Epacadostat Overcomes Cetuximab Resistance in Colorectal Cancer by Targeting IDO-Mediated Tryptophan Metabolism
Funding: This work was supported by the National Natural Science Foundation of China (No. 82202934), Shanghai Sailing Program (No. 22YF1407600), China Postdoctoral Science Foundation (No. 2022M710752), Outstanding Resident Clinical Postdoctoral Program of Zhongshan Hospital Affiliated to Fudan University (No. 2023ZYYS-005), Shanghai Sailing Program (No. 22YF1431200), and the National Natural Science Foundation of China (No. 82203651).
Yimin Zhou, Qiongyan Tao and Chubin Luo contributed equally to this work.
ABSTRACT
Primary or acquired mutations in RAS/RAF genes resulting in cetuximab resistance have limited its clinical application in colorectal cancer (CRC) patients. The mechanism of this resistance remains unclear. RNA sequencing from cetuximab-sensitive and -resistant specimens revealed an activation of the tryptophan pathway and elevation of IDO1 and IDO2 in cetuximab-resistant CRC patients. In vitro, in vivo, and clinical specimens confirmed the upregulation of IDO1and IDO2 and the Kyn/Trp after cetuximab treatment. Additionally, the IDO inhibitor, epacadostat, could effectively inhibit the migration and proliferation of cetuximab-resistant CRC cells while promoting apoptosis. Compared to epacadostat monotherapy, the combination of cetuximab and epacadostat showed a stronger synergistic anti-tumor effect. Furthermore, in vivo experiments confirmed that combination therapy effectively suppressed tumor growth. Mechanistically, KEGG pathway analysis revealed the activation of the IFN-γ pathway in cetuximab-resistant CRC tissues. Luciferase reporter assays confirmed the transcriptional activity of IDO1 following cetuximab treatment. Silencing IFN-γ then suppressed the upregulation induced by cetuximab. Moreover, we observed that the combination reduced the concentration of the tryptophan metabolite kynurenine, promoted the infiltration of CD8+ T lymphocytes, and enhanced the polarization of M1 macrophages within the tumor microenvironment, thereby exerting potent anti-tumor immune effects. Overall, our results confirm the remarkable therapeutic efficacy of combining cetuximab with epacadostat in cetuximab-resistant CRC. Our findings may provide a novel target for overcoming cetuximab resistance in CRC.
Abbreviations
-
- CET
-
- cetuximab
-
- CRC
-
- colorectal cancer
-
- CTG
-
- Cell Titer-Glo
-
- DAMPs
-
- damage-associated molecular patterns
-
- DMSO
-
- dimethyl sulfoxide
-
- EGFR
-
- epidermal growth factor receptor
-
- ELISA
-
- enzyme-linked immunosorbent assay
-
- EPA
-
- epacadostat
-
- GSEA
-
- gene set enrichment analysis
-
- H&E
-
- hematoxylin and eosin
-
- IDO
-
- indoleamine 2,3-dioxygenase
-
- IFN-γ
-
- interferon-gamma
-
- IHC
-
- immunohistochemistry
-
- Kyn
-
- kynurenine
-
- MAPK
-
- mitogen-activated protein kinase
-
- MSI
-
- microsatellite instability
-
- siRNA
-
- small interfering RNA
-
- TCGA
-
- The Cancer Genome Atlas
-
- TME
-
- tumor microenvironment
-
- Trp
-
- tryptophan
1 Introduction
Colorectal cancer (CRC) is the third most diagnosed cancer and the fourth leading cause of cancer-related deaths worldwide [1]. Its epidemiological profile reflects a concerning trend of increasing incidence and prevalence across diverse geographical regions and populations. Standard treatment approaches for CRC include surgery, combined targeted chemotherapy, and radiation therapy [2]. For patients with metastatic colorectal cancer (mCRC) who have wild-type KRAS/NRAS/BRAF, particularly when the tumor is unresectable, chemotherapy regimens such as FOLFOX or FOLFIRI with cetuximab have become one of the standard first-line therapy options [3]. Multiple clinical trials and studies have demonstrated that for mCRC patients with wild-type KRAS/NRAS/BRAF, the use of cetuximab in combination with chemotherapy significantly improves treatment outcomes, including longer progression-free survival (PFS) and overall survival (OS), compared to chemotherapy alone [3, 4].
Cetuximab is a monoclonal antibody against the epidermal growth factor receptor (EGFR), inhibiting the growth and division of cancer cells. Additionally, cetuximab also exerts its effects through antibody-dependent cellular cytotoxicity (ADCC) to kill cancer cells [5-7]. However, clinical evidence shows that the rapid development of primary or acquired resistance, especially mutations in genes such as KRAS/NRAS/BRAF, limits the duration of drug efficacy, with most patients experiencing a PFS period of 6–12 months [8, 9]. Changes in the tumor microenvironment (TME) are also closely related to mechanisms of resistance to cetuximab. For instance, tumor-associated fibroblasts and angiogenesis can support tumor cell growth and survival by secreting growth factors, thereby reducing the efficacy of cetuximab [10]. Additionally, cancer cells may evade immune system attacks by reducing antigen expression or producing immunosuppressive molecules [11]. Due to the complex mechanisms of resistance limiting cetuximab's clinical application, new therapeutic approaches need to be explored to overcome this issue [9, 12].
In recent years, immunotherapy has emerged as a promising treatment modality with immense potential across various types of cancer. Among immunotherapy targets, indoleamine 2,3-dioxygenase (IDO) has undergone extensive research in many tumor types. The enzyme IDO, including IDO1, IDO2, and TDO, is an immunometabolic enzyme involved in the metabolism of the essential amino acid l-tryptophan (Trp) to l-kynurenine (Kyn), while IDO1 plays a crucial role in the initiation and progression of tumors [13]. Overexpression of IDO1 is observed across various cancer types, including CRC, and correlates with poor prognosis regardless of microsatellite stability [14]. Furthermore, studies suggest that inhibiting IDO1 can enhance the therapeutic efficacy in models of lung, melanoma, breast, and brain cancer [15]. Epacadostat, an IDO1 inhibitor, represents a promising avenue in cancer immunotherapy. By targeting the IDO pathway, epacadostat aims to modulate immune responses within TME, potentially overcoming immune evasion mechanisms employed by cancer cells [16]. Its unique mechanism of action and preclinical efficacy in various tumor types have generated considerable interest in evaluating its clinical utility, particularly in combination with other therapeutic agents [17]. However, the role of IDO inhibitors in cetuximab-resistant CRC remains to be explored.
To investigate the mechanisms of cetuximab resistance, we focused on cetuximab-resistant CRC tumor tissues. RNA sequencing from cetuximab-resistant tissues revealed that the IDO-mediated tryptophan metabolism pathway was significantly activated. Subsequently, we found that the IDO1 inhibitor epacadostat effectively overcame cetuximab resistance in both in vitro and in vivo models. Notably, epacadostat treatment in an immune-proficient mouse model transformed “cold” tumors into “hot” tumors by promoting the infiltration of tumor-infiltrating immune cells. These results suggest a novel therapeutic approach for overcoming cetuximab resistance in CRC.
2 Material and Methods
Tumor and blood samples from CRC patients treated with either standard chemotherapy or cetuximab-targeted therapy were collected. Cell lines and mouse models were used to evaluate the effects of cetuximab and epacadostat. In vitro and in vivo experiments were divided into four groups: control, cetuximab, epacadostat, and the combination of cetuximab and epacadostat. For the in vitro experiments, the concentration of cetuximab was 10 μg/mL, and the concentration of epacadostat was 10 μM. In the in vivo experiments, cetuximab was administered via intraperitoneal injection at a concentration of 50 mg/kg, and epacadostat was administered via oral gavage at a concentration of 300 mg/kg. The control group was treated with the same concentration of DMSO as the experimental groups. Methods included qRT-PCR, western blot, ELISA, clonogenic and transwell assays, luciferase reporter assays, si-RNA transfection, immunofluorescence, and immunohistochemistry. Gene expression data from GEO and TCGA were analyzed to explore mechanisms of cetuximab resistance. Statistical analyses were conducted using t-tests, ANOVA, and Pearson correlations to determine significance. Detailed information could be found in Appendix S1.
3 Results
3.1 Cetuximab Increases IDO1/IDO2 Expression in CRC Patients
To investigate the mechanism of cetuximab resistance in CRC, RNA sequencing from four liver metastatic biopsies of CRC from two patients with pre-treated and acquired resistance to cetuximab was analyzed (GSE84267). GO pathway analysis of differentially expressed genes (DEGs) revealed significant enrichment in the tryptophan metabolism pathway (Figure 1A). Notably, key enzymes in the tryptophan metabolism pathway, including IDO1 and IDO2, were significantly upregulated among the top 40 most dysregulated genes, shedding light on the potential alterations in tryptophan metabolism associated with cetuximab resistance (Figure 1B).
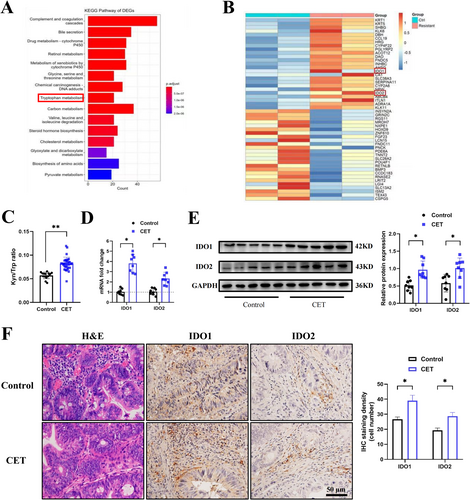
To validate our findings, we collected serum from CRC patients treated with or without cetuximab. Following cetuximab treatment, the levels of the kynurenine/tryptophan (K/T) ratio in patient serum, serving as a measure of IDO activity, showed a significant increase compared to the patients with only neoadjuvant chemotherapy (Figure 1C). Detailed ELISA kit information could be found in Table S1. Furthermore, CRC patients treated with cetuximab exhibited increased IDO1 and IDO2 in tumor tissues, as determined by qPCR (Figure 1D) and Western blot (Figure 1E). Detailed sequences of qPCR primers and antibodies for Western Blot analysis could be found in Table S2 and S3. IHC analysis also revealed a significant elevation in the expression levels of IDO1 and IDO2 in tumor tissues after treatment (Figure 1F). Detailed information on antibodies for IHC analysis could be found in Table S4. These observed phenomena in tumor tissues were consistent with the changes in IDO1 and IDO2 expression levels in patient serum, indicating that cetuximab treatment could promote IDO expression in CRC patients.
3.2 Cetuximab Increases IDO1/IDO2 Expression in RAS/RAF Mutant CRC Cells and Mouse Models
To investigate whether cetuximab induced IDO1 and IDO2 expression in vitro and in vivo, we employed genetically mutated CRC cell lines, HCT116 (KRAS mutant) and HT29 (BRAF mutant), as our cellular and mouse models. These cells were treated with DMSO, 10 μg/mL cetuximab, followed by the assessment of cell viability using CTG. Notably, cetuximab treatment did not significantly alter cell viability after 96 h (Figure S1A). Furthermore, flow cytometry (FC) results demonstrated that cetuximab did not considerably induce apoptosis in HCT116 and HT29 cells (Figure S1B). Likewise, clonogenic assays and transwell assays revealed that cetuximab had no significant effect on the proliferation and migration ability of HCT116 and HT29 cells (Figure S1C). These results indicated that cetuximab monotherapy may not produce cytotoxic effects on HCT116 and HT29 RAS/RAF mutant CRC cells by inhibiting cell proliferation, viability, or migration, or inducing apoptosis, in contrast to the effects seen in RAS/RAF wild-type CRC cells like Caco-2 (Figure S2A–E).
However, HCT116 and HT29 cells treated with cetuximab significantly upregulated the IDO1 and IDO2 mRNA expression (Figure 2A). Consistent with these findings, ELISA analysis revealed an increase in the K/T ratio in the cell culture supernatants following cetuximab treatment, suggesting enhanced cellular IDO activity (Figure 2B). In addition, the protein expression levels of both IDO1 and IDO2 were markedly elevated in the cetuximab-treated group (Figure 2C). These results indicated that cetuximab upregulated IDO1 and IDO2 expression in vitro.
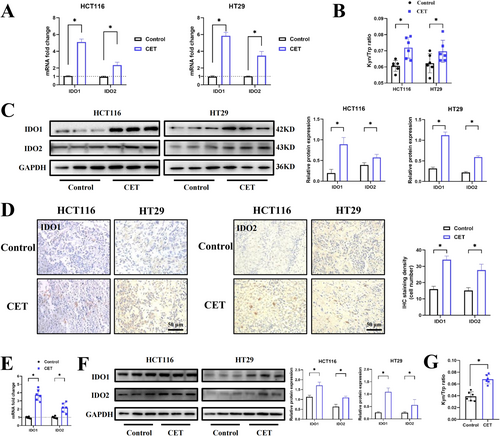
Consistent with the in vitro findings, cetuximab treatment in HCT116 and HT29 subcutaneous xenograft immune-deficient mice did not significantly inhibit tumor growth compared to the control group, indicating limited anti-tumor efficacy of cetuximab monotherapy (Figure S2F). Similarly, H&E staining showed no significant morphological changes in tumor tissues from cetuximab-treated mice (Figure S2G). Notably, IHC staining revealed a marked increase in IDO1 and IDO2 protein levels in cetuximab-treated tumors (Figure 2D). This upregulation was further confirmed by qPCR and western blot analyses, which demonstrated elevated mRNA and protein expression of IDO1 and IDO2, respectively (Figure 2E,F). Additionally, the K/T ratio in cetuximab-treated mice was significantly increased, consistent with observations in patient serum samples following cetuximab therapy (Figure 2G). Collectively, these in vitro and in vivo results demonstrate that cetuximab upregulates IDO1 and IDO2 expression in RAS/RAF mutant CRC cells and mouse models.
3.3 IDO Inhibitor Overcomes Cetuximab Resistance In Vitro
By inhibiting IDO1, epacadostat reduces the consumption of tryptophan and the accumulation of kynurenine, thereby enhancing the immune response to tumors and potentially slowing tumor growth or even inducing tumor regression. It has been investigated as a potential treatment for various types of cancer, particularly in combination with other immunotherapies or chemotherapy agents [18]. Accordingly, we hypothesized that cetuximab upregulates IDO1 and IDO2, thereby promoting CRC progression and immune evasion.
To evaluate the effects of IDO1 inhibition, we first examined the migratory capacity of CRC cells. Under the combined treatment of cetuximab and epacadostat, the migratory ability of CRC cells was significantly reduced compared to the epacadostat group (Figure 3A). Next, we assessed the clonogenic ability of CRC cells and found that the combination of cetuximab and epacadostat significantly inhibited colony formation compared to epacadostat treatment alone (Figure 3B). We then evaluated cell viability using CTG assays. Following 96 h of treatment, cetuximab monotherapy had no significant impact on the viability of CRC cells, while epacadostat alone reduced cell viability compared to the control group. Notably, the combination of cetuximab and epacadostat resulted in a significantly greater reduction in cell viability compared to epacadostat alone (Figure 3C). To further investigate the mechanisms underlying the observed effects, we analyzed apoptosis-related markers. The combination treatment markedly increased the Bax/Bcl-2 ratio and the levels of cleaved-caspase3/caspase3, indicating enhanced apoptosis compared to epacadostat alone (Figure 3D). Consistent with these results, FC analysis demonstrated that epacadostat sensitized HCT116 and HT29 cells to cetuximab, with a higher apoptotic cell rate observed in the combination group compared to epacadostat monotherapy (Figure 3E). In summary, these findings demonstrate that the combination of cetuximab and epacadostat exerts a significant anti-tumor effect by enhancing cytotoxic and antiproliferative activities in vitro.
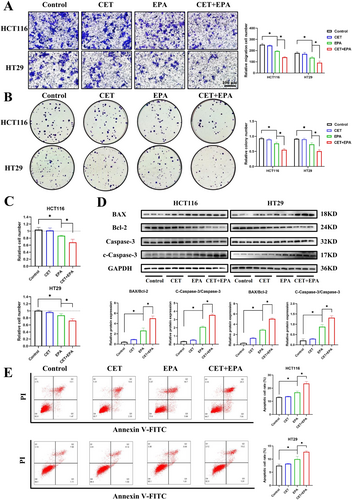
To further validate the role of IDO1 in CRC progression, we conducted IDO1 knockdown experiments using small interfering RNA (siRNA; si-IDO1). Consistent with the effects observed with epacadostat, si-IDO1 significantly attenuated the migratory capacity of HCT116 and HT29 cells in transwell assays compared to the control group (Figure S3A). Moreover, the combination of cetuximab and si-IDO1 further reduced cell migration compared to si-IDO1 alone (Figure S3A). CTG assay results demonstrated a significant reduction in cell viability upon IDO1 depletion, and the combination showed a more pronounced decrease in cell viability than si-IDO1 alone (Figure S3B). Additionally, FC analysis revealed increased apoptosis in the cetuximab-treated group with IDO1 knockdown, suggesting that cetuximab exerts stronger anti-tumor effects in the absence of IDO1 (Figure S3C). These findings collectively demonstrate that IDO1 plays a pivotal role in promoting CRC cell survival, migration, and resistance to apoptosis.
3.4 IDO Inhibitor Overcomes Cetuximab Resistance In Vivo
Based on the previous findings, we next investigated whether inhibition of IDO sensitized CRC to cetuximab in vivo. To achieve this, we established a subcutaneous tumor model using the mouse cell line MC38. Once the tumors were palpable (100–150 mm3), mice were divided into four treatment groups: control (DMSO), cetuximab (50 mg/kg, once weekly), epacadostat (300 mg/kg, once daily), or a combination of cetuximab and epacadostat (Figure 4A).
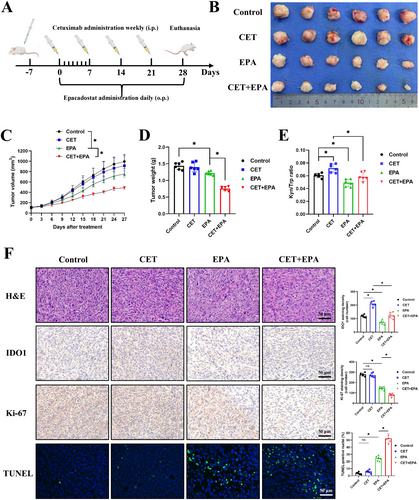
After 28 days of treatment, the tumor size gradually increased in both the control and cetuximab treatment groups. However, the administration of epacadostat alone and the combination group both led to a suppression in tumor growth (Figure 4B), and the combination group exhibited a remarkable reduction in tumor volume and tumor weight (Figure 4C,D). Additionally, the K/T ratio in the serum of mice in the epacadostat and combination therapy groups decreased, indicating that epacadostat inhibits tryptophan metabolism (Figure 4E). Next, we conducted H&E staining on mouse tumor tissues to assess the pathological status, followed by the evaluation of tumor proliferation and apoptosis using Ki-67 and TUNEL staining, respectively. The results of H&E staining indicated a decrease in tumor cell count and proliferation of peritumoral fibrous tissue in the epacadostat group, while these post-treatment pathological features were even more pronounced in the combination group (Figure 4F). Then, from the results of the Ki-67 and TUNEL assay, we found that compared to the inapparent therapeutic effect of cetuximab monotherapy, epacadostat showed significant efficacy, whereas the combination therapy exhibited higher apoptosis and lower proliferation than the epacadostat monotherapy group (Figure 4F). Additionally, compared to the control group, IDO1 expression was significantly reduced in the epacadostat monotherapy group, and a further decrease was observed in the combined treatment group compared to the cetuximab monotherapy group (Figure 4F). Detailed information on antibodies and the TUNEL kit for IHC and IF analysis could be found in Table S4. These in vivo results further supported the in vitro evidence that IDO inhibition overcame cetuximab resistance in CRC.
3.5 Cetuximab-Induced IFN-γ Signaling Promotes the Expression of IDO
To elucidate the mechanism by which cetuximab promotes IDO1 and IDO2 expression, we performed GSEA analysis on RNA sequencing data obtained from liver metastatic biopsies of CRC patients who acquired resistance to cetuximab. The GSEA analysis indicated an upregulation of the IFN-γ signaling pathway in the cetuximab-resistant group (Figure 5A). Evidence suggested that cetuximab could activate immune cells, resulting in the release of various cytokines, including IFN-γ. This has been validated in models such as breast cancer [19]. Furthermore, elevated levels of IFN-γ in the TME are often associated with the release of damage-associated molecular patterns (DAMPs), which are generated in response to or damage induced by therapies such as cetuximab. DAMPs, in turn, can further amplify IFN-γ production, creating a positive feedback loop that promotes immune escape mechanisms [20]. Therefore, we aimed to investigate the impact of cetuximab on IFN-γ signaling and to elucidate the regulatory role of IFN-γ in modulating IDO expression in CRC. We examined the expression of IFN-α, IFN-β, and IFN-γ mRNA in the tumor tissues of the subjects. The results showed that the expression of IFN-γ mRNA was significantly increased in the cetuximab treatment group (Figure 5B), while the levels of IFN-α and IFN-β remained relatively unchanged (Figure S4A). Additionally, serum IFN-γ concentrations were significantly elevated in CRC patients treated with cetuximab (Figure 5C), and IHC staining of tumor tissues corroborated these findings (Figure 5D). To investigate whether cetuximab-induced IDO upregulation was associated with the MAPK pathway, a key resistance mechanism in cetuximab-resistant tumors [1, 3, 5, 6, 16], we treated CRC cells with the MEK inhibitor trametinib. Our results demonstrated that trametinib did not attenuate cetuximab-induced IDO expression, suggesting that the upregulation of IDO by cetuximab is independent of the MAPK pathway (Figure S4B,C). Notably, we observed increased expression of calreticulin (CRT), a well-established marker of DAMPs, in HCT116 and HT29 (Figure S4D). This suggests that cetuximab may also enhance IFN-γ levels in CRC through the release of DAMPs.
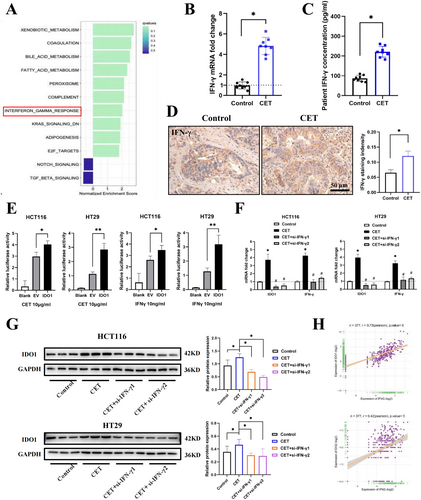
To further elucidate the relationship between IFN-γ and IDO in CRC, we investigated whether IFN-γ directly regulated IDO1 transcription and expression. Dual luciferase reporter assays conducted in HCT116 and HT29 cells revealed that cetuximab activated the transcription of IDO1. Additionally, treatment with the IFN-γ cytokine (10 ng/mL) also activated IDO1 transcription in both cell lines, confirming that cetuximab might promote IDO1 transcription activity via upregulating IFN-γ (Figure 5E). Subsequently, siRNA-mediated knockdown of IFN-γ resulted in the downregulation of IDO1 and IFN-γ expression in HCT116 and HT29 cells (Figures 5F,G and S4E). Next, we examined the type II IFN signaling and the IDO (IDO1 and IDO2) in the TCGA-CRC cohort. As anticipated, IFN-γ showed a strong positive correlation with the expression of IDO1 and IDO2 (Figure 5H). These data indicated that cetuximab induced type II IFN signaling activation, enhancing IDO expression in cetuximab-resistant CRC.
3.6 Epacadostat Overcomes Cetuximab-Resistant Tumor by Promoting the Polarization of Macrophage M1
Tryptophan and its metabolites influence tumor growth and immune evasion. Increased IDO activity could lead to local depletion of tryptophan and accumulation of immunosuppressive kynurenine metabolites, creating an immunosuppressive milieu that promotes tumor immune evasion and tolerance [21]. Therefore, immune cells were isolated by FC and stained with specific markers for further analysis (Figure S5A).
In peripheral blood, no significant differences were observed in the populations of CD4+ T lymphocytes (CD45+/CD3+/CD4+) across the treatment groups (Figure 6A). However, epacadostat treatment alone increased the levels of CD8+ T lymphocytes (CD45+/CD3+/CD8+), with a more pronounced elevation observed in the combination treatment group compared to epacadostat monotherapy (Figure 6A). Consistent with this, the levels of IFN-γ+ CD8+ T cells mirrored the changes in CD8+ T lymphocytes (Figure 6B). Furthermore, the combination treatment significantly increased the proportion of M1 macrophages while reducing the proportion of M2 macrophages in peripheral blood (Figure 6C). In tumor tissues, similar patterns were observed. The combination treatment significantly enhanced the infiltration of CD8+ T lymphocytes and IFN-γ+ CD8+ T cells compared to epacadostat alone (Figure 6D,E). Additionally, the combination therapy shifted the macrophage polarization toward an M1 phenotype, as evidenced by an increased M1/M2 ratio (Figure 6F). In the spleen, the immune cell profiles aligned with those in peripheral blood and tumor tissues. The combination treatment elevated the levels of CD8+ T lymphocytes and IFN-γ+ CD8+ T cells (Figure S5B,C) and promoted M1 macrophage polarization (Figure S5D). However, neutrophil (CD45+/Ly6G+/CD11b+) populations remained unchanged across all treatment groups in peripheral blood, spleen, and tumor tissues (Figure S5E–G).
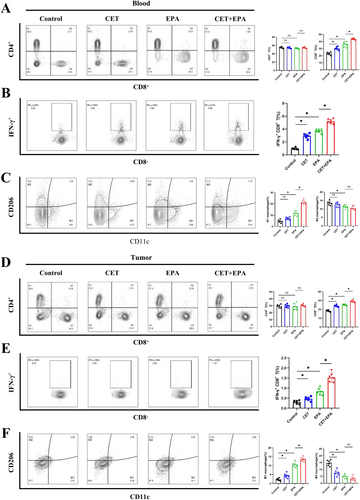
CD8+ T lymphocytes are pivotal effector cells in tumor immunity, capable of directly killing tumor cells and secreting cytokines such as IFN-γ to promote anti-tumor immune responses and inhibit tumor growth [22]. These findings demonstrated that the increases in infiltration of CD8+ T lymphocytes and IFN-γ+ CD8+ T cells in the TME were involved in the process of combination therapy against CRC. Moreover, these results also suggested that the polarization of M1 macrophages was involved in the anti-tumor immune response, whereas the polarization of M2 macrophages exhibited a tumor-promoting effect. Detailed information on antibodies and related reagents for FC analysis could be found in Table S5. FC analysis collectively indicated a positive correlation between therapeutic response and the activation of CD8+ T lymphocytes and M1 macrophages in our models, suggesting that combination therapy enhanced apoptosis in cetuximab-resistant CRC by activating immune effector cells in the TME (Figure 7).
4 Discussion
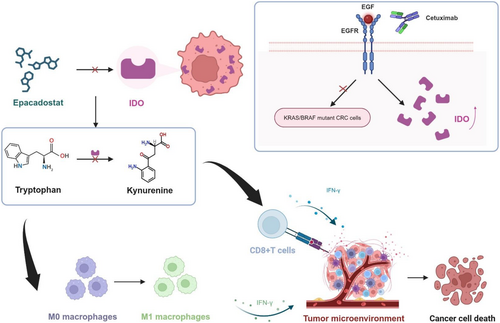
Cetuximab targeted therapy is currently the first-line clinical treatment for KRAS/NRAS/BRAF wild-type CRC patients, especially for patients with mCRC [23]. However, the emergence of cetuximab resistance mechanisms, particularly in the context of KRAS or BRAF mutations, undermines the efficacy of cetuximab-based therapies, limiting treatment options and impacting patient outcomes. Thus, various therapeutic agents are being explored in combination with cetuximab to overcome cetuximab resistance, control CRC progression, and achieve better therapeutic outcomes. Our results validated that epacadostat could sensitize cetuximab-resistant CRC to cetuximab by mechanistically targeting the IFN-γ pathway, tryptophan metabolism, and the TME.
The presently well-researched mechanisms of cetuximab resistance include gene mutations (KRAS/NRAS/BRAF), EGFR mutations, bypass activation (such as HER2 amplification), and alterations in downstream signaling pathways (such as PTEN loss or mutation) [24, 25]. Among these, KRAS/NRAS/BRAF mutations are the most common reasons in colorectal, pancreatic, and lung cancers [25, 26], with KRAS G12C and BRAF V600E mutations being the most common forms of mutated oncogenes in CRC [27]. Although the mechanisms of cetuximab resistance are still undergoing further investigation, Johnson et al. found that patients with ARID1A mutations were linked to worse outcomes when treated with cetuximab, which suggested that ARID1A might be a novel target for cetuximab therapy [28]. Geng et al. linked circHIF1A to cetuximab resistance via HIF1α-mediated glycometabolic alterations in CRC [29]. Based on the integrative analysis of CRC cell lines and clinical samples, Li et al. summarized that immune regulation was significantly associated with drug sensitivity, including that high expression of HDAC6 was negatively associated with the response of cetuximab [30]. Although some other scholars have also been trying to explain the mechanisms from various perspectives, such as ferroptosis or different non-coding RNAs [31, 32], there is no effective treatment to specifically treat CRC expressing KRAS or BRAF mutations [25, 33]. To overcome cetuximab resistance in CRC, various types of agents targeting current resistance mechanisms are undergoing clinical trials at different phases. Rona Yaeger et al. demonstrated that adagrasib exhibited anti-tumor activity in mCRC patients harboring mutant KRAS G12C, achieving a median response duration of over 6 months in the combination therapy group with cetuximab in a phase II trial [34]. Despite the addition of urelumab (a monoclonal antibody agonist that binds to CD137 receptors) with cetuximab being tolerable, Khushalani et al. considered that preliminary response rates did not indicate an evident additive benefit in advanced solid tumors [35]. Koyama et al. tried to combine paclitaxel with biweekly cetuximab in R/M-HNSCC patients, which demonstrated highly encouraging efficacy and manageable toxicities [36].
In recent years, immunotherapy is gaining increasing attention in the treatment of various types of cancer, while IDO is regarded as a novel potential target for immunotherapy [37]. Indeed, by performing RNA sequencing on clinical CRC specimens, we observed significantly elevated IDO expression in cetuximab-resistant CRC. Based on these clinical statistics, we next confirmed the activation of the IDO-mediated tryptophan metabolism pathway in both cetuximab-resistant CRC cell lines and clinical specimens. Furthermore, we demonstrated that the IDO inhibitor sensitized RAS/RAF-mutated CRC cells to cetuximab treatment. These results suggested a promising immunotherapy strategy for overcoming cetuximab resistance. IDO activity has been proposed to be a possible mechanism of resistance to numerous treatments, including PD-1 inhibitors [38]. Epacadostat aims to enhance the immune response by blocking the IDO1-mediated tryptophan metabolism pathway, thereby inhibiting tumor growth and spread [39, 40]. Despite the lack of distinguished clinical benefits in a phase III clinical trial (ECHO301) in melanoma patients, IDO remains a promising immune checkpoint [41]. Our study fortunately found that combining cetuximab with epacadostat could potentially enhance the efficacy of cetuximab by preventing IDO-mediated immunosuppression, achieving promising results in both in vitro and in vivo models. However, clinical trials are indeed required for further validation.
Although previous studies have reported elevated IDO expression in CRC, the mechanisms remain poorly understood. In other tumor models, IDO elevation is regulated by multiple factors and biological processes. For instance, cetuximab has been shown to activate immune effector cells primarily through ADCC, leading to the release of cytokines into the TME [42-45]. The MAPK pathway, which is constitutively activated in cetuximab resistance due to KRAS or BRAF mutations, bypasses EGFR inhibition and contributes to therapeutic resistance [46]. Our results indicate that cetuximab promotes IDO upregulation independently of MAPK activation. Although the precise mechanism remains unclear, cetuximab may induce partial signaling alterations, triggering the upregulation of immune-related genes. The observed increase in calreticulin, a marker of immunogenic cell death (ICD), suggests that cetuximab promotes the release of DAMPs, which may stimulate IFN-γ production through autocrine or paracrine signaling. IFN-γ serves as a key inducer of IDO1/2 expression, promoting the transcription of IDO1/IDO2 genes through the activation of signaling pathways such as JAK–STAT [47]. As anticipated, we found that cetuximab not only upregulated IFN-γ expression in CRC but also enhanced the transcriptional activity of IDO1, thereby validating the regulatory relationship among cetuximab, IFN-γ, and IDO. Collectively, these findings suggest that cetuximab likely activates immune effector cells through DAMPs, which induce type II IFN signaling and subsequently promote IDO expression by enhancing its transcriptional activity in cetuximab-resistant CRC.
Elevated levels of kynurenine and increased serum Kyn/Trp ratios are commonly observed in advanced-stage CRC patients and are associated with poor prognosis, irrespective of MSI status [37, 48, 49]. Notably, our study found that cetuximab induced an increase in IDO, associated with the tryptophan metabolites and TME in CRC. Mechanistically, both the accumulation of kynurenine and the depletion of tryptophan in TME inhibit T cell activation and proliferation, weakening the immune system's ability against tumors [50, 51]. CD8+ T lymphocytes, often referred to as cytotoxic T lymphocytes (CTLs), are crucial anti-tumor immune cells that enhance the immune response and promote the killing of tumor cells by secreting cytokines like IFN-γ [52]. Therefore, the increase in CD8+ T lymphocytes and IFN-γ is generally indicative of an immune response against the tumor [22, 53].
It is also noteworthy that IFN-γ signaling plays a pivotal role in promoting the polarization of macrophages toward the M1 phenotype, characterized by enhanced anti-tumor activities [54]. Through activation of the JAK–STAT signaling pathway, IFN-γ induces the expression of M1-associated markers while suppressing M2-associated markers, ultimately contributing to effective anti-tumor immune responses [55-57]. Fong et al. also found that inhibition of the IDO1/Kyn/AHR axis can improve the anti-PD1 efficacy of CRC through the study of Lactobacillus gallinarum-derived metabolites [58]. Likewise, our study found that inhibiting IDO1 with epacadostat, as well as the Kyn/Trp ratio, could also enhance cetuximab efficacy in CRC. Consistently, we found that the decrease of the Kyn/Trp ratio in the epacadostat group and combination group mice serum. Meanwhile, the decrease in kynurenine concentration led to the higher levels of infiltration of CD8+ T lymphocytes in TME, especially for the populations of IFN-γ+ CD8+ T cells. Furthermore, polarization of M1 macrophages synergistically enhanced the anti-tumor immune environment along with increased infiltration of CD8+ T lymphocytes. The pronounced infiltration of T cells and M1 macrophages in the combination group resulted from multiple synergistic mechanisms. Cetuximab induced tumor cell apoptosis, releasing DAMPs, which activated dendritic cells, recruited T cells, and promoted M1 macrophage polarization [59]. Simultaneously, epacadostat inhibited IDO1, reducing immunosuppressive metabolites and enhancing IFN-γ production, further amplifying T cell infiltration and M1 polarization. These combined actions reshaped the TME into an immunostimulatory state, significantly enhancing anti-tumor efficacy.
In summary, our findings suggested that combining cetuximab with the IDO1 inhibitor epacadostat might represent a promising chemotherapy-immunotherapy strategy for cetuximab-resistant CRC treatment. Moreover, epacadostat, as a novel IDO1 inhibitor, might play a crucial role in CRC immunotherapy by affecting tryptophan metabolism and altering the TME.
Author Contributions
Yimin Zhou: conceptualization, data curation, formal analysis, investigation, project administration, software, validation, visualization, writing – original draft, writing – review and editing. Qiongyan Tao: data curation, formal analysis, investigation, software. Chubin Luo: data curation, software, validation, visualization, writing – review and editing. Jinsong Chen: data curation, formal analysis. Genwen Chen: funding acquisition, methodology, project administration, resources, software, writing – review and editing. Jianyong Sun: funding acquisition, methodology, project administration, supervision, validation, writing – review and editing.
Acknowledgments
I would like to express my heartfelt gratitude to Prof. Sun Jianyong for his guidance and support in both my research and life. I am deeply thankful to Chen Genwen for his meticulous guidance during my experiments and to Tao Qiongyan, Luo Chubin, and Chen Jinsong for their invaluable assistance in the experimental work.
Ethics Statement
The use of clinical specimens with informed consent was approved by the Medical Ethics Committee of Zhongshan Hospital, Fudan University (F2020-035). The experiment was approved by the Experimental Animal Ethics Committee of Zhongshan Hospital, Fudan University (2023-304).
Consent
All informed consents were obtained from the subjects and/or guardians.
Conflicts of Interest
The authors declare no conflicts of interest.




