Autologous HSCT with novel agent-based induction and consolidation followed by lenalidomide maintenance for untreated multiple myeloma
Abstract
Triplet regimen comprising proteasome inhibitors, immunomodulatory drugs, and dexamethasone (DEX) is a recommended induction/consolidation therapy for multiple myeloma (MM) patients eligible for transplant. In this Japanese phase II study conducted from 2017 to 2019, newly diagnosed MM patients aged 20–65 received four induction cycles with bortezomib (Bor), lenalidomide (Len), and DEX (VRD), followed by Bor and high-dose melphalan with autologous stem cell rescue. Subsequently, they underwent four consolidation cycles with carfilzomib, Len, and DEX (KRD), followed by Len maintenance until disease progression. A total of 141 patients were analyzed. In an intent-to-treat population, the complete or better response post induction was 19.9%, rising to 39.7%, 58.9%, and 62.4% after transplant, consolidation, and 1-year maintenance, respectively. With a median follow-up of 38 months, the 3-year progression-free survival (PFS) rate was 83.5% and the 3-year overall survival rate was 92.5%. Severe adverse events (≥grade 3) occurred in ~30% of patients; however, there was no treatment-related mortality. These findings clearly showed the tolerability and effectiveness of this protocol. Nevertheless, patients with high-risk cytogenetics showed a trend toward lower 3-year PFS than those without (77.8% vs. 89.4%, p = 0.051), and ultra-high-risk cytogenetics (≥2 high-risk cytogenetics) had an even worse prognosis, with 61.2% 3-year PFS. To overcome this situation, a more potent treatment strategy incorporating novel agents such as the CD38-antibody should be assessed in future studies.
Abbreviations
-
- AE
-
- adverse event
-
- ALT
-
- alanine aminotransferase
-
- ANC
-
- absolute neutrophil count
-
- ASCT
-
- autologous hematopoietic stem cell transplantation
-
- ASO
-
- allele-specific oligonucleotide
-
- AST
-
- aspartate aminotransferase
-
- BM
-
- bone marrow
-
- Bor
-
- bortezomib
-
- CCr
-
- creatinine clearance
-
- Cfz
-
- carfilzomib
-
- CI
-
- confidence interval
-
- CR
-
- complete response
-
- DEX
-
- dexamethasone
-
- ECOG
-
- Eastern Cooperative Oncology Group
-
- FISH
-
- fluorescence in situ hybridization
-
- IMiDs
-
- immunomodulatory drugs
-
- IMWG
-
- International Myeloma Working Group
-
- Len
-
- lenalidomide
-
- LTD
-
- last tolerated dose
-
- Mel
-
- melphalan
-
- MM
-
- multiple myeloma
-
- MRD
-
- minimal/measurable residual disease
-
- NDMM
-
- newly diagnosed multiple myeloma
-
- OS
-
- overall survival
-
- PBSC
-
- peripheral blood stem cell
-
- PD
-
- progressive disease
-
- PFS
-
- progression-free survival
-
- PI
-
- proteasome inhibitor
-
- PN
-
- peripheral neuropathy
-
- PS
-
- performance status
-
- sCR
-
- stringent CR
-
- TTF
-
- time to treatment failure
-
- ULN
-
- upper limit of normal
-
- VGPR
-
- very good partial response
1 INTRODUCTION
Multiple myeloma (MM) is a B-cell malignancy marked by abnormal plasma cell proliferation in the bone marrow (BM), elevated monoclonal protein, and numerous osteolytic lesions. Previously, MM was deemed incurable, with a median survival of about 3 years post diagnosis before the advent of novel agents.1 Two key drug classes, proteasome inhibitors (PIs) and immunomodulatory drugs (IMiDs), have shown rapid and sustained responses. Consequently, the triplet regimen of bortezomib (Bor), lenalidomide (Len), and dexamethasone (DEX) (VRD) is a promising induction therapy prior to autologous hematopoietic stem cell transplantation (ASCT) for newly diagnosed multiple myeloma (NDMM) patients.2-4
However, many patients eventually relapse; therefore, further efforts are needed to improve survival. Attempts to achieve better outcomes included intensification of consolidation5 and/or maintenance therapies.6-8 Carfilzomib (Cfz), a next-generation PI, irreversibly binds to the β5 subunit of the 20S proteasome and selectively inhibits its chymotrypsin-like activity. Preclinical and clinical studies have demonstrated that Cfz overcomes resistance to Bor and elicits a more profound and sustained proteasome inhibition than Bor.9, 10 Several phase II studies have indicated that Cfz, combined with Len and DEX (KRD) is highly effective with tolerable adverse events (AEs) for NDMM as both induction and consolidation therapy, improving patients' outcome.11-13
In Japan, both the VRD regimen for NDMM and Cfz for relapsed/refractory MM were first approved in 2016. Thus far, limited data were available on the triplet-based regimen combined with ASCT among Japanese patients. In this phase II multicenter study with NDMM patients, we assessed the safety and efficacy of the combined treatment strategy, including VRD induction, ASCT, KRD consolidation, and Len maintenance until disease progression.
2 MATERIALS AND METHODS
2.1 Patients and eligibility
A total of 143 patients from 44 institutions were enrolled in this study between March 2017 and July 2019 after providing written informed consent. The Kyushu University Hospital Institutional Review Board approved the study (approval number; 20181005, UMIN registration number; 000024165), which was conducted by the Japan Study Group for Cell Therapy and Transplantation.
The inclusion criteria for this study were as follows: patients with NDMM as per the International Myeloma Working Group (IMWG) criteria; aged between 20 and 65 years; measurable levels of serum and/or urinary M-protein; Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0–2 (grades 3–4 acceptable if pain is caused by myeloma); adequate hematological parameters (e.g., absolute neutrophil count [ANC] ≥1.0 × 109/L, hemoglobin level ≥8 g/dL without transfusion, platelet count ≥75 × 109/L); creatinine clearance (CCr) ≥30 mL/min estimated by the Cockcroft–Gault formula; serum total bilirubin level ≤1.5 times the upper limit of normal (ULN); levels of aspartate aminotransferase (AST) and/or alanine aminotransferase (ALT) values ≤3 × ULN; left ventricular ejection fraction ≥50%; oxygen saturation ≥93% measured by pulse oximetry in room air; expected prognosis of more than 3 months; adequate contraception available. Patients were excluded if they had myeloma-related disorders (e.g., solitary plasmacytoma, plasma cell leukemia, Waldenstrom's macroglobulinemia, amyloidosis, POEMS syndrome), recent treatment history (surgery, radiation, and ≥30 mg/day of corticosteroids) within the last 14 days, central nervous system invasion, active concurrent malignancies, active systemic infection, positive viral screening tests (hepatitis B surface antigen, hepatitis C antibody, or human immunodeficiency virus), potential pregnancy, or other severe medical or psychiatric conditions unsuitable for study inclusion.
2.2 Protocol
2.2.1 Induction therapy
In this study, the VRD regimen was initially administered every 3 weeks for four cycles. It contained subcutaneous (sc) Bor (1.3 mg/m2 on days 1, 4, 8, and 11), Len (25 mg/day from day 1 to 14), and oral DEX (40 mg/day on days 1, 4, 8, and 11). The initial Len dose reduced to 10 mg/day for patients with impaired renal function (CCr <60 mL/min). It was recommended to reduce the dose of Len and/or temporarily discontinue it in the case of neutropenia, thrombocytopenia, or renal dysfunction. The Bor dosage was adjusted according to PN grading as follows. If patients developed grade 2 or painful grade 1 PN, Bor administration was reduced from twice weekly to once weekly; then, its dose was reduced in steps from 1.3 to 1.0 mg/m2 and 0.7 mg/m2. If patients developed grade 3 or painful grade 2 PN, Bor administration was discontinued and then resumed at a reduced dose of 0.7 mg/m2 after recovery.
The next cycle was initiated if the ANC was ≥1.0 × 109/L, platelet count was ≥75 × 109/L, and nonhematologic AEs were ≤grade 2. The treatment was considered intolerable and discontinued upon a 3-week schedule delay.
2.3 Peripheral blood stem cell (PBSC) harvest
A mobilization procedure was scheduled for patients with PS 0–2, adequate vital organ functions, and no evidence of progressive disease (PD). For efficient PBSC harvest, high-dose cyclophosphamide was administered (1.5 g/m2 × 2 days), combined with four doses of sc Bor (1.3 mg/m2 or last tolerated dose [LTD]). Granulocyte colony-stimulating factor was initiated during myelosuppression to mobilize CD34+ hematopoietic stem/progenitor cells to peripheral blood. When planning this trial, plerixafor was not yet available. The minimum required amount of collected CD34+ cells was 2 × 106 cells per patient body weight, with a second apheresis allowed if the first did not yield enough cells.
2.4 Autologous hematopoietic stem cell transplantation
High-dose chemotherapy with autologous stem cell rescue was performed on patients with PS of 0–2, adequate vital organ functions, no evidence of PD, and preservation of ≥2 × 106/kg of CD34+ cells. Patients underwent a conditioning regimen comprising melphalan (Mel) (total dose of 200 mg/m2 over two consecutive days) and four doses of sc Bor (1.3 mg/m2 or LTD). Reduced Mel dose (total of 140 mg/m2) was adjusted for patients with impaired renal function.
2.5 Consolidation and maintenance therapy
Patients with good PS, adequate vital organ functions, and no PD 90 days post ASCT underwent four consolidation cycles with Cfz (20 mg/m2 on days 1 and 2 and 27 mg/m2 on days 8, 9, 15, and 16 for the first consolidation course; 27 mg/m2 on days 1, 2, 8, 9, 15, and 16 for the subsequent two to four courses, respectively), Len (25 mg/day or LTD on days 1 to 21), and DEX (40 mg/day on days 1, 8, and 15). Subsequently, maintenance with Len monotherapy (10 mg/day or LTD from day 1–21) was continued every 4 weeks until PD.
2.6 Fluorescence in situ hybridization (FISH) analysis
Plasma cells were purified from BM samples of initial diagnosis by using anti-CD138-coated magnetic beads (EasySep Human CD138 Positive Selection Kit, STEMCELL Technologies Inc.). FISH analyses were conducted to detect (i) chromosome 17p deletion (del[17p]), (ii) IgH/FGFR3 fusion due to t(4;14)(p16;q32), (iii) IgH/MAF fusion due to t(14;16)(q32; q23), and (iv) gain of chromosome 1q (1q gain) using commercially available methods (LSI Medience Corporation).
2.7 Minimal/measurable residual disease (MRD) analysis
High-molecular-weight DNA from BM plasma cells was analyzed to identify IgH rearrangements to generate allele-specific oligonucleotide (ASO) primers. MRD analysis was conducted in cases where an ASO primer for IgH-PCR could be established. The sensitivity of our MRD test was 10−5. We assessed the MRD status post induction therapy, post ASCT, post consolidation therapy, and 1 year after starting maintenance therapy in patients who achieved a very good partial response (VGPR) or better.
2.8 Endpoints
The primary endpoint of this study was the complete response (CR) rate following consolidation therapy with KRD. Secondary endpoints included CR rate post VRD induction therapy, CR rate 100 days post ASCT, stringent CR (sCR) rate post consolidation therapy, CR rate 3 years after treatment protocol initiation, 3-year progression-free survival (PFS), 3-year overall survival (OS), time to treatment failure (TTF), rate of MRD negativity at specific timepoints, and toxicities.
The response to treatment was assessed according to the IMWG criteria14 at the appropriate time. The AEs were monitored throughout the study and categorized according to the Common Terminology Criteria for Adverse Events (version 4.0). The treatment was discontinued in cases of unacceptable toxicities, disease progression, patient consent withdrawal, or patient mortality due to any cause.
2.9 Statistics
Progression-free survival, defined as the time from study enrollment to disease progression or death from any cause, OS, defined as the time from study enrollment to death from any cause, and TTF, defined as the time from study enrollment to disease progression, death from any cause, or early treatment discontinuation for any reason, were estimated using the Kaplan–Meier method. The confidence interval (CI) was calculated using Greenwood's formula.
Referring to prior studies2, 15-18 by us and others, we set the expected CR rate and the threshold CR rate in this study at 58% and 46%, respectively. The sample size calculation for the study, using a binomial test with normal approximation (one-sided 5% significance level, 80% power), yielded 106 cases. Accounting for a 20% dropout rate after the registration, at least 133 patients were targeted for a reliable statistical analysis. All analyses were conducted using EZR software version 4.0.3 (Saitama Medical Center, Jichi Medical University).19
3 RESULTS
3.1 Patients' characteristics
Among 143 enrolled patients, two cases were excluded due to concurrent nonhematologic malignancy or initiation of radiation therapy for oncologic emergency. The baseline characteristics of the remaining 141 patients are presented in Table 1. The median age was 58 years (range, 38–65 years), with 83.7% of patients at PS 0–1. The M-protein class was IgG in 86 (61.0%), IgA in 32 (22.7%), IgD in 2 (1.4%), and Bence Jones in 21 (14.9%). Clinical stage determined by the International Scoring System was I in 60 (42.6%), II in 61 (43.3%), and III in 20 (14.2%). The median plasma cells in the BM at the beginning of the study were 27.4%. Extramedullary disease was observed in 16 (11.3%) patients. Cytogenetic analyses by FISH, feasible in 121 (85.8%) patients, revealed abnormal FGFR3 expression linked to t(4;14) in 17 cases, abnormal expression of the MAF associated with t(14;16) in 3 cases, TP53 impairment related to del 17p in 24 cases, and 1q gain in 51 cases.
| Characteristics | |
|---|---|
| Age, median (range) | 58 (36–65) |
| Sex (male/female), n [%] | 75 [53.2]/66 [46.8] |
| Performance status, n [%] | |
| 0–1 | 118 [83.7] |
| ≥2 | 23 [16.3] |
| Type of M-protein, n [%] | |
| IgGa | 86 [61.0] |
| IgAb | 32 [22.7] |
| IgD | 2 [1.4] |
| Bence Jones | 21 [14.9] |
| International staging system stage at enrollment, n [%] | |
| I | 60 [42.6] |
| II | 61 [43.3] |
| III | 20 [14.2] |
| Bone marrow plasma cells (%), (median range]) | 27.4 (0.0–96.4) |
| Extramedullary disease (yes/no), n [%] | 16 [11.3]/125 [88.7] |
| Creatinine clearance (mL/min), median (range) | 82.3 (31.0–175.5) |
| β2-microglobulin (mg/L), median (range) | 2.8 (1.4–16.2) |
| Hemoglobin (g/dL), median (range) | 10.9 (7.4–16.4) |
| FISH analysisc | (n = 121) |
| Standard-risk/high-risk, n [%] | 52 [43.0]/69 [57.0] |
| IgH-FGFR, n [%] | 17 [14.0] |
| IgH-MAF3, n [%] | 3 [2.5] |
| del 17q, n [%] | 24 [19.8] |
| 1q gain, n [%] | 51 [42.1] |
| ≥2 high-risk, n [%] | 21 [17.4] |
| IgH-FGFR +1q gain, n [%] | 10 [8.3] |
| IgH-MAF3 + 1q gain, n [%] | 1 [0.8] |
| del 17q + 1q gain, n [%] | 5 [4.1] |
| IgH-FGFR + del 17q + 1q gain, n [%] | 3 [2.5] |
| IgH-MAF3 + del 17q + 1q gain, n [%] | 2 [1.7] |
- a Included five cases with also Bence Jones protein.
- b Included three cases with also Bence Jones protein.
- c Including duplicate cases.
Overall, 69 patients (57.0% of those available for analysis) were classified as high-risk cytogenetics, and 21 patients (17.4%) possessed two or more high-risk cytogenetic abnormalities (Table 1).
3.2 Treatment status
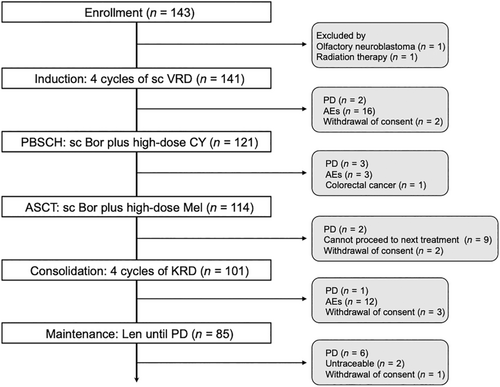
Our patients followed the treatment protocol outlined in Figure 1. A total of 121 (85%) patients completed four induction cycles, while 2 experienced PD and 18 withdrew due to AEs (n = 16) or consent withdrawal (n = 2). Of the 121 patients who completed induction, 7 did not proceed to ASCT due to PD (n = 3), AEs (n = 3), or early-stage colorectal cancer detection (n = 1). The median number of collected CD34+ cells was 6.40 × 106 (range, 2.0–85.7) cells/kg. Only seven cases required 2 days of apheresis, and no patient failed to reach the target cell count. After ASCT, all patients achieved prompt neutrophil engraftment, with a median of 11 (range, 9–21) days; however, 13 patients exited the protocol due to PD (n = 2), consent withdrawal (n = 2), or not meeting the criteria to start the next treatment (n = 9). Thus, 101 (72%) patients received four consolidation cycles. Subsequently, 85 (60%) patients, excluding 16 who dropped out due to PD (n = 1), AEs (n = 12), or consent withdrawal (n = 2) progressed to maintenance with Len; 71 (50%) patients continued over a year.
3.3 Response assessment
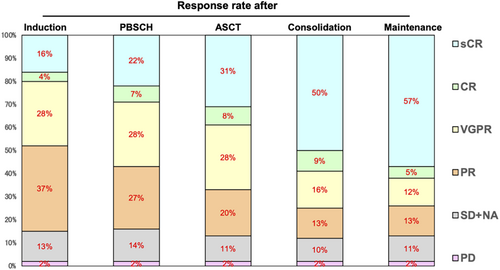
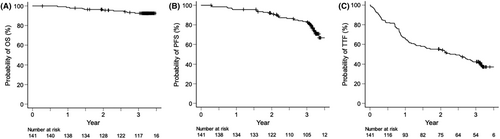
Responses among the intent-to-treat population are summarized in Figure 2. After completing the KRD consolidation therapy, 83 (58.9% [90% CI, 51.6%–65.8%]) patients achieved CR or better, meeting this study's primary endpoint. Response rates continued to improve throughout the treatment protocol. CR/sCR was observed in 28 (19.9%) patients post induction, 41 (29.1%) after PBSC collection, 56 (39.7%) following ASCT, 83 (58.9%) after consolidation, and 88 (62.4%) during maintenance. Overall, 14 patients (9.9%) experienced disease progression during the treatment period. With a median follow-up of 38 months, the 3-year estimates for OS, PFS, and TTF were 92.5% (95% CI, 86.6%–95.9%), 83.5% (95% CI, 76.0%–88.8%), and 42.3% (95% CI, 33.9%–50.4%), respectively (Figure 3).
3.4 Subgroup analysis
We further compared the outcomes among patients with varying cytogenetic risks. Patients with at least one of the following high-risk cytogenetic abnormality (del(17p), t(4;14), t(14;16), or 1q gain) showed a marginal association with lower 3-year PFS compared with those without (77.8% vs. 89.4%, p = 0.051) (Figure 4A). For each high-risk cytogenetic abnormality, del(17p) (p = 0.125), t(4;14) (p = 0.327), or 1q gain (p = 0.119) did not affect 3-year PFS. Patients with t(14;16) had significantly inferior 3-year PFS (p = 0.037), although this abnormality was detected only in three cases (data not shown). Moreover, a statistically significant association was found between ≥2 high-risk cytogenetics and PFS (p = 0.0027, as shown in Figure 4B).
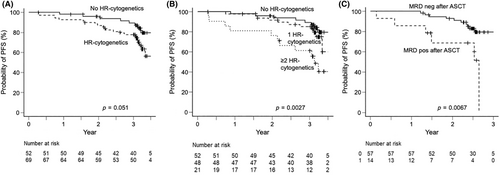
Allele-specific oligonucleotide primer for MRD detection could be established in 106 (75.2%) patients. Among them, we assessed the MRD status upon achieving a VGPR or better. Approximately 60% of patients achieved MRD negativity post induction, increasing to 80% after ASCT and 90% following consolidation/maintenance (data not shown). MRD status after ASCT (pre-consolidation) significantly correlated with improved 2-year PFS (92.3% [95% CI, 82.0%–97.2%] vs. 68.8% [95% CI, 35.7%–87.3%], p = 0.0067) (Figure 4C). Notably, among 30 patients who achieved BM-sustained MRD negativity (≥12 month) in this ASO-PCR method, all but one patient demonstrated durable response with a median 1142 (range, 956–1484) days of PFS (data not shown).
3.5 Safety profile
Regarding safety data, serious AEs of ≥ grade 3 during each treatment were collected and are listed in Table 2. The most common toxicity linked to scVRD induction was skin rash (15.6%), followed by myelosuppression (13.5%), PN (10.6%), elevated liver enzymes (9.2%), and infections (8.5%). A total of 12 patients developed severe and/or persistent PN (≥grade 2) requiring reduction or discontinuation of Bor dosing. Of them, one patient suffered from grade 3 PN and another patient suffered from persistent painful grade 2 PN and discontinued this treatment protocol. During consolidation with KRD, most toxicities were hematologic (34.7%), with only 4% of patients developing PN. Common AEs reported during Len maintenance included myelosuppression (17.6%) and elevated liver enzymes (3.5%). Overall, the treatment protocol was discontinued in 31 (22.0%) patients due to unacceptable AEs, including myelosuppression (n = 9), skin rash (n = 5), PN (n = 4), fever (n = 3), and so on. Half of the AEs that led to protocol discontinuation were during remission induction therapy (n = 16) mainly due to nonhematologic toxicity, followed by those during consolidation therapy (n = 12) mainly due to hematologic toxicity.
| VRD induction (n = 141) | KRD consolidation (n = 101) | Len maintenance (n = 85) | ||||
|---|---|---|---|---|---|---|
| Grade 3 | Grade 4 | Grade 3 | Grade 4 | Grade 3 | Grade 4 | |
| Myelosuppression, n [%] | 14 [9.9] | 5 [3.5] | 31 [30.7] | 4 [4.0] | 13 [15.3] | 2 [2.4] |
| Nonhematologic toxicities, n [%] | ||||||
| Fatigue | 0 | 0 | 1 [1.0] | 0 | 0 | 0 |
| Tumor lysis syndrome | 4 [2.8] | 1 [0.7] | 0 | 0 | 0 | 0 |
| Thrombosis | 2 [1.4] | 0 | 1 [1.0] | 0 | 0 | 0 |
| Infections | 12 [8.5] | 0 | 5 [5.0] | 0 | 2 [2.4] | 0 |
| Peripheral neuropathy | 15 [10.6] | 0 | 4 [4.0] | 0 | 1 [1.2] | 0 |
| Liver dysfunction | 11 [7.8] | 2 [1.4] | 3 [3.0] | 0 | 3 [3.5] | 0 |
| Renal dysfunction | 0 | 0 | 0 | 0 | 0 | 0 |
| Gastrointestinal tract dysfunction | 12 [8.5] | 0 | 0 | 0 | 0 | 0 |
| Respiratory tract dysfunction | 1 [0.7] | 0 | 0 | 0 | 0 | 0 |
| Skin eruption | 22 [15.6] | 0 | 0 | 0 | 0 | 0 |
| Edema | 2 [1.4] | 0 | 0 | 0 | 0 | 0 |
| Musculoskeletal disorder | 1 [0.7] | 0 | 1 [1.0] | 0 | 0 | 0 |
| Psychiatric disorder | 2 [1.4] | 0 | 1 [1.0] | 0 | 1 [1.2] | 0 |
| Others | ||||||
| Hyperkalemia | 1 [0.7] | 0 | 0 | 0 | 0 | 0 |
| Abnormal blood pressure | 2 [1.4] | 0 | 0 | 0 | 0 | 0 |
| Dizziness | 0 | 0 | 1 [1.0] | 0 | 0 | 0 |
| Choledocholithiasis | 0 | 0 | 1 [1.0] | 0 | 0 | 0 |
| Vasculitis | 0 | 0 | 1 [1.0] | 0 | 0 | 0 |
- Abbreviations: KRD, carfilzomib, lenalidomide, dexamethasone; Len, lenalidomide; VRD bortezomib, lenalidomide, dexamethasone.
4 DISCUSSION
This Japanese nationwide prospective study (JSCT MM16) assessed the efficacy of combined therapy with triplet regimens and ASCT in a large cohort of NDMM patients (n = 143). Our study showed an encouraging outcome, with responses consistently deepening throughout the program. Notably, the CR/sCR rate upgraded from 39.7% (n = 56) to 58.9% (n = 83) at before and after KRD consolidation therapy in the intent-to-treat population.
While direct comparisons are not feasible, the response rate in this study was higher than that of the control arm (VRD induction/consolidation) in the GRIFFIN trial20 with 42% of CR/sCR at the end of consolidation, or compared with our previous trials in the era of doublet-based regimens (JSCT MM1015 or JSCT MM1216). Moreover, it was consistent with previous reports where KRD was used as both induction and consolidation therapy. A response rate of at least CR was observed in 64% of patients in the IFM KRd trial (n = 46),11 54% in the KRD plus ASCT cohort (n = 158) of the FORTE trial,12 and 65% in another multicenter trial13 with 76 NDMM cases. Intensified consolidation with the KRD regimen may lead to a higher rate of deeper response.
Several groups have reported that achieving MRD negativity is associated with improved PFS and OS.3, 21, 22 With the current protocol of four cycles of scVRD, followed by transplantation and four cycles of KRD, 80% of patients reached undetectable MRD at 90 days post transplant, confirming a positive impact on PFS (Figure 4C). Moreover, 51 out of 56 (91%) and 32 out of 35 (91%) patients assessed at the end of consolidation and after 1 year of maintenance initiation achieved MRD negativity (data not shown). Expectedly, all but a single case with BM-sustained MRD negativity demonstrated long-term PFS, indicating the efficacy of this protocol. However, in this study, MRD status in some participants was assessed by ASO-PCR, but not by gold-standard multicolor flowcytometry or deep sequencing. We should assess the MRD status in all patients using more sensitive methods in future studies.
Overall, the clinical outcomes of the current study are promising. With a median follow-up of 38 months, the estimated 3-year PFS was 84%, and OS was 93%. The outcomes were comparable to those in the aforementioned previous reports11-13, 20, 23 as well as a more recent DETERMINATION trial.24 The median age and prevalence of high-risk cytogenetics of patients enrolled in the DETERMINATION trial were similar to our patients, and the prognosis of patients randomized to the ASCT arm in the trial was comparable to our patients, with 3-year PFS and 3-year OS of approximately 80% and 90%, respectively. However, there is still room for improvement. First, the treatment did not achieve adequate PFS in patients with two or more high-risk cytogenetic abnormalities (Figure 4B). These patients are identified as having ultrahigh-risk MM with an extremely poor survival prognosis. Previous analysis by CIBMTR25 indicated 3-year PFS and OS rates of 27% and 67%, respectively. Our group and another group26 noted a slightly improved 3-year PFS in this very-high-risk subgroup (61% in our study and 48% in a retrospective study at MD Anderson Cancer Center), likely due to more patients receiving triplet-based induction and post-transplant consolidation/maintenance.
Second, we noted that not a few patients discontinued the protocol due to unacceptable AEs, not disease progression, yet overall, the protocol was well tolerated with no treatment-related mortality. The incidence of AEs leading to study discontinuation in our study was slightly higher as compared with that in the DETERMINATION trial; however, we think some of the study discontinuations due to investigator judgment/patients' decision in the DETERMINATION trial may include those due to nonserious but problematic AEs. In our study, among 16 unacceptable AEs developed during induction with scVRD, 14 (87.5%) were nonhematologic toxicities, including skin rash, PN, and fever. On the other hand, most AEs leading to study discontinuation during consolidation with KRD were hematological (7/12, 58.3%). With regard to concerning cardiovascular events associated with KRD, no patients experienced grade 3 or higher. Nonetheless, we should closely monitor cardiovascular function and tightly control blood pressure to prevent this well-established serious AE.11-13, 27, 28 Future investigation is needed to see whether anti-CD38 antibody-combined treatment29 and/or novel agents (e.g., BCMA-targeted bispecific antibody30, 31 and BCMA-directed CAR-T cell32, 33) can address the two issues raised. With regard to anti-CD38 antibody, encouraging data have been demonstrated in multiple clinical trials among transplant-eligible NDMM patients: Daratumumab-combined regimen before and after ASCT could provide deeper and more sustained response.20, 23, 34-36 Thus, data accumulation also in Japanese patients is warranted.
Our study has several limitations. Since we conducted this clinical trial as a single-arm phase II study, we cannot ascertain the true benefit of intensified consolidation with KRD without comparing it with a control arm that continued scVRD therapy. In the current study, the median age of patients enrolled was 58 years, and more than 80% of patients maintained fair general condition with PS 0–1. It may suggest the possibility of bias in patient selection; thus, attention should be paid when interpreting the obtained results. Although this study covers a larger Japanese NDMM patient cohort than previous reports, the patient subset remains relatively small, potentially explaining the limited statistical power in subgroup analyses (e.g., PFS in high-risk vs. standard-risk cytogenetics, PFS by MRD status post consolidation, etc.).
In summary, our sequential treatment program comprising scVRD induction, ASCT, KRD consolidation, and Len maintenance until disease progression is feasible and highly effective for Japanese patients with NDMM. The depth of the response improved with each phase. Future studies should assess whether the innovative therapeutic strategy incorporating the anti-CD38 antibody increases protocol completion rates and thereby enhances survival outcomes, particularly in patients with high-risk or ultrahigh-risk cytogenetics.
AUTHOR CONTRIBUTIONS
Yasuo Mori: Data curation; formal analysis; investigation; project administration; resources; validation; visualization; writing – original draft; writing – review and editing. Jun Takizawa: Data curation; formal analysis; investigation; resources; supervision; validation. Yuna Katsuoka: Data curation; formal analysis; investigation; resources; supervision; validation. Naoki Takezako: Data curation; formal analysis; investigation; resources; supervision; validation. Koji Nagafuji: Data curation; formal analysis; investigation; resources; supervision; validation. Hiroshi Handa: Data curation; formal analysis; investigation; resources; supervision; validation. Junya Kuroda: Data curation; formal analysis; investigation; resources; supervision; validation. Kazutaka Sunami: Data curation; formal analysis; investigation; resources; supervision; validation. Tomohiko Kamimura: Data curation; formal analysis; investigation; resources; supervision; validation. Ryosuke Ogawa: Data curation; formal analysis; investigation; resources; supervision; validation. Yoshikane Kikushige: Formal analysis; investigation; validation; visualization; writing – original draft; writing – review and editing. Mine Harada: Conceptualization; investigation; project administration; supervision; validation. Koichi Akashi: Conceptualization; data curation; formal analysis; funding acquisition; investigation; project administration; resources; supervision; validation. Toshihiro Miyamoto: Conceptualization; data curation; formal analysis; funding acquisition; investigation; project administration; resources; supervision; validation; visualization; writing – original draft; writing – review and editing.
ACKNOWLEDGMENTS
We thank the medical and nursing staff who cared for the patients and provided patient information. We are grateful to professor Koji Yonemoto (Ryukyu University) for supporting the data analysis.
FUNDING INFORMATION
ONO PHARMACEUTICAL CO. LTD. and Bristol Myers Squibb K.K. funded this study.
CONFLICT OF INTEREST STATEMENT
Yasuo Mori has received honoraria/fees from MSD. Hiroshi Handa has received honoraria/fees from MSD Janssen, Takeda, and Bristol Myers Squibb and research funding from Pfizer and has accepted a researcher from Kyowa Kirin. Junya Kuroda has received honoraria/fees from Ono Pharmaceutical, Sanofi, Bristol Myers Squibb, Janssen Pharmaceutical, Asahikasei Pharma, Pfizer, and Chugai Pharmaceutical and research funding from Kyowa Kirin, Eisai, Chugai Pharmaceutical, Daiichi Sankyo, Ono Pharmaceutical, Sumitomo Pharma, Shionogi, and Bristol Myers Squibb. Kazutaka Sunami has received honoraria/fees from Ono Pharmaceutical, Janssen, Sanofi, and Bristol Myers Squibb and research funding from Ono, MSD, Abbvie, Takeda, Sanofi, Janssen, GSK, Chugai, Novartis, Otsuka, Bristol Myers Squibb, Pfizer, Kyowa-Kirin, Parexel, and Astellas-Amgen. Tomohiko Kamimura has received honoraria/fees from Janssen, Ono, and Abbvie. Yoshikane Kikushige has received honoraria/fees from Astellas and Kyowa Kirin. Koichi Akashi is an Editorial Board member of Cancer Science and has received honoraria/fees from Asahikasei, Astellas, Astrazeneca, Abbvie, Kyowa Kirin, Chugai, Bristol Myers Squibb, and Janssen; research funding from Abbvie and Kyowa Kirin; and scholarship endowments/academic research funding from Otsuka, Nippon Shinyaku, TAIHO, Asahikasei, Kyowa Kirin, CHUGAI, Sumitomo, AbbVie, Eisai, and Takeda. Toshihiro Miyamoto has received honoraria/fees from Takeda, Otsuka, MSD, Astellas, Janssen, Abbvie, and Kyowa Kirin. Other authors do not have a conflict of interest.
ETHICS STATEMENT
Approval of the research protocol by an Institutional Review Board: Kyushu University Hospital (approval number; 20181005).
Informed consent: Informed consent was obtained from all the subjects.
Registry and the Registration No. of the study/trial: UMIN registration number: 000024165 (JSCT-MM16).
Animal Studies: N/A.




