Anticancer activity of gallic acid template-based benzylidene indanone derivative as microtubule destabilizer
Abstract
Benzylidene indanones have been designed and synthesized from gallic acid, a plant phenolic acid as possible anticancer agent. The best analogue of the series, that is, 3-(3′,4′,5′-trimethoxyphenyl)-4,5,6-trimethoxy-2-(4˝-nitrobenzylidene)-indan-1-one (8) exhibited potent cytotoxicity (IC50=3–10 μm) against several human cancer cell lines through microtubule destabilization (IC50=1.54 μm) after occupying colchicine-binding site of β-tubulin. In cell cycle analysis, compound 8 exerted G2/M phase arrest in both MCF-7 and MDA-MB-231 cells and induced apoptosis. It reduced 34.8% solid tumor in in vivo Ehrlich ascite carcinoma in Swiss albino mice at 30 mg/kg dose. In acute oral toxicity experiment, it was tolerable up to 300 mg/kg doses in Swiss albino mice. The lead compound 8 needs to be optimized for better activity.
Cancer is a public health menace. The disease is of a great concern to both developed and developing countries due to its high morbidity and mortality. In many countries, it has become second largest killer after cardiovascular disease. In 2012, there were 14 million new cases added on and 8.2 million deaths were due to cancer.1 Among men, lung cancer was the most predominant, while among women, it was breast cancer. Africa, Asia, and Central and South America diagnosed more than 60% cancer cases and 70% of total cancer deaths. There will be 24 million cancer cases annually and 14.6 million annual deaths by the end of 2015.2 These troubling figures compel policy makers and the researchers to combat this disease. Nevertheless, the complexity of the disease is too much and still today, cancer is a challenge to the researchers.
Microtubules are considered as an attractive target in cancer chemotherapeutics.1, 2 Microtubules are rigid hollow cylinders built by polymerization of tubulin dimers, a globular protein. Microtubules play a key role in various cellular processes including mitosis. Some of the compounds known as antitubulins interfere in microtubule dynamics either through stabilizing or destabilizing (inhibiting) microtubule assembly. In both the cases, the equilibrium of this process is disturbed and this interference in microtubule dynamics has a significant impact on cell division which ultimately causes cell death.3, 4 Several natural molecules such as paclitaxel, epothilones, laulimalide, peruloside, and discodermolide are microtubule stabilizers while podophyllotoxin, combretastatins, vinca alkaloids, dolastatins etc. are microtubule destabilizers.5-7
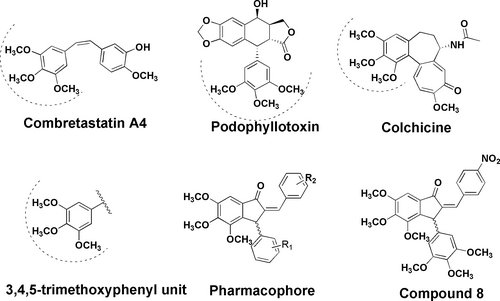
In this study, we modified gallic acid, a plant phenolic acid, to a basic pharmacophore based on structure activity relationship of some natural antitubulins such as combretastatin A-4 (CA-4), colchicine, and podophyllotoxin (PDT; Figure 1).8, 9 In these natural antitubulins, a 3,4,5-trimethoxyphenyl unit is very crucial to interact with tubulin. This motif is essentially required to induce antitubulin effect in these compounds.6, 8-10 Thus, we designed a benzylidene indanone unit with a 3,4,5-trimethoxyphenyl unit in ring A in the pharmacophore. The mode of action of these compounds was expected through tubulin polymerization inhibition. Based on these facts, we synthesized several benzylidene indanones with a 3,4,5-trimethoxyphenyl fragment and restricted rotation around C–C bond as in case of natural antitubulin combretastatin A4.11 The compounds have been evaluated against human cancer cell lines for cytotoxicity. The best analogue of the series has been evaluated for extensive biology, that is, tubulin polymerization assay, cell cycle analysis, in vivo anticancer activity, and in vivo acute oral toxicity. Molecular docking studies revealed that it occupies colchicine-binding pocket of β-tubulin.
1 Experimental Section
1.1 Chemical synthesis
1.2 General procedure for the synthesis of benzylidene indanones 8, 9, 10, and 12
To a stirred solution of hexamethoxyindanone 7 (200 mg, 0.51 mmol) in 3% methanolic KOH (6 mL) respective benzaldehyde (0.60 mmol) was added. The reaction mixture was stirred at room temperature for 2–4 h. The organic solvent was evaporated and residue was taken in ethyl acetate (20 mL) and acidified with dil. HCl (10%, 10 mL). Organic layer was washed with water, dried over anhydrous sodium sulfate, and evaporated in vacuo. The residue thus obtained was purified through silica gel column (230–400 mesh) by flash chromatography (glass column, length 130 mm, i.d. 18 mm, flow rate = 18 mL/min) using hexane-ethyl acetate as eluents. The desired benzylidene indanones were obtained in good yields (72–89%).
1.2.1 3-(3′,4′,5′-trimethoxyphenyl)-4,5,6-trimethoxy-2-(4″-nitrobenzylidene)-indan-1-one (8)
Yield = 82%; m.p. = 162–64 °C; 1H NMR (300 MHz, CDCl3): δ 3.45 (s, 3H, OCH3), 3.73 (s, 9H, 3 × OCH3), 3.93 (s, 6H, 2xOCH3), 5.28 (s, 1H, 3-CH), 6.38 (s, 2H, 2″ & 6″-CH of 3-phenyl), 7.15 (s, 1H, 7-CH), 7.24 (s, 1H, CH=benzylidene double bond), 7.62 (d, 2H, 2′ & 6′-CH of 2-benzylidene ring, J = 8.7 Hz), 8.12 (d, 2H, 3′ & 5′-CH of 2-benzylidene ring, J = 7.2 Hz); 13C NMR (75 MHz, CDCl3): δ 46.5, 56.6, 56.7, 60.7, 61.3, 101.9, 106.3, 123.8, 131.6, 131.8, 132.0, 136.8, 137.5, 141.3, 143.9, 150.4, 153.6, 155.6, 192.6; HR-ESI-MS (MeOH): for C28H28NO9 [M + H]+; calcd, 522.1764; found, 522.1755.
1.2.2 3-(3′,4′,5′-trimethoxyphenyl)-4,5,6-trimethoxy-2-(4″-benzyloxybenzylidene)-indan-1-one (9)
Yield = 89%; m.p. = 190–92 °C; 1H NMR (300 MHz, CDCl3): δ 3.56 (s, 3H, OCH3), 3.81 (s, 9H, 3 × OCH3), 3.98 (s, 6H, 2 × OCH3), 5.04 (s, 2H, Benzylic), 5.27 (s, 1H, 3-CH), 6.49 (s, 2H, 2″ & 6″-CH of 3-phenyl), 6.94 (d, 2H, 3′ & 5′-CH of 2-benzylidene ring, J = 8.7 Hz), 7.23 9s, 1H, 7-CH), 7.34 (bs, 5H, 5 × CH of benzyl ring), 7.51 (d, 2H, 2′ & 6′-CH of 2-benzylidene ring, J = 8.4 Hz), 7.66 (s, 1H, CH=benzylidene); 13C NMR (75 MHz, CDCl3): δ 46.7, 56.6, 56.7, 60.6, 61.2, 70.4, 101.8, 106.5, 115.2, 127.6, 127.8, 128.6, 129.0, 132.6, 133.6, 134.8, 136.8, 137.3, 137.8, 141.2, 148.9, 150.4, 153.4, 155.2, 160.4, 194.2; ESI-MS (MeOH): 583.2 [M + H]+, 605.2 [M + Na]+, 621.2 [M + K]+.
1.2.3 3-(3′,4′,5′-trimethoxyphenyl)-4,5,6-trimethoxy-2-(4″,4″-dimethylaminobenzylidene)-indan-1-one (10)
Yield = 72%; m.p. = 182–85 °C; 1H NMR (300 MHz, CDCl3): δ 2.98 (s, 6H, N-(CH3)2), 3.53 (s, 3H, OCH3), 3.75 (s, 9H, 3xOCH3), 3.96 (s, 6H, 2xOCH3), 5.24 (s, 1H, 3-CH), 6.53 (s, 2H, 2″ & 6″-CH of 3-phenyl), 6.63 (d, 2H, 3′ & 5′-CH of 2-benzylidene ring, J = 8.4 Hz), 7.21 (s, 1H, 7-CH), 7.47 (d, 2H, 2′ & 6′-CH of 2-benzylidene ring, J = 8.4 Hz); 13C NMR (75 MHz, CDCl3): δ 30.0, 40.9, 47.0, 56.6, 60.6, 61.2, 101.8, 106.5, 112.6, 133.0, 135.38, 135.7, 137.1, 137.5, 140.9, 148.5, 150.3, 150.8, 153.4, 155.1, 194.2; ESI-MS (MeOH): 520.4 [M + H]+, 542.4 [M + Na]+, 558.2 [M + K]+.
1.2.4 3-(3′,4′,5′-trimethoxyphenyl)-4,5,6-trimethoxy-2-(4″-carboxymethylbenzylidene)-indan-1-one (12)
Yield = 78%; m.p. = 160–62 °C; 1H NMR (300 MHz, CDCl3): δ 3.44 (s, 6H, OCH3 and COOCH3), 3.72 (s, 9H, 3 × OCH3), 3.94 (s, 6H, 2 × OCH3), 5.30 (s, 1H, 3-CH), 6.39 (s, 2H, 2″ & 6″-CH of 3-phenyl), 7.23 (s, 1H, 7-CH), 7.54 (d, 2H, 2′ & 6′-CH of 2-benzylidene ring, J = 8.1 Hz), 7.69 (s, 1H, CH=benzylidene); 7.93 (d, 2H, 3′ & 5′-CH of 2-benzylidene ring, J = 8.1 Hz); 13C NMR (75 MHz, CDCl3): δ 46.5, 52.6, 56.6, 56.7, 60.6, 61.2, 61.3, 101.8, 106.4, 129.8, 130.7, 131.1, 132.2, 133.4, 137.1, 137.3, 139.2, 141.4, 142.4, 149.5, 150.4, 153.3, 155.4, 166.8, 193.8; ESI-MS (MeOH): 535.1 [M + H]+, 557.1 [M + Na]+, 573.3 [M + K]+.
1.2.5 Synthesis of 3-(3′,4′,5′-trimethoxyphenyl)-4,5,6-trimethoxy-2-(4″,4″,4″-trimethylaminium benzylidene)-indan-1-one- iodide (11)
Compound 10 (100 mg, 0.19 mmol) was taken in dry acetone (10 mL) and anhydrous potassium carbonate (300 mg) and methyl iodide (0.2 mL, 3.21 mmol) were added and reaction mixture was stirred at room temperature for 2 h. Reaction mixture was filtered and washed with acetone. The filtrate was evaporated and residue was recrystallized with chloroform-acetone to get 11.
Yield = 84%; m.p. = 269 °C (decompose); 1H NMR (300 MHz, CDCl3): δ 3.32 (s, 3H, OCH3), 3.83 (s, 6H, N-(CH3)2), 3.92 (s, 9H, 3 × OCH3), 3.94 (s, 6H, 2 × OCH3), 5.29 (s, 1H, 3-CH), 6.36 (s, 2H, 2″ & 6″-CH of 3-phenyl), 7.22 (s, 1H, 7-CH), 7.68 (d, 2H, 3′ & 5′-CH of 2-benzylidene ring, J = 8.4 Hz), 7.78 (d, 2H, 2′ & 6′-CH of 2-benzylidene ring, J = 8.4 Hz); ESI-MS (MeOH): 534.3 [M − I]+, 535.3 [M + H − I]+.
Synthesis of compound 13: Ester 12 (100 mg, 0.19 mmol) was taken in 5% KOH (methanol:water = 9:1, 6 mL) and heated at 50 °C for an hour. Solvent was evaporated and residue was taken in ethyl acetate. It was acidified with dil. HCl (5%, 10 mL). Organic phase was washed with water and dried over anhydrous sodium sulfate. Organic solvents were evaporated to give a residue which on recrystallization with chloroform-hexane afforded compound 13.
1.2.6 3-(3′,4′,5′-trimethoxyphenyl)-4,5,6-trimethoxy-2-(4″-carboxybenzylidene)-indan-1-one (13)
Yield = 89%; m.p. = 230–31 °C; 1H NMR (300 MHz, CDCl3): δ3.46 (s, 3H, OCH3), 3.74 (s, 9H, 3 × OCH3), 3.93 (s, 6H, 2 × OCH3), 5.33 (s, 1H, 3-CH), 6.41 (s, 2H, 2″ & 6″-CH of 3-phenyl), 8.03 (d, 2H, 3′ & 5′-CH of 2-benzylidene ring, J = 8.7 Hz), 7.27 (s, 1H, 7-CH), 7.59 (d, 2H, 2′ & 6′-CH of 2-benzylidene ring, J = 8.4 Hz), 7.72 (s, 1H, CH= of benzylidene); 13C NMR (75 MHz, CDCl3): δ 46.5, 56.6, 61.3, 61.4, 101.9, 106.35, 129.9, 130.4, 131.1, 132.2, 133.4, 137.1, 137.3, 140.0, 141.5, 142.7, 149.5, 150.3, 153.4, 155.5, 171.1, 193.9; ESI-MS (MeOH): 521.2 [M + H]+, 543.2 [M + Na]+, 559.1 [M + K]+.
1.2.7 Synthesis of 3-(3′,4′,5′-trimethoxyphenyl)-4,5,6-trimethoxy-2-(4″-carbamoylbenzylidene)-indan-1-one (14)
Ester 12 (100 mg, 0.19 mmol) was taken in methanol (10 mL) and stirred with cooling (5–10 °C). Aqueous ammonia (1 mL) was added to it and stirred for 2 h. Thereafter, reaction mixture was stirred at room temperature overnight. Reaction mixture was evaporated and residue was taken in ethyl acetate. It was washed with water, and organic layer was dried over anhydrous sodium sulfate and evaporated. The crude mass was purified through column chromatography over silica gel using ethyl acetate-hexane system to get amide 14.
Yield = 42%; m.p. = oil; 1H NMR (300 MHz, CDCl3): δ3.44 (s, 3H, OCH3), 3.76 (s, 9H, 3 × OCH3), 3.92 (s, 6H, 2 × OCH3), 5.31 (s, 1H, 3-CH), 6.40 (s, 2H, 2″ & 6″-CH of 3-phenyl), 7.21 (s, 1H, 7-CH), 7.54 (d, 2H, 2′ & 6′-CH of 2-benzylidene ring, J = 8.1 Hz), 7.67 (s, 1H, CH=benzylidene); 7.91 (d, 2H, 3′ & 5′-CH of 2-benzylidene ring, J = 8.1 Hz); ESI-MS (MeOH): 520.2 [M + H]+.
1.3 Biological evaluation
The detail of biological assays is in Supporting information.
1.4 Antiproliferative activity by sulforhodamine assay
Human cancer cell lines, THP-1 (leukemia), HCT-116 (colon), MCF-7 (breast), T-47D (breast), and A549 (lung), were originally obtained from American type of cell culture collection (ATCC), USA, and assay was performed as per reported method.14
1.5 Soft agar colony assay
Human breast cancer MCF-7 and MDA-MB-231 cells (5 × 104/mL) were seeded separately in 24-well plates with or without compound 8 treatment for 24 h.15 Combretastatin A-4 (CA-4) and tamoxifen (TAM) were used as standard anticancer drugs (positive control) for cytotoxicity.
1.6 Tubulin polymerization assay
Tubulin polymerization experiment was carried out as per reported protocol using ‘assay kit’ from Cytoskeleton, USA.16, 17 Podophyllotoxin (PDT) was used as standard inhibitor of tubulin polymerization.
1.7 Molecular docking studies
In silico docking and visualization studies were completed with Discovery Studio version 3.5 (Accelrys, USA, 2013), a software for molecular modeling.18 To prepare target protein, the structure of beta-tubulin (PDB ID 4O2B) has been retrieved from RCSB PDB site (http://www.rcsb.org/pdb). For pharmacokinetic, metabolism and toxicity (ADMET) prediction, ADMET Predictor™ was used.
1.8 Cell cycle analysis
The effect of benzylidene indanone 8 on cell division cycle of MCF-7 and MDA-MB-231 cells was assessed by flow cytometry with PI-stained cellular DNA.19
1.9 In vivo anticancer activity of 8 against Ehrlich ascites tumor (solid)
Ehrlich ascites carcinoma (EAC) experiment was performed20 on Swiss albino mice using compound 8 at 30 mg/kg given intraperitoneal (i.p.) after dissolving in carboxymethylcellulose (CMC) from day 1 to 9. 5-Fluorouracil (22 mg/kg) was used as positive control.
1.10 Acute oral toxicity of benzylidene indanone 8
The acute oral toxicity compound 8 was evaluated in accordance with the Organization for Economic Co-operation and Development (OECD) test guideline No 423 (1987) in Swiss albino mice.21, 22 Compound 8 was suspended in double-distilled water and given through oral route at various doses, that is, 5, 50, 300, and 1000 mg/kg (groups II–IV) on body weight.
1.11 Statistical analysis
Data expressed as mean or representative of one of three similar experiments unless otherwise indicated. Both the soft agar colony formation and cell cycle experiment were performed in duplicates and results are expressed as Mean ± SD. For multiple comparisons, each value was compared by one-way anova following Dunnett's test, Tukey's test and Student's t-test in graphpad instat version 3.06. Data with p value < 0.05 were considered as significant.
2 Results and Discussion
2.1 Chemistry
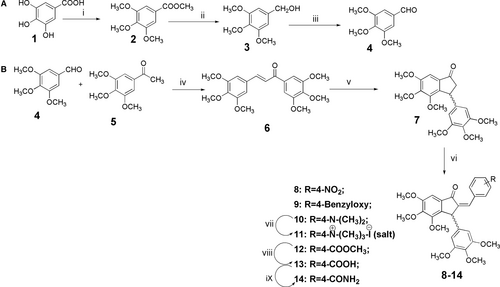
The synthetic strategy was as depicted in Scheme 1. The starting substrate gallic acid (1) was modified to ester 2 using dimethyl sulfate. Ester 2 was reduced with lithium borohydride to get benzyl alcohol 3, which on oxidation with pyridinium chlorochromate (PCC) yielded 3,4,5-trimethoxybenzaldehyde (4). Aldehyde 4 was treated with 7% methanolic KOH to afford hexamethoxy chalcone 6 through Claisen–Schmidt reaction. On treatment with trifluoroacetic acid, chalcone 6 underwent Nazarov's cyclization reaction12 to give a 3-(3,4,5-trimethoxyphenyl)-hexamethoxy indanone (7). Indanone 7 was treated with various aromatic aldehydes to get 2-benzylidene indanones 8, 9, 10, 12, and 13. 2-(4-N,N-Dimethylamino)-benzylidene indanone (10) was transformed to its corresponding quaternary ammonium salt (compound 11) by treating it with methyl iodide in potassium carbonate-methyl iodide system. 2-(4-Carboxymethyl)-benzylidene indanone (12) was hydrolyzed with 5% KOH in aqueous methanol (1:9) to afford corresponding carboxylic acid derivative 13. Benzylidene 13 on treatment with aqueous ammonia-THF system yielded the corresponding amide derivative 14. All the derivatives were as isomeric mixtures and were confirmed through spectroscopy (Supporting Information).
2.2 Biological evaluation
The benzylidene indanones (8–14) were evaluated for cytotoxicity against a panel of human cancer cell lines by sulforhodamine assay.14 Paclitaxel (taxol) and 5-fluorouracil (5-FU) were used as standard anticancer drugs (positive control) for cytotoxicity. Compounds exhibiting IC50 > 50 μm were considered as inactive. For structure and activity relationship, mainly the IC50 data in MCF-7 were taken in to consideration. In the series, presence of an electron-withdrawing group at 2-benzylidene ring exhibited good cytotoxicity. Compound 8 with 4-nitro group (IC50 = 4 μm) and compound 12 with 4-carboxylic ester (IC50 = 24 μm) possessed good cytotoxicity. However, compound 13 with a 4-carboxylic acid group was weak cytotoxic (IC50 = 48 μm). Compounds 8, 10, 12, and 13 exhibited IC50 < 10 μm against one or two cell lines, which is considered as good activity. Compound 8, the best compound of the series, exhibiting good activity against all the cell lines (IC50 = 3–10 μm) was extensively evaluated further. However, the cytotoxicity (MCF-7) of compound 8 was comparable to podophyllotoxin but less active than combretastatin A4, paclitaxel, and 5-fluorouracil. Compounds 8, 10, 12, and podophyllotoxin were evaluated for interaction with tubulin polymerization process (Cytoskeleton, USA).16, 17 The cytotoxicity and antitubulin data are represented in Table 1.
| Compound No. | Human cancer cell linesa | Tubulin polymerization inhibition, IC50 (μm) | ||||
|---|---|---|---|---|---|---|
| THP-1, IC50 (μm) | HCT-116, IC50 (μm) | MCF-7, IC50 (μm) | T-47D, IC50 (μm) | A549, IC50 (μm) | ||
| 8 | 3 ± 0.2 | 7 ± 0.4 | 4.0 ± 0.3 | 5.0 ± 0.3 | 10 ± 0.6 | 1.54 ± 0.12 |
| 9 | 50 ± 1.8 | >50 | 42.0 ± 2.4 | 50 ± 3.4 | >50 | n.d. |
| 10 | 4.0 ± 0.3 | 15.0 ± 0.6 | 44 ± 1.8 | 40 ± 2.3 | 4.0 ± 0.23 | 2.08 ± 0.16 |
| 11 | 12 ± 0.7 | 20 ± 0.5 | 44 ± 2.2 | 50 ± 3.6 | >50 | n.d. |
| 12 | 18 ± 0.6 | 40 ± 1.2 | 24 ± 1.4 | 4 ± 0.2 | 30 ± 1.6 | 1.55 ± 0.09 |
| 13 | 30 ± 1.4 | 12.5 ± 0.2 | 48 ± 2.2 | 32 ± 1.2 | 8 ± 0.5 | n.d. |
| 14 | >50 | >50 | >50 | >50 | >50 | n.d. |
| PDT | — | — | 3.5 ± 0.6 | — | <1.3 | 0.62 ± 0.12 |
| CA4 | — | — | 0.01 ± 0.003 | — | <1.87 | 1.20 ± 0.26 |
| Standard drugs | Human cancer cell lines | |||||
|---|---|---|---|---|---|---|
| Concentration, μm | THP-1, % inhibition | HCT-116, % inhibition | MCF-7, % inhibition | T-47D, % inhibition | A549, % inhibition | |
| Taxol | 1.0 | 60 ± 3 | 45 ± 2 | 77 ± 2 | 78 ± 5 | 88 ± 4 |
| 5-FU | 5.0 | 90 ± 7 | 88 ± 3 | 50 ± 2 | 60 ± 3 | 54 ± 2 |
- a IC50 > 50 μm considered inactive.
Further, in soft agar colonization assay,15 compound 8 suppressed the growth of MCF-7 cells by 10–96% at 1.5–50 μg/mL concentrations (IC50 = 8.35 μg/mL) and in MDA-MB-231 cells by 28–90% (IC50 = 9.68 μg/mL; Table 2). Combretastatin A4 exhibited 23–96% and 25–97% growth inhibition in MCF-7 and MDA-MB-231, respectively, under the same conditions. Tamoxifen exerted 62–100% growth reduction at 1.5–50 μg/mL concentration in MCF-7 cells. These results indicate that the cytotoxicity of compound 8 is quite significant and concentration dependent. Soft agar colonization assay is used to confirm cellular anchorage-independent growth in vitro and it is an astringent method to detect the tumorigenic potential of transformed cells and tumor suppressive effects on transformed cells.23
| Condition | Concentration, μg/mL | MCF-7, % Live cells | MCF-7, IC50 (μg/mL) | MDA-MB-231, % Live cells | MDA-MB-231, IC50 (μg/mL) |
|---|---|---|---|---|---|
| Control | — | 100 | 100 | ||
| Compound 8 | 50 | 3.62 ± 0.23a | 8.35 (16 μm) | 10.19 ± 0.87a | 9.68 (18.57 μm) |
| 20 | 9.69 ± 0.15a | 19.22 ± 0.45a | |||
| 10 | 44.25 ± 0.58a | 52.36 ± 1.43a | |||
| 5 | 80.76 ± 0.66a | 66.12 ± 1.09a | |||
| 1.5 | 90.43 ± 0.18a | 72.45 ± 0.35a | |||
| CA-4 | 50 | 3.72 ± 0.24a | 4.32 (10 μm) | 3.14 ± 0.08a | 5.37 (12.43 μm) |
| 20 | 4.81 ± 0.43a | 22.76 ± 1.61a | |||
| 10 | 16.27 ± 0.16a | 34.4 ± 5.55a | |||
| 5 | 49.48 ± 8.14a | 53.54 ± 2.34a | |||
| 1.5 | 76.75 ± 4.11a | 76.67 ± 1.05a | |||
| Tam | 50 | 0 | 0.82 (2.2 μm) | n.d. | n.d. |
| 20 | 1.25 ± 0.25a | n.d. | |||
| 10 | 5.03 ± 0.35a | n.d. | |||
| 5 | 19.73 ± 0.71a | n.d. | |||
| 1.5 | 38.56 ± 0.17a | n.d. |
- n.d., means not determined.
- a p < 0.01 (Dunnett's test).
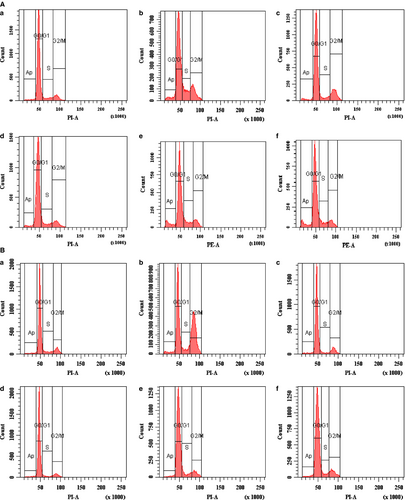
In cell cycle analysis,19 compound 8 exerted cell cycle arrest and induced apoptosis in both cells lines, that is, MCF-7 and MDA-MB-231 (Table 3). In both the cancer cell lines, cell cycle was arrested at G2/M phase (Figure 2A,B). Thus, compound 8 exhibits significant increase in number of subdiploid cells at all concentrations in both cell lines, indicating induction of apoptosis. Cell cycle is controlled by a complex series of signaling pathways by which cell grows, replicates its DNA, and divides. Cell cycle regulation ensures the fidelity of genomic replication and cell division.24 Cell cycle arrest is prevention of normal progression of the cell cycle. It is induced via various mechanisms. Some compounds interfere with CDK/cyclin complexes leaving cells stuck at the G2/M phase border, while others affect CaMKII phosphorylation, inducing G1 phase arrest. Among several other mechanisms, interference with RNA function and inhibition of protein synthesis are important. As a result of cell cycle interruption, many compounds ultimately induce apoptosis.25 In cell cycle analysis such as other antitubulins, compound 8 induced mainly G2/M phase arrest in both MCF-7 and MDA-MB-231 cells (8). Induction of cell cycle arrest in cancer cells constitutes one of the most prevalent strategies to stop or limit cancer spreading.26, 27
| Concentration of 8 (μg/mL) | MCF-7 | MDA-MB-231 | ||||||
|---|---|---|---|---|---|---|---|---|
| % of Cells | % of Cells | |||||||
| G0/G1 | S | G2/M | Apoptosis | G0/G1 | S | G2/M | Apoptosis | |
| Control | 76.25 ± 4.03 | 8.85 ± 1.20 | 12 ± 2.83 | 2.9 ± 0.00 | 74.8 ± 0.14 | 9.3 ± 0.00 | 11.95 ± 0.21 | 3.2 ± 0.42 |
| 50 | 61.6 ± 0.14 | 13.9 ± 1.70 | 17.35 ± 1.77 | 7.05 ± 0.21 | 43.45 ± 0.92 | 8.35 ± 0.21 | 43.25 ± 0.21 | 4.85 ± 0.92 |
| 20 | 64.8 ± 8.77 | 14.45 ± 9.26 | 14.2 ± 5.52 | 5.45 ± 3.46 | 78.25 ± 0.07 | 5.6 ± 0.28 | 12.2 ± 0.00 | 3.85 ± 0.07 |
| 10 | 74.45 ± 4.31 | 12.2 ± 2.69 | 10.55 ± 0.64 | 4.8 ± 2.26 | 83.9 ± 0.00 | 4.65 ± 0.07 | 8.3 ± 0.14 | 3.1 ± 0.28 |
| 5 | 57.45 ± 13.22 | 13.05 ± 5.16 | 14.2 ± 3.11 | 5.95 ± 2.19 | 78.1 ± 0.28 | 9.35 ± 0.92 | 9.7 ± 1.13 | 2.75 ± 0.07 |
| 1.5 | 52.3 ± 10.61 | 14.15 ± 8.41 | 13.9 ± 2.69 | 9.1 ± 3.11 | 78.25 ± 2.19 | 7.85 ± 2.33 | 11.15 ± 0.21 | 2.65 ± 0.07 |
In tubulin polymerization assay, compound 8 inhibited polymerization (IC50 = 1.54 μm), while the standard antitubulin podophyllotoxin showed higher effect (IC50 = 0.62 μm). Microtubules are tube-like hollow filamentous structures formed on association of α/β-tubulin heterodimers. They serve as cytoskeletons of the cells and form framework for structures such as spindle apparatus useful in chromosome segregation during cell division. Microtubules play diverse roles in cellular structure and function.28 Thus, their crucial role in mitotic process makes them an attractive target, and the drugs that affect microtubule assembly by disturbing the machinery are useful especially in the treatment of cancer. Antitubulins have been one of the most successful approaches in cancer chemotherapeutics (4), and many drug candidates are under various phases of clinical trials (8).
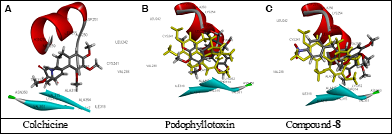
Molecular docking studies (Table 4) revealed that compound 8 occupies colchicine-binding pocket of β-tubulin. It has LibDock score of 83.66 comparable to colchicine (92.22), but lower than podophyllotoxin (120.80). The residual amino acids confirmed the same binding site of compound 8 like colchicine. The docking pose in Figure 3 shows that amino acid residue of β-tubulin is close proximity of compound 8.
| Compound | LibDock score | H-bond | Binding pocket amino acid residues (bold represents the similar amino acid residues with colchicine) |
|---|---|---|---|
| Colchicine | 92.22 | No | VAL238, CYS241, LEU242, LEU248, ALA250, ASP251, LYS254, LEU255, ASN258, MET259, THR314, VAL315, ALA316, ALA317, ILE318, ASN350, VAL351, LYS352, ALA354 |
| Podophyllotoxin | 120.80 | No | CYS241, LEU248, ALA250, LYS254, LEU255, ASN258, MET259, THR314, VAL315, ALA316, ALA317, ILE318, ASN350, VAL351, LYS352, THR353, ALA354 |
| Compound 8 | 83.66 | No | VAL238, CYS241, LEU242, LEU248, ALA250, ASP251, LYS254, LEU255, ASN258, MET259, THR314, VAL315, ALA316, ILE318, ASN349, ASN350, LYS352, THR353, ALA354 |
In our pharmacophore designing, a 3,4,5-trimethoxyphenyl motif has been placed in ring A. This motif is supposed to interact with tubulin. Based on calculations of ligand–tubulin association constants, it was reported that residue β:316 (ALA316) of tubulin is directly involved in binding the common trimethoxyphenyl unit of ring A of colchicine.29 Later, it was reported that residue β:318 (VAL318) is more crucial and has strong binding with the middle methoxy group of trimethoxyphenyl unit of colchicine with various isotypes (βIV, βII, and βIII) of β-tubulin.30
In silico Pharmacokinetic studies showed that compound 8 slightly violates Lipinski rule for one parameter (molecular weight = 521 > 500). It is lipophilic in nature, lacks water solubility, and has poor oral absorption. It has high tendency to cross blood brain barrier. Its various PK parameters volume of distribution (0.88–1.56 L/kg), blood-to-plasma concentration (0.64), and clearance rate (7.47 mL/min) etc. have been determined. All these parameters seem to be in normal range. Five probable metabolites (M1–M5) are mostly demethylated phenolic derivatives. CYP 2C8 and CYP 3A4 are mainly involved in the metabolism of indanone 8. The details have been provided in Supporting information.
Ehrlich ascites carcinoma is a spontaneous murine mammary adenocarcinoma adapted to ascites form and carried out by serial intraperitoneal passages in outbred mice.31 From day 1 to 9, first treatment group was treated with benzylidene indanone 8 (30 mg/kg i.p.). The second treatment group was treated with 5-fluorouracil, a standard drug of solid tumors (22 mg/kg, i.p.) from day 1 to 9. The control group was administered normal saline (0.2 mL, i.p.) from day 1 to 9. Compound 8 exhibited only 34.8% tumor reduction, while 5-fluorouracil exerted 53.5% tumor reduction (Table 5). There was no mortality throughout the experimental period. Hence, the anticancer effect of compound 8 was moderate as compared to clinical drug, 5-fluorouracil. EAC is referred to as an undifferentiated carcinoma, originally hyperdiploid, high transplantable capability, no-regression, rapid proliferation, short life span, 100% malignancy.32 However, Ehrlich ascites tumor cells are much more difficult to break than most non-malignant somatic cells.33
| Treatment groups | Av. Body weights (g) of animals on days | Day 13 | % Tumor growth inhibition | Mortality | |||
|---|---|---|---|---|---|---|---|
| 1 | 5 | 9 | Av. Body weights (g) | Av. Tumor weights (mg) | |||
| Compound 8 (30 mg/kg i.p.) | 20.71 | 21.85 | 22.14 | 21.85 | 1006.71 ± 100.75 | 34.80 | 0/7 |
| Fluorouracil (22 mg/kg i.p.) | 20.71 | 21.57 | 20.28 | 20.0 | 718.71 ± 67.11 | 53.49 | 0/7 |
| Normal controlNS (0.2 mL i.p.) | 21.5 | 22.9 | 23.4 | 23.6 | 1545.5 ± 105.25 | – | 0/10 |
Finally, compound 8 was evaluated for acute oral toxicity in Swiss albino mice in accordance with the OECD test guideline No 423 (1987).21, 22 Acute oral toxicity is used to assess the ability of a substance to cause adverse effects within a short period of time following dosing or exposure. When in vivo testing is performed, estimation of rodent acute toxicity is an important task in safety assessment of drug candidates. Compound 8 was suspended in double-distilled water and given through oral route at various doses, that is, 5, 50, 300, and 1000 mg/kg (groups II–IV) on body weight. Control animals (group I) received only vehicle.
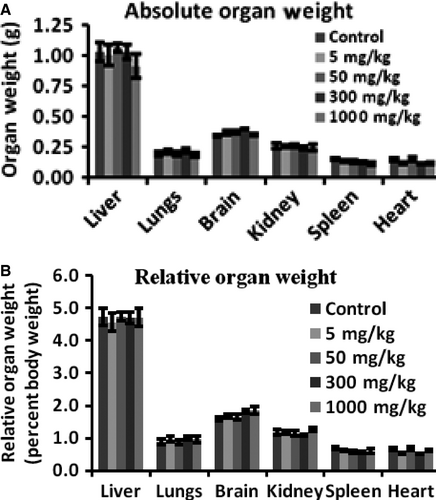
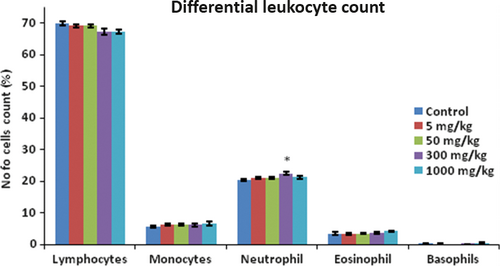
There were no observational changes, morbidity, and mortality throughout the experimental period in all the groups of experimental animal up to the dose levels of 300 mg/kg including body weight. However, at 1000 mg/kg dose, there was loss in body weight. However, animals on gross pathological study showed insignificant changes in any of the organs studied including their absolute and relative weight (Figure 4A,B). In most of the cases, blood and serum samples upon analysis showed non-significant changes in all the blood and liver parameters studied such as red blood cells (RBC) count, white blood cells (WBC) count, serum glutamate-pyruvate transaminase (SGPT), serum glutamic oxaloacetic transaminase (SGOT), alkaline phosphatase (ALKP) activity, and creatinine, triglycerides, cholesterol, and albumin levels (Table 6) and also differential leukocyte count (Figure 5). Benzylidene indanone 8 was well tolerable up to 300 mg/kg dose in Swiss albino mice. However, there was some indication of higher levels of albumin, creatinine, triglycerides, and total cholesterol at 1000 mg/kg dose which were statistically insignificant. It indicated caution for safety at higher dosage of compound 8 and suggests evaluating it for longer duration toxicity experiments.
| Parameters | Dose of compound 8 at mg/kg body weight as a single oral dose | ||||
|---|---|---|---|---|---|
| Control | 5 mg/kg | 50 mg/kg | 300 mg/kg | 1000 mg/kg | |
| Body wt. 7th day (g) | 21.74 ± 0.53 | 21.85 ± 0.77 | 22.60 ± 0.50 | 21.92 ± 0.84 | 19.25 ± 1.40 |
| Hemoglobin (g/dL) | 17.75 ± 0.17 | 18.25 ± 0.30 | 17.19 ± 0.46 | 16.93 ± 0.11 | 16.01 ± 0.56a |
| RBC (millions/mm3) | 6.73 ± 0.28 | 6.60 ± 0.31 | 6.72 ± 0.04 | 6.34 ± 0.34 | 5.92 ± 0.37 |
| WBC (thousands/mm3) | 12.06 ± 0.66 | 13.36 ± 1.14 | 11.60 ± 0.37 | 12.11 ± 0.69 | 12.50 ± 0.42 |
| ALKP (U/L) | 225.10 ± 20.32 | 225.26 ± 13.82 | 202.41 ± 31.04 | 200.59 ± 19.19 | 190.29 ± 22.47 |
| SGOT (U/L) | 25.09 ± 1.86 | 27.84 ± 1.22 | 28.21 ± 1.08 | 31.28 ± 1.05 | 28.06 ± 2.40 |
| SGPT (U/L) | 26.78 ± 2.25 | 25.64 ± 0.95 | 28.86 ± 1.32 | 28.42 ± 1.60 | 29.92 ± 1.00 |
| Albumin (g/dL) | 2.77 ± 0.19 | 2.84 ± 0.20 | 3.00 ± 0.13 | 3.25 ± 0.13 | 3.20 ± 0.13 |
| Creatinine (mg/dL) | 0.11 ± 0.03 | 0.18 ± 0.07 | 0.21 ± 0.04 | 0.20 ± 0.05 | 0.24 ± 0.05 |
| Triglycerides (mg/dL) | 156.5 ± 5.28 | 179.0 ± 4.69 | 197.2 ± 24.73 | 199.6 ± 9.92 | 196.7 ± 17.87 |
| Serum protein (mg/mL) | 6.18 ± 0.07 | 6.74 ± 0.11 | 6.22 ± 0.13 | 6.87 ± 0.15a | 6.89 ± 0.21a |
| Total cholesterol (mg/dL) | 101.94 ± 3.27 | 104.06 ± 5.41 | 112.83 ± 7.03 | 110.42 ± 6.05 | 118.47 ± 5.87 |
- a p < 0.05 compared to control.
3 Conclusion
In conclusion, benzylidene indanones are potential anticancer agents. The representative compound 8 of this series exhibits potent in vitro anticancer activity against various human cancer cell lines. Its anticancer activity is attributed through microtubule destabilization. Compound 8 shows moderate in vivo anticancer activity against solid tumors at 30 mg/kg, and in toxicity profile, it seems tolerable up to 300 mg/kg dose. It can be optimized for better activity, and soft drug design approach may also be used in future to optimize it for safer anticancer lead molecule.
Acknowledgments
The financial support from SERB-DST India is duly acknowledged.
Conflict of interest
Authors declare no conflict of interest.




