Mitochondrial fission enhances IL-6-induced metastatic potential in ovarian cancer via ERK1/2 activation
Abstract
Ovarian cancer is a lethal gynecologic cancer mostly diagnosed in an advanced stage with an accumulation of ascites. Interleukin-6 (IL-6), a pro-inflammatory cytokine is highly elevated in malignant ascites and plays a pleiotropic role in cancer progression. Mitochondria are dynamic organelles that undergo fission and fusion in response to external stimuli and dysregulation in their dynamics has been implicated in cancer progression and metastasis. Here, we investigate the effect of IL-6 on mitochondrial dynamics in ovarian cancer cells (OVCs) and its impact on metastatic potential. Treatment with IL-6 on ovarian cancer cell lines (SKOV3 and PA-1) led to an elevation in the metastatic potential of OVCs. Interestingly, a positive association was observed between dynamin-related protein 1 (Drp1), a regulator of mitochondrial fission, and IL-6R in metastatic ovarian cancer tissues. Additionally, IL-6 treatment on OVCs was linked to the activation of Drp1, with a notable increase in the ratio of the inhibitory form p-Drp1(S637) to the active form p-Drp1(S616), indicating enhanced mitochondrial fission. Moreover, IL-6 treatment triggered the activation of ERK1/2, and inhibiting ERK1/2 mitigated IL-6-induced mitochondrial fission. Suppressing mitochondrial fission through siRNA transfection and a pharmacological inhibitor reduced the IL-6-induced migration and invasion of OVCs. This was further supported by 3D invasion assays using patient-derived spheroids. Altogether, our study suggests the role of mitochondrial fission in the metastatic potential of OVCs induced by IL-6. The inhibition of mitochondrial fission could be a potential therapeutic approach to suppress the metastasis of ovarian cancer.
Abbreviations
-
- CAMK1
-
- calcium/calmodulin-dependent protein kinase 1
-
- CDK1
-
- cyclin-dependent kinase 1
-
- Drp1
-
- dynamin-related protein 1
-
- ERK1/2
-
- extracellular signal-regulated kinase 1/2
-
- GEO
-
- gene expression omnibus
-
- GSEA
-
- gene set enrichment analysis
-
- IHC
-
- immunohistochemistry
-
- IL-6
-
- interleukin-6
-
- IL-6R
-
- interleukin-6 receptor
-
- Mfn1
-
- Mitofusin 1
-
- NOSE
-
- normal ovary surface epithelial cell
-
- OVC
-
- ovarian cancer cell
-
- p-Drp1(S616)
-
- phospho-Drp1(Ser616)
-
- p-Drp1(S637)
-
- phospho-Drp1(Ser637)
-
- ROCK1
-
- Rho-associated coiled-coil kinase 1
-
- siRNA
-
- small interfering RNA
-
- TCGA
-
- The Cancer Genome Atlas
-
- TMA
-
- tissue microarray
1 INTRODUCTION
Ovarian cancer is the fifth most common cause of cancer death in females and the leading cause of death among gynecological cancer in the United States.1 Patients with ovarian cancer show no palpable symptoms making it difficult to be detected at early stages. About 80% of patients with ovarian cancer are diagnosed at an advanced stage that the cancer cells have already formed a niche at secondary sites distant from the origin of the cancer, leading to high mortality of ovarian cancer.2 Despite patients receive cytoreductive surgery and chemotherapy, 70% of the advanced-stage patients eventually experience relapse resulting in a 5-year survival rate below 45%.3, 4 Unlike other solid tumors, OVCs disseminate mainly through the transcoelomic routes, gaining access to various secondary organs in the peritoneal cavity via ascites.5 Ascites is the most common finding in patients with advanced and recurrent ovarian cancer.6, 7 It has been shown that while only 17% of early-stage ovarian cancer produced malignant ascites, 89% of advanced-stage patients produced malignant ascites, demonstrating that ascites may work as an indicator of bad prognosis in ovarian cancer patients.8, 9 Ascites not only decrease the quality of life of ovarian cancer patients but also play a crucial role in the process of metastasis of ovarian cancer constituting the tumor microenvironment of ovarian cancer. The malignant ascites function as a mediator of metastasis and also the reservoir of growth factors and cytokines including IL-6, providing a tumor-promoting pro-inflammatory microenvironment for cancer cells.10, 11
Interleukin-6 (IL-6) is one of the major pro-inflammatory cytokines along with tumor necrosis factor-α (TNF-α) and IL-1β.12, 13 IL-6 can be secreted from immune cells, stromal cells and tumor cells in the tumor microenvironment promoting cancer progression.14, 15 IL-6 activates downstream pathways including JAK/STAT3, PI3K/AKT and MAPK/ERK pathways which affect cancer hallmarks such as proliferation, angiogenesis, metastasis and inflammation.16-20 Many cancer studies have shown that IL-6 is enriched in the microenvironment of cancers such as gastric, lung and ovarian cancer.14, 21-24 Consistently, in our previous study, excessive IL-6 concentration was demonstrated as a critical feature of ovarian cancer ascites that distinguished from benign ascites.23 High level of IL-6 leads to inflammation and persistent activation of inflammatory signals in the tumor microenvironment may contribute to cancer development, progression and metastasis.25, 26
Mitochondria are double-membrane-bound organelles that generate adenosine triphosphate (ATP), the energy source of cells. The morphology of mitochondria is highly dynamic and continuously undergoes fission and fusion processes.27 The alteration of mitochondrial dynamics occurs in response to various cellular stimuli and regulates cells' metabolic homeostasis and energy distributions.28 Defective mitochondrial fission/fusion balance has been reported to be involved in several diseases including metabolic and age-related diseases, neurodegenerative diseases, muscle atrophy and cancer.28, 29 Recently, many cancer studies have shown that the expression of mitochondrial dynamics-related genes promotes mitochondria fission.30-32 This indicates a potential role of mitochondrial dynamics in tumorigenesis. Dynamin-related protein 1 (Drp1), which is a dynamin-like GTPase encoded by the DNM1L gene, is regarded as a major component of the mitochondrial fission machinery33, 34 and is suggested to be involved in the metastatic processes of cancer by mediating the subcellular distribution of mitochondria.35, 36 Drp1 activity can be modulated by the serine phosphorylation site. Ser616 phosphorylation activates Drp1 activity,37 whereas Ser637 phosphorylation decreases Drp1 activity.38 Upon Drp1 activation, Drp1 translocated from the cytoplasm and oligomerized into a ring structure around the mitochondrial outer membrane and mediates fragmentation of mitochondria.39, 40 Mitochondrial dynamics can be differentially regulated in response to multiple stimuli in the tumor microenvironment, such as reactive oxygen species (ROS), starvation, chemotherapy, hypoxia, and inflammation, resulting in modulation of oncogenic signaling.41-45 While the pro-metastatic role of IL-6 is increasingly evident, the effect of IL-6-enriched conditions on mitochondrial dynamics has not been explored in cancer.
In this study, we investigated the effects of IL-6 on the mitochondrial dynamics of OVCs. IL-6 treatment can induce mitochondrial fission resulting in enhanced metastatic potential of OVCs by Drp1 activation rather than upregulation. As the tumor microenvironment of ovarian cancer contains high levels of IL-6 in ascites, ovarian cancer is much more vulnerable to IL-6-induced metastasis than other types of cancer. Regulation of IL-6-induced mitochondrial fission could be a promising therapeutic target for the metastasis of OVCs.
2 MATERIALS AND METHODS
2.1 Ovarian cancer cell culture
Human ovarian cancer cell lines SKOV3 and PA-1 were obtained from American Type Culture Collection (Rockville, MD) and cultured in RPMI1640 (WELGENE) supplemented with 10% fetal bovine serum (Gibco) and 100 U/mL penicillin and 100 μg/mL streptomycin (Invitrogen). Both cell lines were seeded in 100 mm culture dishes (SPL) and maintained at 37°C in a humidified atmosphere of 5% CO2 incubator.
2.2 Wound healing assay
The migration activity was measured using wound healing assay. Cells were seeded in a 6-well plate and wound was given when cells reached 70%–80% confluence. Using a 1 mm Scar™ Scratcher (SPL), straight line of artificial wound was created on the cell monolayer and PBS washing was followed. The wound healing was observed and photographed after 24 h. Cells were examined under a microscope (Olympus). Images were taken with GalaxyS8 (Samsung) and the remaining wound size was analyzed using the ImageJ software.
2.3 Invasion assay
The invasive capacity was assessed by Boyden chamber-based transwell assay. 8.0 μm pore permeable membrane supports for 12-well plate (Falcon) were pre-coated with serum-free medium diluted growth factor reduced Matrigel (30 μg/support; Corning). After being dried at 37°C, cells were resuspended in serum-free medium and seeded to the upper chamber support (30,000 cells/200 μL), and 800 μL of complete medium was added to the bottom of the well. After 24 h 37°C incubation, cells were washed with PBS three times and fixed with 4% formaldehyde for 1 h at room temperature (RT) and stained with 0.5% crystal violet. Continue to PBS washing, Matrigel with non-invaded cells was removed with a cotton swab. Invaded cells were photographed and counted using ImageJ software.
2.4 Immunoblotting analysis
Both cell lines were incubated in 60 mm culture dishes and harvested when the cells reach about 80% confluence. Harvested cells were lysed with protein extraction buffer (0.5 M NaCl, 0.5 M Tris–HCl, 50 mM EDTA, 50 mM EGTA, 10% triton X-100, 1 mg sodium deoxycholate, 1 mM Na3VO4, 1 mM phenylmethylsulphonyl fluoride, EDTA-free protease inhibitor, and distilled water). Equal amounts of proteins were loaded to 6%–12% SDS-PAGE and transferred onto a nitrocellulose membrane. After 2 h blocking with 5% skim milk in TBS-T in RT, membranes were 4°C overnight incubated with specific primary antibodies. Membranes were then washed with TBS-T and incubated with horseradish peroxidase-conjugated secondary antibodies diluted in 5% skim milk TBS-T (1:5000) for 2 h at RT. After TBS-T washing, WESTSAVE up (AbFrontier, Korea) was used as a detection reagent and ChemiDoc MP (Bio-Rad) detected chemiluminescence signals. Protein bands were quantified using ImageLab software and ImageJ. Primary and secondary antibodies used in this study are listed followed; Drp1 (Cell Signaling Technology, Cat# 8570, 1:1000), p-Drp1(S616) (Cell Signaling Technology, Cat# 3455, 1:1000), p-Drp1(S637) (Cell Signaling Technology, Cat#6319, 1:1000), Mfn1 (Santa Cruz Biotechnology, sc-50330, 1:1000) and α-tubulin (Santa Cruz Biotechnology, sc-8035, 1:5000).
2.5 MTT assay
Cell viability of ovarian cancer cell lines was measured by MTT assay. SKOV3 (2500 cells) and PA-1 (2300 cells) were seeded into each well of a 96-well plate with 100 μL medium and incubated. After 48 h from seeding, the medium was removed, and upto 20 μL of Mdivi-1 (Merck-Millipore, 338967-87-6) or upto 100 nM PD325901 (MedChemExpress, HY-10254) diluted culture medium was pre-added. After 1 h, 50 μL of IL-6 (Cell Signal Technology, Cat# 8904SC) was added to each well and incubated for 24 h. To measure cell viability, 2 mg/mL of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) (ThermoFisher) dissolved in PBS was added and incubated at 37°C for 3 h and then removed. Subsequently, 100 μL of dimethyl sulfoxide (DMSO) was added to a 96-well plate and incubated on a shaker for 30 min at RT. The absorbance of formazan was recorded at 540 mm using a spectrophotometer (Labsystem, Helsinki, Finland).
2.6 Immunofluorescent staining and confocal microscopy
Immunofluorescent staining was performed based on the previous study (Han et al., 2019). In brief, 1–2 × 104 cells were seeded on Nunc™ Lab-Tek™ II four-well chamber slide™ w/ removable wells (Thermo Scientific). Cells were washed with PBS and stained with 250 nM MitoSpy™ Orange CMTM ROS dye (BioLegend, San Diego, CA) for 20 min at 37°C according to the manufacturing protocols. Then cells were fixed with 4% paraformaldehyde (PFA) and permeabilized with 0.1% Triton X-100. After permeabilization, cells were stained with 1 μg/mL DAPI (Thermo Scientific), then mounted with ProLong™ Glass Antifade Mountant (Thermo Scientific). Prepared samples were visualized with a 63× oil immersion objective lens of an LSM800 confocal fluorescence microscope (Carl Zeiss, Oberkochen, Germany).
2.7 Analysis of mitochondrial dynamics
Mitochondrial length from at least 50 cells per group was analyzed. Cancer cells were divided into three groups (fission, intermediate and fusion) depending on the mitochondrial dynamics state and the proportion of the three groups was obtained. The state of mitochondrial dynamics was decided as follows: fission (<5 μm), intermediate (5–10 μm), and fusion (>10 μm). The length and dynamics state of mitochondria were analyzed by the three individuals.
2.8 IHC staining and assessment
At Seoul National University Hospital, specimens of patients who have agreed to donate human materials are stored in the pathology department after surgery. We were provided with tissue microarray (TMA) blocks with personal information removed through hospital regulations. (IRB: 1807-037-956). All of the cases had available formalin-fixed paraffin-embedded (FFPE) specimens of primary and metastatic tumors and adjacent normal tissue which were obtained from biopsy. 4 μm thick tissue section from FFPE tissue TMA blocks were used for Drp1, p-Drp1(s616) and p-Drp1(s637). TMA sections were stained with following antibodies and dilution ratios: anti-Drp1 (1:50, DF7037 Affinity Bioscience); anti-p-Drp1(s616) (1:50, AF8470, Affinity Bioscience); anti-p-Drp1(s637) (1:50, DF2980, Affinity Bioscience); anti-IL-6R (1:500, sc-373708, Santa Cruz). The intensity of positively stained cells was scored as follows: 0 (negative), 1 (weekly positive), 2 (moderately positive), and 3 (strongly positive).
2.9 3D spheroid culture and 3D invasion assay
Primary cancer cells (A037 and A038) from malignant ascites were used for tumor spheroid formation and 3D invasion assay. Cells were seeded in the Ultra-Low Attachment 96 well-plate (Corning, USA) and cultured for three days to form spheroids. Three days later, Matrigel (Corning) was added to the 3-day-old spheroids for the 3D invasion assay. Images were taken after 90 h and analyzed using ImageJ.
2.10 RNA extraction and quantitative real-time PCR
Total RNA was extracted from the SKOV3 cells and PA-1 cells using RNAiso Plus reagent (Takara, Tokyo, Japan). Then, 1 μg of RNA was reverse transcribed into cDNA with the PrimeScript Reverse Transcriptase (Takara, Tokyo, Japan). Quantitative real-time PCR was performed using a QuantiSpeed SYBR Kit (Phile Korea, South Korea), according to the manufacturer's instructions. Relative expression was evaluated by the 2−ΔΔCt method with GAPDH as an endogenous control. Primer sequences were listed as follows: DNM1L forward 5′-GAT GCC ATA GTT GAA GTG GTG AC-3′, reverse 5′-CCA CAA GCA TCA GCA AAG TCT GG-3′, CDK1 forward 5′-CGC GGA TCC ATG GAA GAT TAT ACC AAA A-3′, reverse 5′-CCG GAA TTC CTA CAT CTT CTT AAT CTG AT-3′, CAMK1 forward 5′-GGA TTG CTG GTC CAT AGG TGT C-3′, reverse 5′-CAG AGA TGT CGT CCC AGT AAG G-3′, ROCK1 forward 5′-GGT GGT CGG TTG GGG TAT TTT-3′, reverse 5′-CGC CCT AAC CTC ACT TCC C-3′, and GAPDH forward 5′-GAG TCA ACG GAT TTG GTC GT-3′, reverse 5′-TTG ATT TTG GAG GGA TCT CG-3′.
2.11 Seahorse assay
PA-1 cells were seeded onto the Seahorse XF96 cell culture microplate at a density of 2.5 × 104 cells per well and incubated overnight. On the day of the assay, cells were washed with Seahorse XF Base Medium (Agilent Technologies) supplemented with 1 mM pyruvate, 2 mM glutamine, and 10 mM glucose, and then incubated at 37°C for 1 h in a CO2-free incubator. The mitochondrial respiration rate was measured using the Seahorse XF Cell Mito Stress Test Kit (Agilent Technologies) according to the manufacturer's instructions. The assay measures the oxygen consumption rate (OCR) in real-time, which is an indicator of mitochondrial respiration. The mitochondrial respiration rate was calculated by subtracting the OCR in the presence of rotenone and antimycin A (which inhibits mitochondrial respiration) from the basal OCR.
2.12 Quantification and statistical analysis
Data were presented as mean ± SEM of triplicate experiments. One-way ANOVA and, when appropriate, Student's t-test were used for statistical analyses. Significant difference among experimental groups was analyzed by Bonferroni's posthoc test. All analyses were conducted using GraphPad Prism9 software. p values of <0.05 were considered statistically significant.
3 RESULTS
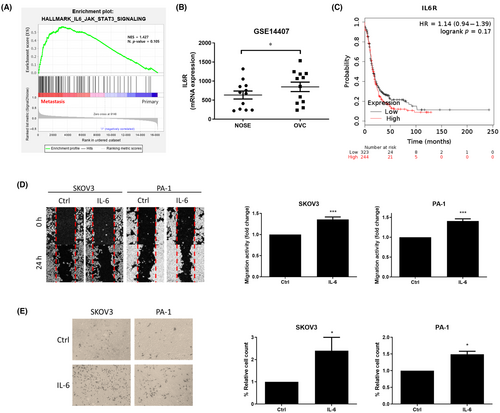
3.1 IL-6 promotes the migration and invasion of OVCs
To investigate the core relationship between IL-6 and metastasis, the gene set enrichment analysis (GSEA) on the gene expression omnibus (GEO) database was applied. Using the GSE30587 dataset, we found that the IL-6 signaling pathway-related gene set was enriched in the metastatic tissues compared to the matched primary tissues (Figure 1A). Additionally, we compared the transcript levels of IL-6R using the GSE14407 dataset. IL-6R level was significantly elevated in the ovarian cancer tissues than the normal ovarian epithelial tissues (Figure 1B). As a validation, we performed immunohistochemistry (IHC) on tissue microarray (TMA) containing 221 cases of ovarian cancer tissue along with adjacent normal tissue for IL-6R protein (Figure S1A). It was shown that IL-6R was more highly expressed in primary and metastatic cancer tissues than the normal tissue (Figure S1B) corresponding to the previous GEO dataset. Ovarian cancer patients from the The Cancer Genome Atlas (TCGA) database were divided into two groups according to the transcript level of IL-6 receptor (IL-6R) and the progression-free survival (PFS) of each group was compared using Kaplan–Meier Plotter (https://kmplot.com/analysis/).46 The group with higher IL-6R levels exhibited a tendency of poorer PFS compared to the low IL-6R group with a significant difference of p = 0.17 (Figure 1C). Next, we assessed the effect of IL-6 on the metastatic potential of OVCs. Using in vitro migration (wound healing) and invasion (Matrigel-coated transwell) assay, we found IL-6 (10 ng/mL) elevated migration (Figure 1D) and invasion (Figure 1E) capacity in both SKOV3 and PA-1 ovarian cancer cell lines.
3.2 Drp1 expression shows a positive correlation with IL-6R in OVCs
Based on the prognosis of patients with ovarian cancer, the GSE17296 dataset was divided into a bad prognosis group (n = 29) that relapsed within 12 months and a good prognosis group (n = 16) that did not recur until 36 months. Using GSEA, we found ‘regulation of mitochondrial fission’ gene set was significantly enriched in the bad prognosis group compared with the good prognosis group (Figure 2A). We further analyzed whether an increase in metastatic potential due to IL-6 is related to the change of mitochondrial dynamics in ovarian cancer. GEO data confirmed the association between IL-6R and Drp1 in ovarian cancer patient-derived tissue samples. Pearson's correlation analyses based on the GSE73168 dataset showed a positive correlation between IL-6R and Drp1 mRNA expression in ovarian cancer patients (Figure 2B). In the same manner, Pearson's correlation analysis using the GSE143897 dataset elucidated a significant positive correlation between IL-6R and Drp1 (Figure 2C).
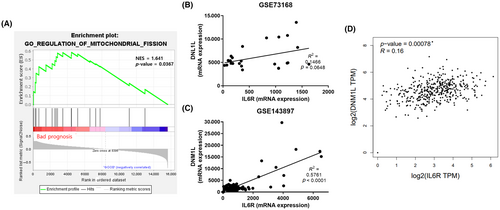
The results from the TCGA data analysis also showed that IL-6R is correlated with Drp1 (DNM1L). The results were calculated using the online web service GEPIA (http://gepia.cancer-pku.cn/index.html)47 (Figure 2D). These bioinformatics data suggest the potential relationship of Il-6 and mitochondrial fission in the prognosis of ovarian cancer.
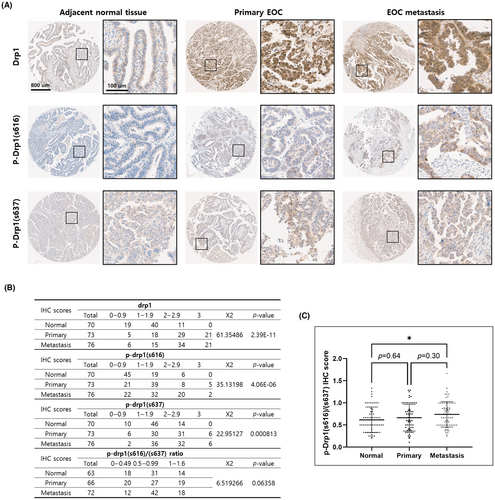
3.3 Drp1 and p-Drp1 protein expressions in ovarian cancer
To validate transcriptomic findings at the protein level, we performed quantitative immunohistochemistry (IHC) on tissue microarray (TMA) containing 221 cases of ovarian cancer tissue along with adjacent normal tissue for the following genes: Drp1, p-Drp1(s616), and p-Drp1(s637). Using the quantitative assessment of antibody staining in these histological samples, the overexpression of Drp1, p-Drp1(s616), and p-Drp1(s637) in TMA sections was scored. It was shown that Drp1, p-Drp1(s616), and p-Drp1(s637) were more highly expressed in cancer tissues than the normal tissue (Figure 3A). The IHC results were statistically analyzed using the Chi-square test and significant differences were identified in the expression of Drp1 forms in each tissue (Figure 3B). In particular, the ratio value between Drp1s616/Drp1s637, which means the activity of Drp1, was highest in metastatic tissue, followed by primary and normal tissue (Figure 3C).
3.4 IL-6 induces Drp1-mediated mitochondrial fission in OVCs
To confirm whether IL-6 induces fission of mitochondria in OVCs, SKOV3 and PA-1 cells were treated with 10 ng/mL of recombinant human IL-6 for different durations (3, 6 and 24 h). Mitochondrial dynamics was visualized by immunofluorescence assay using MitoSpy™ Orange and DAPI staining. Representative confocal microscopic images showed modulated mitochondrial dynamics on IL-6 (Figure 4A). The mitochondrial length in cancer cells was analyzed into three groups (fusion, intermediated and fission) and displayed as a percent stacked bar plot. Interestingly, the percentage of cells with elongated mitochondria decreased while those with mitochondria fission increased upon treatment with IL-6 in a time-dependent manner (Figure 4B).
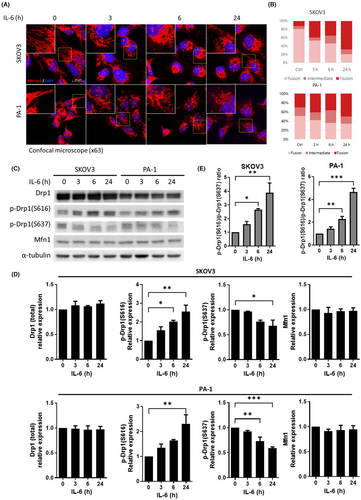
We next investigated the molecular mechanisms that regulate mitochondrial fusion/fission states when exposed to IL-6. As previous studies have emphasized the importance of the expression ratio between the two key proteins (Drp1 and Mfn1) in the fission and fusion mechanisms,48-50 protein expression of Drp1, p-Drp1(S616), p-Drp1(S637) and Mfn1 were assessed by western blot (Figure 4C). Upon IL-6 treatment, both mRNA and protein expression of the Drp1 (total form) did not exhibit significant changes in both cell lines (Figure S2 and Figure 4C). The protein expression levels of p-Drp1(S616), an active form of Drp1, increased on IL-6 treatment while the expression levels of p-Drp1(S637), an inhibitory form of Drp1, mildly decreased and. Mfn1, a mitochondrial fusion protein, did not exhibit changes in the expression level in both cell lines (Figure 4D). Overall, the ratio of active form and inactive form of Drp1 was confirmed. In both cell lines, the ratio of p-Drp1(S616)/p-Drp1(S637) was significantly increased in a time-dependent manner after IL-6 treatment. (Figure 4E).
3.5 ERK1/2 inhibition restores IL-6-induced mitochondrial fission in OVCs
As Drp1 activity is regulated by phosphorylation, we conducted a screening of select genes associated with Drp1 phosphorylation, including Cyclin-dependent kinase 1 (Cdk1),51 Calcium/Calmodulin Dependent Protein Kinase I (Camk1),52 Rho Associated Coiled-Coil Containing Protein Kinase 1 (Rock1),53 and extracellular signal-regulated kinase 1/2 (ERK1/2).30 Among these genes, Cdk1, Camk1, and Rock1, which their expressions are directly linked to DRP1 phosphorylation, were screened using qRT-PCR. Cdk1 transcription level did not exhibit significant changes upon IL-6 treatment, Camk1 transcription level displayed a decrease in SKOV3 whereas increased levels in PA-1 cells. Additionally, Rock1 exhibited a significant decrease in SKOV3 cells but no significant alteration in PA-1 cells (Figure S3).
Of particular interest is ERK1/2, a well-known protein regulated by the IL-6 signaling pathway.54 ERK1/2 has been reported to induce p-Drp1(S616) and consequently promote mitochondrial fission.30, 55 To validate this pathway, we conducted western blot analysis to assess ERK1/2 activation through phosphorylation. Remarkably, both SKOV3 and PA-1 cell lines exhibited increased p-ERK1/2 levels following IL-6 treatment (Figure 5A). To directly investigate the role of ERK1/2 on Drp1 activity, we employed a MEK inhibitor (PD325901) to block upstream ERK1/2 activation in OVCs. Upon IL-6 treatment with PD325901, IL-6-induced ERK1/2 and Drp1 activations were restored (Figure 5B). To determine whether ERK1/2 activated by IL-6 is involved in mitochondrial fission, we treated cells with IL-6 and PD325901 and examined mitochondrial morphology using confocal microscopy (Figure 5C). Subsequent analysis of mitochondrial morphology demonstrated that the IL-6-induced fission was indeed suppressed by pre-treatment with PD325901 (Figure 5D).
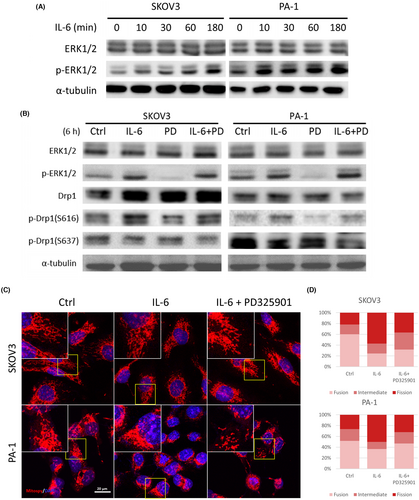
3.6 Inhibition of mitochondrial fission impairs IL-6-induced migration and invasion of OVCs
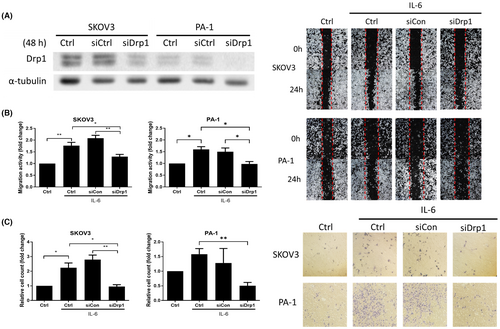
To investigate the link between mitochondrial fission and IL-6-induced migration and invasion, we inhibited mitochondrial fission with small interfering RNA (siRNA) transfection. Drp1 expression in SKOV3 and PA-1 cell lines was silenced through Drp1 targeting siRNA transfection. The Drp1 knockdown efficiency with siRNA was confirmed by western blot (Figure 6A). Subsequently, we examined whether the metastatic potential caused by IL-6 was changed in Drp1 knockdown OVCs. On IL-6 treatment, invasion and migration increased in non-transfected cells or siCtrl cells transfected with scrambled RNA but not in Drp1 knockdown cells (Figure 6B,C).
We also suppressed mitochondrial fission in OVCs with a pharmacologic inhibitor. Mdivi-1 is an inhibitor of mitochondrial fission that reduces the fusion of mitochondria by inhibiting the self-assembly of Drp1. The morphological change of SKOV3 and PA-1 on IL-6 treatment or IL-6 with Mdivi-1 (10 μM) pre-treatment was visualized by immunofluorescent staining (Figure 7A). On Mdivi-1 pre-treated IL-6 treatment, increased cell percentage with mitochondria fission was diminished in both cell lines (Figure 7B). We have also localized Drp1 in PA-1 cells using immunofluorescence assay on IL-6 treatment or IL-6 with Mdivi-1 pre-treatment. The results showed that the cytoplasmic Drp1 expression in PA-1 cells was increased upon IL-6 treatment. The Drp1 expression at the end of the cells and overall fluorescence intensity of Drp1 were further increased and restored with Mdivi-1 pre-treatment (Figure S4). The population counting was performed in the same manner as in Figure 2B. We next identified the metastatic potential of OVCs on Mdivi-1 pre-treatment. Mdivi-1 pre-treatment repressed IL-6-induced migration (Figure 7C) and invasion (Figure 7D) increase in SKOV3 and PA-1. To assess the association with epithelial-mesenchymal transition (EMT), we evaluated the protein expression involved with an epithelial marker (E-cadherin) and mesenchymal markers (N-cadherin and Vimentin) on IL-6 treatment. IL-6 treatment on OVCs resulted in an increased E-cadherin expression while causing a decreased N-cadherin and Vimentin expression. Additionally, Drp1 inhibition using Mdivi-1 and siRNA transfection restored IL-6-induced EMT which may indicate EMT-dependent invasion and migration (Figure S5).
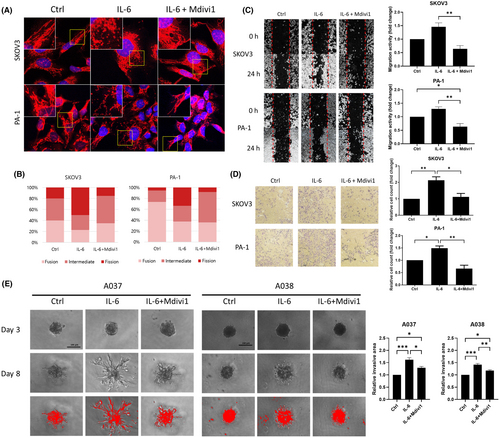
OVCs in the ascites of the abdominal cavity are detached from cancer tissue and float in the form of single cells or spheroids. Based on these characteristics, we formed 3D spheroids using OVCs derived from ascites from ovarian cancer patients and carried out a 3D invasion assay. IL-6 treatment induced invasion in both A037 and A038 spheroids and the increased invasion was restored when pre-treated with Mdivi-1 (Figure 7E).
4 DISCUSSION
In this study, we showed for the first time that IL-6 induces the elongation of mitochondria in cancer cells. IL-6-induced mitochondrial fission has been demonstrated in several studies using skeletal muscle cells and cardiomyocytes.56, 57 However, there was no evidence of mitochondrial fission induced by IL-6 in cancer cells. IL-6 is a pro-inflammatory cytokine specifically abundant in malignant ascites, which is a major component of the ovarian cancer tumor microenvironment.23 Metastasis of ovarian cancer mainly occurs through the peritoneal cavity, and high concentrations of IL-6 may become more effective in ovarian cancer than other types of cancer. Herein, our results provide an association between IL-6 and mitochondrial dynamics, which has been rarely understood in cancer studies, and suggest inhibition of IL-6-induced mitochondrial fission for the suppression of ovarian cancer metastasis (Figure 8).
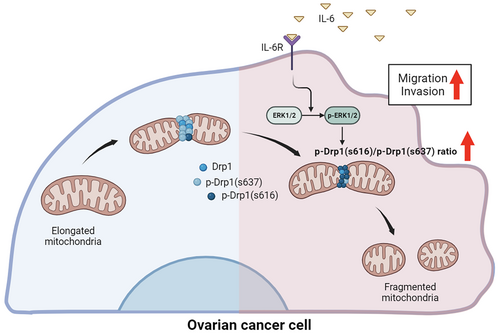
First, we found out that IL-6 increased migration and invasion of OVCs throughout the in vitro experiments with supportive bioinformatic analysis. In the same manner, multiple studies provided evidence that IL-6 in tumor microenvironment exerts enhanced cancer metastasis. Kim et al.58 showed that IL-6 treatment stimulated the migration of breast cancer cells. Also, IL-6 promoted metastasis by inducing EMT via JAK/STAT3 pathway in head and neck cancer cells and gastric cancer cells.59, 60 In non-small cell lung cancer, expression levels of IL-6 and TNF-α were significantly upregulated compared to normal controls in both serum and tissue. The expression levels were also positively correlated with the metastasis and EMT markers.61 Although most studies report that IL-6 modulates cancer metastasis,16, 19, 59, 62 it is still hard to define the mechanism of how metastasis is induced due to numerous indirect effects stemming from IL-6 signaling pathways.
Mitochondria play major roles in cancer progression with the regulation of metabolic pathways, ROS, extracellular pH, apoptosis and energy production.36, 63 The role of mitochondria in cancer has already ripened as a subject with clinical therapeutic potential. However, it remains debatable due to its complexity. In particular, the most prominent example of this intricacy is the role of mitochondrial dynamics in cancer, which was overlooked until even a few years ago.64 It has been found that mitochondrial fission is upregulated in metastatic tumor cells and is considered to be associated with the migration and invasion of cancer cells.35, 65-69 Accumulating evidence shows that deregulated mitochondrial dynamics is an important driver of metastatic potential and may work as a therapeutic target. Recent studies show that the expression of Drp1 has a positive correlation with the migration and invasion of lung cancer and breast cancer.35, 65 Furthermore, mitochondrial fission-induced metastasis of hepatocellular carcinoma by increasing focal-adhesion turnover and lamellipodia formation through Ca2+-induced ERK/FAK pathway activation.70 On the other hand, in triple-negative breast cancer (TNBC), phosphatidylserine decarboxylase (PISD)-induced mitochondrial fission reduced cellular processes and signaling pathways including Akt and ERK, resulting in metastasis inhibition.71 There is still debate but the prevailing view is that mitochondrial fission increases cancer metastasis.
Numerous studies elucidate that mitochondrial fission influences the motility and metastasis of tumor cells via metabolic changes,66 calcium-driven motility,70 and lamellipodia formation, which provides tracks to mitochondria to generate directional energy.35 There are several molecules known to modulate mitochondrial dynamics through an indirective mechanism. Cdk1/cyclin B, Cdk5 and ERK can lead to mitochondrial fission by phosphorylating Ser616 of Drp1. ERK is a well-known Drp1 activating kinase and is stimulated in response to different growth factors, transcription factors, and cytokines, including IL-6.30, 72 Similarly, in a recent study, activation of Erk1/2 by SIRT2 increased mitochondrial fission in OVCs.73 Contrarily, cAMP and PKA phosphorylate Ser 637 of Drp1 and induce detachment of Drp1 from mitochondria which inhibits the fission process.74
In this study, we observed that IL-6 can increase mitochondrial fission by increasing the ratio of active/inhibitory form of Drp1 in OVCs (Figure 4E). In addition, the mitochondrial activity increased by IL-6 was inhibited by Mdivi-1 treatment (Figure S6). Similar to our results, the accumulated evidence mostly studied in skeletal muscle and cardiomyocytes has explained the relationship between IL-6 and mitochondrial dynamics. Drp1 and mitochondrial fission 1 protein (Fis1) were significantly increased and Mfn1 was decreased when gp130, the IL-6 signal transducer, was knocked out from skeletal muscle.56 Likewise, IL-6 regulated the biogenesis and fission of mitochondria by reducing PGC-1α and Mfn1/2 and increased Fis1 in the early stage of cancer cachexia.75 In microglia, the resident macrophages in the brain, Drp1-dependent mitochondrial fission occurred through the IL-6 signaling pathway.76 Additionally, another pro-inflammatory cytokine TNF-α also may induce Drp1-mitochondrial fission in cardiomyocytes along with IL-6.57, 77 Collectively, the association between pro-inflammatory IL-6 and Drp1 has been confirmed in some studies on non-cancer cells but not on cancer cells. Mdivi1, a putative inhibitor of Drp1, has exhibited anti-cancer properties through diverse mechanisms. Mdivi1 was found to decrease oxidative metabolism in cancer, impairing cell proliferation.78 Furthermore, Mdivi1 not only induced mitotic spindle abnormalities and mitotic arrest but also cytotoxicity in Taxol-resistant TNBC cells, enhancing its anticancer efficacy.79 These findings collectively suggest the potential utility of Mdivi1 as a promising anti-cancer agent. Ovarian cancer mainly metastasizes along the transcoelomic route and produces ascites which contain high levels of IL-6. Numerous studies have demonstrated the association of IL-6 with cancer metastasis. We have previously reported that IL-6 increased ovarian cancer cell migration through JAK/STAT3 signaling in parallel with increased EMT markers. However, it has not been documented that IL-6 can regulate mitochondrial dynamics in cancer. Our finding highlights that mitochondrial fission can be stimulated by IL-6 and its inhibition could be a potential therapeutic approach to suppressing the metastasis of ovarian cancer.
AUTHOR CONTRIBUTIONS
Juwon Lee: Data curation; formal analysis; investigation; visualization; writing – original draft. Youngjin Han: Conceptualization; methodology; writing – review and editing. Soochi Kim: Writing – review and editing. HyunA Jo: Writing – review and editing. Wenyu Wang: Writing – review and editing. Untack Cho: Writing – review and editing. Se Ik Kim: Writing – review and editing. Boyun Kim: Supervision; writing – review and editing. Yong Sang Song: Conceptualization; funding acquisition; project administration; resources; supervision.
ACKNOWLEDGMENTS
We would like to thank Prof. Woo Ho Kim (Seoul National University, Republic of Korea) and his lab members for technical assistance in this study.
FUNDING INFORMATION
This research was supported by 2022 cancer research support project from the Korea Foundation for Cancer Research (CB-2022-A-2) and a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (HI16C2037).
CONFLICT OF INTEREST STATEMENT
The author Yong Sang Song is an Editorial board member of Cancer Science. The other authors have no conflict of interest to declare.
ETHICS STATEMENTS
Approval of the research protocol by an Institutional Review Board: This study was approved by the Institutional Review Board of Seoul National University Hospital (IRB: 1807-037-956).
Informed Consent: N/A.
Registry and the Registration No. of the study/trial: N/A.
Animal Studies: N/A.




