), the only licensed tetravalent dengue vaccine by Sanofi Pasteur, was made available since 2015. However, administration of CTD-TDV, in general, has not received the prequalification recommendation from the World Health Organization. Having a universal antidengue agent for treatment will therefore beneficial. Accordingly, the development of nucleoside inhibitors specific to dengue viral polymerase that perturb dengue infection has been studied by many. Alternatively, we have used a marketed anti-HCV prodrug sofosbuvir to study its in silico and in vitro effects against dengue. As a result, the active metabolite of sofosbuvir (GS-461203) was predicted to bind to the catalytic motif (Gly-Asp-Asp) of dengue viral polymerase with binding affinity of −6.9 kcal/mol. Furthermore, sofosbuvir demonstrated excellent in vitro viral inhibition with an EC90 of 0.4 μm. In addition, this study demonstrated the requirement of specific liver enzymes to activate the prodrug into GS-461203 to exert its antidengue potential. All in all, sofosbuvir should be subjected to in-depth studies to provide information of its efficacy toward dengue and its lead potential as DENV polymerase inhibitor in human subjects. In conclusion, we have expended the potential of the clinically available drug sofosbuvir as treatment for dengue.
1 INTRODUCTION
Dengue virus (DENV) which causes dengue fever and its severe manifestations, such as dengue hemorrhagic fever (DHF) and dengue shock syndrome (DSS), had been estimated to result in global burden of 390 million (95% credible interval 284–528) dengue infections per year, of which 96 million (67–136) manifest apparently (with any level of clinical or subclinical severity).1 In order to combat the disease, Sanofi Pasteur has successfully developed and obtained the first license to market the tetravalent dengue vaccine Dengvaxia® (CTD-TDV); the administration of the vaccine was subsequently approved in more than 10 countries—Mexico, Brazil, Philippines, El Salvador, Costa Rica, Paraguay, Guatemala, Peru, Indonesia, Thailand, and Singapore.1 However, it is worth mentioning that World Health Organization (WHO) only recommends countries with high prevalence of dengue cases to consider the introduction of the vaccine and has yet to prequalify the use of CTD-TDV in general.2 Furthermore, CTD-TDV has lower protective efficacy against serotypes 1 and 2 of DENV (DENV1 and DENV2),2 and was not engineered to protect against the infection of the fifth serotype of DENV (DENV5) which was newly discovered in 2013.3 The emergence of new virus serotype strongly suggests that the current design of tetravalent vaccine may not provide complete protection against dengue infection. In contrast, candidates with CTD-TDV pose a higher susceptibility to develop the original antigenic sin4 and manifest DHF or DSS if they are infected by DENV5 or other unclassified DENV serotype.5 Hence, rapid development and approval of efficacious antidengue agent as treatment for infected patients can be importantly useful.
As part of the Flaviviridae family, all dengue virus serotypes contain a ~11-kb single-stranded RNA of positive polarity encoding three structural (C-prM-E) and seven non-structural proteins (NS1-NS2A-NS2B-NS3-NS4A-NS4B-NS5).6 Interfering the viral structural protein development demonstrated reduction in subsequent viral infection; disruptions to any of the enzyme activities encoded within the viral non-structural protein region showed a perturbation of the viral replication.7-9 Treating hepatitis C (HCV), another virus from the same family, infected patients with a potent RNA-dependent RNA polymerase (RdRp) inhibitor GS-461203 has resulted in viral clearance and maintained up to 100% sustained virologic response.10 For example, the viral RdRp is located at the C-terminus of NS5 non-structural protein—similar location in the polyproteins of flavivirus genus and hepacivirus genus, which is crucial for the viral RNA replication.6 Further improvements made to the nucleoside triphosphate analogue have resulted in the development of sofosbuvir, a prodrug of GS-461203. As a result, administration of sofosbuvir as antiviral treatment for chronic HCV infection has been approved by the Food and Drug Administration (FDA) of the United States of America.3 As the NS5 catalytic domain region of the flavivirus and hepacivirus shares great similarities,11 we hereby postulate that sofosbuvir can also be used as antidengue treatment, thus providing a rapid solution to the disease.
In this study, we begin with the virtual evaluation of GS-461203 as potential inhibitor to NS5 RdRp of DENV2 via molecular docking analyses. Binding interactions analysis was employed to validate the accuracy of GS-461203 binding conformation toward NS5 RdRp of DENV2 by comparing to that of HCV. Subsequently, the in vitro virucidal activity of the marketed prodrug of GS-461203, sofosbuvir, was tested in both hepatocytes (HepG2) and non-hepatocytes (Vero) models. The antiviral activity was calculated based on 90% effective concentration (EC90) of sofosbuvir treatment against DENV2 infection.
2 METHODS AND MATERIALS
2.1 In silico studies
2.1.1 Homology modeling
Due to the lack of DENV2 polymerase crystal structure, homology modeling is required to be performed. DENV2, strain New Guinea, is selected as the model to synchronize the computational results with the in vitro experiment. Model sequence (polymerase sequence of DENV2, strain New Guinea C/PUO-218 hybrid, was retrieved from GenBank (AAC59274.1) and three Protein Data Bank (PDB) crystal structures (ID: 5K5M, 2J7U, and 4WTG; DENV2, DENV3, and HCV polymerases, respectively) were selected as templates. Three homology models of the polymerase were then generated from the sequence through modeller software embedded in biovia ds 4.512, 13 where each model was constructed via combination of structural data of the three aforementioned templates. The inclusion of HCV polymerase structure as template in this analysis is due to three main reasons. First, an active conformation of the polymerase is required to obtain an accurate result for the docking study. Second, there is no available structure of DENV polymerase which cocrystallized with an RNA substrate to date; and third, the catalytic domain of DENV polymerase folds into a similar conformation in comparison with HCV polymerase despite their low sequence identity. All the generated models were then submitted to UCLA Structure Analysis and Verification Server (University of California, Los Angeles; http://services.mbi.ucla.edu/SAVES/) for quality assessment of their 3D structures via procheck and verify 3d softwares.14, 15 Models that attain a PASS status from both softwares (>90% of favorable residue in procheck and at least 80% of residues attain a score >0.2 in verify 3d) were classified as structurally verified and selected for docking with the standard ligand.
2.1.2 Molecular docking of GS-461203
The active triphosphate form of sofosbuvir (2′-deoxy-2′-α-fluoro-β-C-methyluridine-5′-triphosphate, GS-461203) was drawn and minimized using ds 4.5 employing the prepared ligand function and Smart Minimizer Algorithm. autodocktools 1.5.7 (ADT) was then used to generate the docking input files of the minimized structures, which convert the structure into.pdbqt formats for flexible ligand docking using autodock vina 1.1.2.16, 17 In order to prepare the protein structure, best outcome from the homology modeling was submitted to ADT for addition of hydrogen atoms, merging of non-polar hydrogen atoms, assignment of Gasteiger charges (necessary for pdbqt file preparation but not needed in autodock vina's calculation16) and ADT atom type other than checking, and repairing missing atoms in the homology protein model.17 Furthermore, grid box which defined the area of protein involved in the docking calculation (grid volume, Å3 = 58 × 70 × 60; center x, y, z co-ordinates = −15.355, −39.282, and −17.284) was also determined using ADT. The grid box was set to cover the whole polymerase for blind docking. Subsequently, the prepared homology protein model was saved in.pdbqt format for docking calculation. Both of the ligand and the protein files were then submitted to autodock vina for molecular docking with exhaustiveness set at 100, and the final number of conformations generated was set to 20. Five independent runs with different initial seeding values were used for each docking process. The docking results were then subjected to in situ minimization via ds 4.5 followed by rescoring of the minimized receptor-ligand complex as part of the docking optimization algorithm in order to select the best binding conformation generated during the docking process.18, 19 The best result was then reanalyzed in ds 4.5 for receptor–ligand interactions.
2.2 In vitro studies
2.2.1 Cell culture
HepG2, a human liver hepatocellular carcinoma cell line, and Vero, an African green monkey kidney epithelial cell line (ATCC, USA), were maintained in standard DMEM (Gibco, USA) supplemented with 10% fetal calf serum (FCS) (GIBCO, USA) and antibiotics (Sigma, USA), penicillin and streptomycin 100 U/ml each.
2.2.2 DENV2 virus stock
DENV2, strain New Guinea C/PUO-218 hybrid, was used in this study. The viral stock obtained was mass propagated as previously described.20 The viral strain was confirmed by RT-PCR sequencing (ABI, USA) using the following primers: 5ʹ-ccgggatccgccgatttggaactg-3ʹ as the forward primer and 5ʹ-cccaagcttcaattttctctttcg-3ʹ as the reverse primer.21 Virus stock was titrated via plaque assay prior to infection.
2.2.3 Viral infection and treatment with prodrug sofosbuvir (PSI-7977, MedChem Express, USA)
Infection was performed on the 12-well culture plate. Generally, cell lines were allowed to attach to the culture plate overnight in the humidified incubator. The DENV2 stock was diluted with medium containing 2% FCS to achieve multiplicity of infection (MOI) factor of one for HepG2 and MOI of 0.2 for Vero. Medium containing 2% FCS only was used as mock infection. In order to perform the infection, culture media from the overnight culture were removed, and the preformed monolayer of cells was rinsed with buffered saline prior to the addition of 200 μl of DENV2 infection media. The plate was left for 1–2 hr with occasional rocking to assist viral adsorption. After adsorption, infected cells were again rinsed with buffered saline to remove the residual virus in the infection media. Fresh media containing various concentrations of sofosbuvir (97.7 nm up to 50 μm) were supplied to the cells as treatment, while the untreated groups received only fresh media. The cells were further maintained for 72 hr in the humidified incubator prior to sampling of the culture media for the titration of nascent virus released.
2.2.4 Titration of virus concentration by plaque assay
Plaque assay was performed as previously described with some modifications.22 Generally, virus stock or pooled media samples (n ≥ 3) from the post-infected sets were being quantified. A series of 10-fold dilution were prepared from the virus containing media of interest with fresh media containing 2% of FCS prior to infection. The infection process was performed according to the method mentioned above. After infection, cells were rinsed with buffered saline and overlaid with fresh media containing 2% of FCS and 0.5% agarose (Sigma, USA). Similarly, cells were further maintained for 72 hr in the humidified incubator to allow the formation of plaques by the virus. To visualize the plaques formed, cells were first fixed with 20% trichloroacetic acid (TCA) (Sigma, USA) at room temperature for an hour. Subsequently, the solution was removed, and cells were rinsed with distilled water prior to the addition of 200 μl of crystal violet dye (Sigma, USA) at room temperature. The dye was aspirated after 30 min, and the cells were again rinsed with distilled water to remove the excessive dye. The plate was air-dried, and the numbers of plaques in each well were determined. Finally, the viral concentration in each sample was expressed as plaque-forming units (pfu) per milliliter.23
3 RESULTS
3.1 In silico studies
3.1.1 Homology modeling
Among the three homology models generated, only Model 3 fulfills the prerequisites as “good model” (Procheck's Ramachandran plot with more than 90% of amino acids located in the most favored region and verify 3d score more than 80%, Table 1). Therefore, Model 3 was adopted as the protein model for subsequent molecular docking.
| Ramachandran plot | Homology models | ||
|---|---|---|---|
| Model 1 (%) | Model 2 (%) | Model 3 (%) | |
| Most favored region | 89.6 | 88.8 | 90.6 |
| Additionally allowed region | 7.7 | 7.1 | 7.8 |
| Generously allowed region | 0.9 | 2.1 | 0.2 |
| Disallowed region | 1.8 | 2.0 | 1.4 |
| verify 3d score | 76.31 | 83.17 | 84.94 |
- Each model was constructed independently using combined structural data of three PDB crystal structures—5K5M, 2J7U, and 4WTG.
3.1.2 Molecular docking
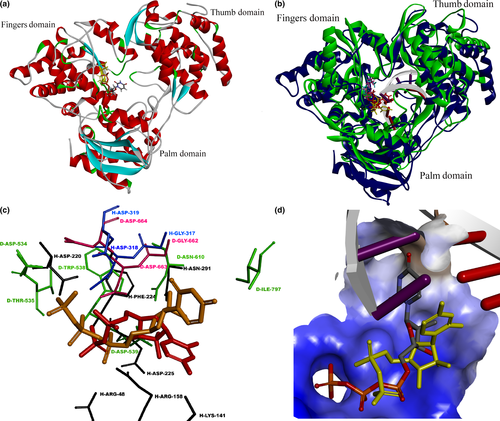
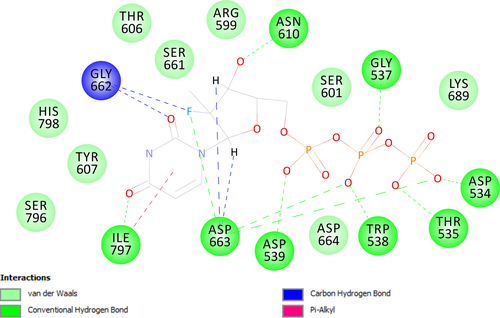
Through molecular docking with autodock vina, most conformations generated for GS-461203 had shown to dock onto the palm domain of DENV polymerase (binding affinity of the best pose = −6.9 kcal/mol, % of conformation in active site = 62%) with the triphosphate group located adjacent to the GDD (Gly-Asp-Asp)24 catalytic motif (Figure 1a). Albeit the lack of available crystal structure to show the protein-substrate complex of DENV polymerase with RNA, we superimposed our homology model with a HCV polymerase crystal structure (PDB id 4WTG) which cocrystalized with a dsRNA and GS-461203 (Figure 1b) for comparison. The best conformation of the docked GS-461203 showed similarities to that of DENV2 polymerase (Model 3) when compared to its native conformation determined by X-ray crystallization with HCV polymerase (4WTG). The putative protein–ligand interaction between GS-461203 and DENV polymerase is illustrated in Figure 2.
3.2 In vitro studies
3.2.1 Evaluation of sofosbuvir-antiviral potential in DENV2-infected HepG2 and DENV2-infected Vero cell lines
A cell-based assay was developed to investigate the activity of prodrug sofosbuvir against DENV2 in vitro replication. As the prodrug requires liver-specific enzymes to be metabolized into its active form GS-461203, the assay was performed on a liver-derived cell line HepG2. As a result, treatment with sofosbuvir demonstrated a remarkable reduction in nascent virus release as compared to those untreated DENV2-infected HepG2 cells (Table 2). In addition, we have also repeated the assay on Vero cell line (non-liver cell) to validate our observation. Treatment with similar concentration of sofosbuvir on DENV2-infected Vero cells displayed no significant inhibitory activity against the release of nascent virus when compared to the untreated infection (Table 2). These findings therefore confirm the results from the in silico study.
| Treatment concentration (μm) | Virus titration (pfu) | |
|---|---|---|
| HepG2 (liver cell line) | Vero (non-liver cell line) | |
| 0 | 3.8 ± 0.6 × 107 | 4.6 ± 0.8 × 107 |
| 12.5 | <100* | 4.0 ± 0.5 × 107 |
| 25 | <100* | 5.4 ± 0.6 × 107 |
| 50 | <100* | 4.9 ± 0.1 × 107 |
- DENV2 was allowed to infect HepG2 (MOI = 1) and Vero (MOI = 0.2) for 2 hr, washed thoroughly with phosphate-buffered saline and either treated with sofosbuvir at indicated concentration for 72 hr or left untreated. The culture medium was collected and subjected to plaque assay to determine the concentration of virus yield. Results are presented as mean ± SD of the pfu. Statistical significance (p < .05) is indicated by an asterisk.
3.2.2 Efficacy of sofosbuvir as anti-DENV2
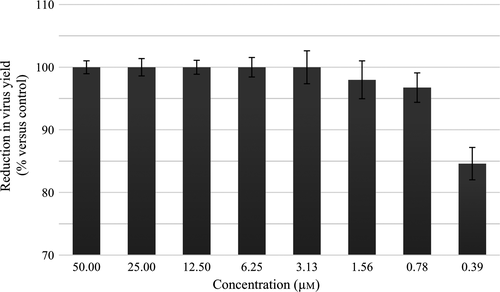
To study the dose–response relationship between the treatment concentration of sofosbuvir and DENV2 replication activity, similar experiment was repeated by treating the DENV2-infected HepG2 cells with various concentrations of sofosbuvir. Virus concentration of each treatment was determined via plaque assay, and percentage of inhibition of the respective treatment was calculated by normalizing the virus number released from untreated infection to 100% yield. Result shown in Figure 3 demonstrated a concentration-dependent inhibition of virus released. The calculated EC90 value was 0.4 μm.
4 DISCUSSION
Molecular docking had recently gained great attention and popularity in the field of drug discovery due to its ability to screen for potential inhibitors in a virtual space with significant cost-effectiveness in comparison with the traditional high-throughput screening (HTS) campaign. In this study, we had utilized an in silico approach as a guidance for the discovery of a novel DENV polymerase inhibitor. Accordingly, sofosbuvir was chosen as test inhibitor for DENV polymerase due to several reasons as follows: sofosbuvir is a marketed inhibitor targeting HCV polymerase with excellent potency across different HCV genotypes; HCV and DENV polymerases possessed similar folding pattern in their catalytic site with RMSD around 1.7 Å; and the similar topology between the binding pocket of both enzymes might allow sofosbuvir to dock on both enzymes via similar mechanism. Through molecular docking and in silico analyses, the active form of sofosbuvir (GS-461203) was showed to have most of its poses docked into the catalytic site of DENV polymerases. On the other hand, through superimposition of the native (GS-461203 in 4WTG, HCV polymerase) and docked conformation (GS-461203 docked onto Model 3) of GS-461203, we observed that both conformations had their phosphate group docked into a hydrophilic clef and were stabilized by a network of hydrogen bond with the amino acids located in the GDD motif (two hydrogen bonds with Gly662 and five hydrogen bonds with Asp663). It is worth mentioning that the GDD motif is highly conserved in the polymerases family and plays an important role in guiding incoming nucleotides into the growing nucleic acid chain during the elongation of transcript.25, 26 Despite the similar docking pose of their phosphate groups, the base and sugar moiety of the docked GS-461203 seems to deviate from the crystal conformation in 4WTG. Therefore, it was postulated that the drift in nucleotide base and sugar moiety was due to the absence of RNA template that would stabilize the nucleotide bases through the pi-stacking interaction between nucleotide bases from the same strand and the Watson–Crick interaction from the complementary strand. With a fair binding affinity, high percentage of docking poses in the active site as well as a good interaction profile, the in silico analyses from this study have showed GS-461203 potential as a potent inhibitor against DENV polymerase activity.
In other words, the in silico analyses forecasted sofosbuvir inhibitory effect toward dengue-infected human cell. Accordingly, results from the in vitro studies exhibited excellent antidengue property with an EC90 of 0.4 μm in DENV2-infected hepatic cell line but no activity in DENV2-infected kidney epithelial (Vero) cell line. This is due to the presence of specific enzymes in the liver cells, namely the carboxylesterase 1 (CES1), cathepsin A (CatA), and histidine triad nucleotide-binding protein 1 (Hint1) that are required for the hydrolysis of the prodrug sofosbuvir prior to phosphorylation into its active triphosphate form, GS-461203.27, 28 The importance of liver-specific enzymes for the hydrolysis and activation of the prodrug was demonstrated in this study when the negative control-infected kidney epithelial cell line that was treated with sofosbuvir, in all three concentrations—12.5, 25, and 50 μm, showed no inhibition of DENV2 propagation when compared to untreated infection (Table 2).
Nevertheless, the prodigious antidengue property of sofosbuvir toward DENV2 infection varied from the report by Lee et al.,29 where sofosbuvir did not show any effects either on DENV replication or its NS5 polymerase activity even with a maximum concentration of 50 μm. The discrepancy may arise from the different study design used where different DENV2 strain was used in both studies (DN454009A by Lee et al.29 and strain New Guinea C/PUO-218 hybrid in this study) that led to different susceptibility toward GS-461203. We have tried to perform structural analysis for the binding affinity of GS-461203 toward DN454009A's NS5 polymerase to no avail as the NS5 protein sequence is still unavailable for DN454009A. Having said so, NMR or X-ray crystal structure are still the most reliable techniques to study the macromolecule–ligand binding mechanism. In addition, different types of hepatic cell lines, Huh-7 cell line by Lee et al.29 and HepG2 cell line in this study, might be the main contribution of the independent observation between studies. Noteworthy, when compared to the primary hepatic cells, immortalized hepatic HepG2 and Huh-7 cell lines were reportedly found to have suppressed CES1 expression30, 31 with low detectable expression in HepG231 and undetectable in Huh-7—clone A.28 Accordingly, sofosbuvir is preferably hydrolyzed by CatA, while its stereoisomer GS-491241 (formerly termed PSI-7976) is preferably hydrolyzed by CES1.28 Moreover, Murakami et al. (2010) have reported that sofosbuvir is relatively active when compared to its stereoisomer GS-491241 in their clone A replicon-based assay.22 They have further concluded that the observed result was because clone A cells express only CatA and not CES1. Therefore, it was postulated that isomerization of sofosbuvir to GS-491241 would prevent activation of the prodrug in Huh-7 cell line and its subsequent clone.
Moreover, many other studies have also reported the potential of using sofosbuvir triphosphate as polymerase inhibitor for other members of flavivirus, zika virus.32-34 These studies have further suggested that the enzyme inhibitor for a particular virus also present as a potential inhibitor for another virus within a family as these viruses share the same morphology and replication mechanism. As sofosbuvir is safe for humans, and it is being used for treating HCV infection, the drug may provide physicians with a treatment option for dengue patients with high risk of DHF/DSS as there is no any antiviral agent available for treating dengue infection.4 Nevertheless, as sofosbuvir is currently used to treat slow-progressing chronic HCV infection, prescribing sofosbuvir to acute infectious diseases such as zika and dengue might give rise to a different outcome than those reported for HCV. Noteworthy, more studies are needed to provide information for the effect of sofosbuvir toward dengue and its lead potential as DENV polymerase inhibitor in human subjects.
5 CONCLUSION AND FUTURE DIRECTIONS
This study was designed to access the potential of sofosbuvir as antidengue agent using in silico and in vitro methods. As a result, sofosbuvir triphosphate was found to dock into the active site of DENV2 polymerase with binding affinity of −6.9 kcal/mol and 62% of active site binding. The in vitro plaque assay further validates the hypothesis of sofosbuvir as a potential DENV2 polymerase inhibitor with an EC90 value of 0.4 μm. However, a specific polymerase assay to elucidate the mechanism of action of sofosbuvir triphosphate against DENV2 polymerase was not performed in this study due to exhausted funding. It is however worth mentioning that the usage of sofosbuvir as a polymerase inhibitor against zika virus has been proposed by others,25-27 hence highlighting the potential of sofosbuvir as universal polymerase inhibitor against members of flavivirus. In conclusion, clinical trial is in dire needs to better understand the effect of sofosbuvir as antidengue in humans.
ACKNOWLEDGMENTS
We thank University Malaya's Postgraduate Research Grant PG076-2016A for supporting this study.
CONFLICT OF INTEREST
None.




