L-type amino acid transporter 1 inhibitor JPH203 prevents the growth of cabazitaxel-resistant prostate cancer by inhibiting cyclin-dependent kinase activity
Junryo Rii and Shinichi Sakamoto contributed equally to this work.
Abstract
L-type amino acid transporter 1 (LAT1, SLC7A5) is an amino acid transporter expressed in various carcinomas, and it is postulated to play an important role in the proliferation of cancer cells through the uptake of essential amino acids. Cabazitaxel is a widely used anticancer drug for treating castration-resistant prostate cancer (CRPC); however, its effectiveness is lost when cancer cells acquire drug resistance. In this study, we investigated the expression of LAT1 and the effects of a LAT1-specific inhibitor, JPH203, in cabazitaxel-resistant prostate cancer cells. LAT1 was more highly expressed in the cabazitaxel-resistant strains than in the normal strains. Administration of JPH203 inhibited the growth, migration, and invasive ability of cabazitaxel-resistant strains in vitro. Phosphoproteomics using liquid chromatography-mass spectrometry to comprehensively investigate changes in phosphorylation due to JPH203 administration revealed that cell cycle-related pathways were affected by JPH203, and that JPH203 significantly reduced the kinase activity of cyclin-dependent kinases 1 and 2. Moreover, JPH203 inhibited the proliferation of cabazitaxel-resistant cells in vivo. Taken together, the present study results suggest that LAT1 might be a valuable therapeutic target in cabazitaxel-resistant prostate cancer.
Abbreviations
-
- CDK1
-
- cyclin-dependent kinase 1
-
- CDK2
-
- cyclin-dependent kinase 2
-
- CRPC
-
- castration-resistant prostate cancer
-
- LAT1
-
- L-type amino acid transporter 1
-
- LC–MS/MS
-
- liquid chromatography-mass spectrometry
-
- PC
-
- prostate cancer
1 INTRODUCTION
Prostate cancer (PC) is the second most common solid tumor in men worldwide.1 Metastatic PC is usually treated with androgen receptor (AR)-axis-targeted (ARAT) drugs that target AR; however, advanced cancers are treated with chemotherapy. Cabazitaxel is the standard chemotherapy for patients with metastatic castration-resistant prostate cancer (CRPC) after docetaxel.2 While cabazitaxel has shown some efficacy, drug resistance remains a challenge as it eventually does occur, and when it does, there are no longer effective drugs for PC.3 We previously cultured PC cells in medium containing cabazitaxel for an extended time and established a cabazitaxel-resistant cell line as a tool to search for effective drugs.4, 5
L-type amino acid transporter 1 (LAT1, SLC7A5) is an amino acid transporter expressed on the plasma membrane of various types of cancer cells.6 Our group has demonstrated that LAT1 plays a pivotal role in cancer cell proliferation through the activation of the mammalian target of rapamycin (mTOR) pathway in urologic cancers, including PC.7-9 We previously reported that LAT1 expression is upregulated in PC and that LAT1 plays an important role in CRPC.7 Therefore, we hypothesized that LAT1 might also have a key role in cabazitaxel resistance. Recently, JPH203, a LAT1-specific inhibitor, was developed,10 and it was reported to have anti-cancer effects in several carcinomas, including urologic cancers.8, 9, 11 In addition, a phase I clinical trial of JPH203 has already been conducted, and the results showed that it has some degree of efficacy against biliary tract cancer.12
Phosphoproteomics is the study of protein phosphorylation through the large-scale and comprehensive identification of target substrates of kinases in cells by liquid chromatography–mass spectrometry (LC–MS/MS), and it has been actively applied in cancer research in recent years.13
In the present study, we investigated the effects of LAT1 inhibition on the functions of cabazitaxel-resistant PC cells. We also used phosphoproteomics to comprehensively analyze the changes in intracellular phosphorylation caused by LAT1 inhibition and attempted to identify novel downstream signaling molecules of LAT1.
2 MATERIALS AND METHODS
2.1 Cell cultures and transfection
The human PC cell lines PC-3 (RRID: CVCL_0035) and DU145 (RRID: CVCL_0105) were obtained from the Cell Resource Centre for Biomedical Research, Institute of Development, Aging and Cancer, Tohoku University (Miyagi, Japan). Cabazitaxel-resistant PC-3-TxR/CxR (RRID: CVCL_ZX06) and DU145-TxR/CxR (RRID: CVCL_ZX04) cells were generated previously.4 Cabazitaxel-resistant 22Rv1-CR cells were also generated previously.5 Cell culturing was basically performed as previously reported.14 PC-3-TxR/CxR and DU145-TxR/CxR cells were cultured in RPMI-1640 medium (Fujifilm Wako, Osaka, Japan) with 3 nM cabazitaxel (Fujifilm Wako) to maintain their drug resistance. All human cell lines have been authenticated using short tandem repeat (STR) profiling within the past 3 years. The CycleavePCR Mycoplasma Detection Kit (Takara Bio, Shiga, Japan) was used to confirm that the cultures were mycoplasma-free.
2.2 Reagents and antibodies
Lipofectamine RNAiMax Transfection Reagent, OPTI-MEM, the small interfering RNA (siRNA) SiLAT1 (Stealth siRNAs: HSS112004 and HSS112005), SiCDK1 (Silencer Select SiRNAs: s464 and s465), SiCDK2 (Silencer Select SiRNAs: s205 and s206), and Stealth RNAi siRNA Negative Control Med GC Duplex #3 were obtained from Thermo Fisher Scientific (Waltham, MA). The plasmid vectors containing CDK1 and CDK2 were provided by Kazusa Genome Technologies (Clone ID oc00221 and oc00080; Chiba, Japan). The LAT1-specific inhibitor JPH203 was provided by J-Pharma (Yokohama, Japan). The catalog numbers and dilutions of all used antibodies are summarized in Table S1.
2.3 Reverse transcription-polymerase chain reaction
Total RNA isolation and reverse transcription-polymerase chain reaction (RT-PCR) were performed as described previously.14 The GAPDH mRNA level was quantified as a control for normalization. The PCR primers used in this study are listed in Table S2.
2.4 Western blot analysis
Protein extraction and western blotting were performed as described previously.7 The protein assay bicinchoninic acid kit (Nacalai Tesque, Kyoto, Japan) was used to quantify the protein content, and 20-mg protein samples were subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis using a gel prepared with the TGX Fastcast Acrylamide Kit (10%; Bio-Rad, Hercules, CA) according to the manufacturers’ protocols. Blocking One-P (Nacalai Tesque) was used as a blocking agent for the detection of phosphorylation, and Bullet Blocking One (Nacalai Tesque) was used as a blocking agent for all of the other experiments. Original full images of membranes are shown in Figures S1–S3.
2.5 Cell growth inhibition assay
Cells were seeded into 96-well plates (1500 cells/well), and at each time point, cells were incubated with Cell Counting Kit-8 (CCK8; Dojindo, Kumamoto, Japan) for 1.5 h, then the absorbance was measured using Microplate Manager v6.0 (Bio-Rad). To evaluate the inhibitory effect of JPH203, 24 h after seeding, the cells were treated with the inhibitors at the indicated concentrations or the same amount of dimethyl sulfoxide (DMSO; 0.5%; vehicle control). The half-maximal inhibitory concentration (IC50) definition and measurements were as previously reported.8
To examine the effects of JPH203 in combination with a cyclin-dependent kinase (CDK) inhibitor (dinaciclib, flavopiridol, or milciclib), combination assays were performed using a fixed concentration of JPH203 and three concentrations of each CDK inhibitor based on previous literature.15 Of the three concentrations tested, only the one that had the largest effect in combination with JPH203 is shown in the results.
2.6 Cell migration and invasion assay
The protocol and reagents used for the migration and invasion assay were as described previously.14 A wound healing assay was also performed as described previously.16 In each assay, cells were incubated with 5 μg/mL of mitomycin C (Nacalai Tesque) for 1 h at the start to inhibit cell proliferation.
2.7 Phosphoproteomics analysis
2.7.1 Preparation of protein samples and phosphopeptide enrichment
The proteins were extracted with RIPA buffer (Santa Cruz, Dallas, TX). Acetone was then added to the protein extract (final acetone concentration: 80% v/v), and the sample was incubated for 2 h at −20°C. After removing the supernatant by centrifugation at 15,000 ×g for 15 min at 4°C, the precipitate was redissolved in 0.5% sodium dodecanoate and 100 mM Tris–HCl (pH 8.5) in a water bath-type sonicator (Bioruptor UCD-200; SonicBio, Kanagawa, Japan). The redissolved proteins were adjusted to 5 mg/mL with 0.5% sodium dodecanoate and 100 mM Tris–HCl (pH 8.5). Next, 500 μg of protein extract was treated with 10 mM dithiothreitol at 50°C for 30 min, then subjected to alkylation with 30 mM iodoacetamide in the dark at room temperature for 30 min. The reaction of iodoacetamide was stopped by treating the sample with 60 mM cysteine for 10 min. The mixture was then diluted with 150 μL of 50 mM ammonium bicarbonate and digested by a 50-μg mixture of Trypsin/Lys-C mix (Promega, Madison, WI) overnight at 37°C. The digested sample was acidified with 150 μL of 5% trifluoroacetic acid, followed by sonication for 5 min (Bioruptor UCD-200). Then, the mixture was shaken for 5 min and centrifuged at 15,000 ×g for 5 min. Subsequently, the supernatant was desalted using a MonoSpin C18 (GL Sciences, Tokyo, Japan), followed by drying with a centrifugal evaporator. Phosphopeptides were enriched using the Titanosphere Phos-TiO Kit (GL Sciences) according to the manufacturer's instructions.
2.7.2 Liquid chromatography–mass spectrometry/mass spectrometry analysis
Phosphopeptides were directly injected onto a 75 μm × 12 cm nanoLC nano-capillary column (Nikkyo Technos, Tokyo, Japan) at 40°C, then separated for 80 min through a gradient at a flow rate of 100 nL/min using an UltiMate 3000 RSLCnano LC system (Thermo Fisher Scientific). Phosphopeptides eluted from the column were analyzed on a Q Exactive HF-X (Thermo Fisher Scientific). MS data acquisition was performed in the data-dependent acquisition (DDA)-MS mode and overlapping-window data-independent acquisition (DIA)-MS mode.17, 18 In the DIA-MS mode for quantification, MS1 spectra were collected in the range of 390 to 1010 m/z at 30,000 resolution to set an AGC target of 3 × 106. MS2 spectra were collected in the range of >200 m/z at 30,000 resolution to set an AGC target of 3 × 106. The isolation width was set to 10 m/z with stepped normalized collision energies of 22%, 26%, and 30%. Isolation window patterns in 400 to 1000 m/z were used as window placements optimized by Skyline 4.1.19 In the DDA-MS mode for the spectral library, the pool of all samples was analyzed using the gas-phase fractionation method. We used three MS ranges (395–555, 545–705, and 695–1005 m/z), and each was measured in the DDA mode. MS1 spectra were collected at 120,000 resolution to set an AGC target of 3 × 106. The 20 most intense ions with charge states of 2+ to 5+ that exceeded 5.0 × 103 were fragmented by collision-induced dissociation with a normalized collision energy of 22%, 26%, and 30%, and tandem mass spectra were acquired on the Orbitrap mass analyzer with a mass resolution of 60,000 at 200 m/z to set an AGC target of 2 × 105.
2.7.3 Data analysis
A spectral library was generated by searching DDA-MS data against the mouse UniProt reference proteome (Uniprot id UP000000589, reviewed, canonical) using Proteome Discoverer v2.3 (Thermo Fisher Scientific). The setting parameters were as follows: experimental data search enzyme, trypsin; maximum missed cleavage sites, 1; precursor mass tolerance, 8 ppm; fragment mass tolerance, 0.02 Da; static modification, carbamidomethylation [C]; variable modification, phosphorylation [S, T, Y]; and site probability threshold, 75. The peptide identification threshold was a peptide false discovery rate (FDR) <1%. The quantitative analysis of phosphopeptides was performed by Scaffold DIA v2.2. The setting parameters were as follows: spectral library, the generated spectral library; enzyme, trypsin; maximum missed cleavage sites, 1; precursor mass tolerance, 9 ppm; fragment mass tolerance, 9 ppm; static modification, carbamidomethylation [C]; and variable modification, phosphorylation [S, T, Y]. The peptide identification threshold was a peptide FDR <1%. Peptide quantification was performed by Scaffold DIA using the EncyclopeDIA algorithm.20
2.7.4 In silico analysis
Gene ontology (GO), biological process, and molecular network analyses were performed using KeyMolnet software (KM Data, Tokyo, Japan).21 For the GO and biological process analyses, the cutoff values were set as follows: fold change >5 or <0.2. For the molecular network analysis, the cutoff values were set as follows: fold change >10 or <0.1. The Kinase-Substrate Enrichment Analysis (KSEA) online tool (https://casecpb.shinyapps.io/ksea/) was used to perform a comprehensive analysis, including an analysis of the changes in each phosphorylation site22 with the PhosphoSitePlus kinase-substrate database.23 The cutoff for the p-value was set at 0.05, and the cutoff for the substrate count was set at 10.
2.8 Cell cycle and apoptosis assay
Cabazitaxel-resistant PC cells were cultured in six-well dishes for 24 h, then treated with JPH203 or DMSO as a control for 24 h before collection. The reagents and analysis methods used were as previously reported.16
2.9 L-Leucine uptake assay
Cabazitaxel-resistant PC-3 cells (1.5 × 105 cells/well) were cultured in 24-well dishes for 48 h and examined by transporter assay using L-Leucine (containing [14C] leucine at 0.1 mCi/mL) (PerkinElmer, Boston, USA). The precise protocols of the experiment was reported previously.24
2.10 Immunohistochemistry
Paraffin-embedded sections (4 mm in thickness) were used. The detailed immunohistochemistry (IHC) methods were as previously reported.25 Anti-Cdc6 (phospho S54, 1:200 dilution) and anti-phospho Rb (Ser807/811, 1:100 dilution) were used as the primary antibodies. To assess the expression of these phosphoproteins, we employed a scoring method that we have used previously.9 The intensity of tumor cell staining was evaluated in at least five fields of view at 400× magnification. Two independent evaluators (AF and JR), who were blinded to the sample information, scored the intensity of tumor cell staining in the specimens.
2.11 Analysis of a clinical prostate cancer patient dataset
A dataset of clinical PC data from Grasso et al. (GSE35988)26 was analyzed in this study as previously reported.14 Gene expression correlations were examined from reports of expression analysis in patients with advanced prostate cancer27 (https://github.com/cBioPortal/datahub/tree/master/public/prad_su2c_2019.).
2.12 Mouse xenografts
Six-week-old male SCID mice (C.B-17/Icr-scid/scidJcl) were obtained from CLEA (Tokyo, Japan). After an acclimatization period, 2 × 106 PC-3-TxR/CxR cells were implanted with 50% Matrigel (354,230, Corning, Corning, NY) in the dorsal subcutaneous region of the mice. When the tumor volumes reached approximately 100 mm3, the mice were divided into two groups: a control group and a JPH203-treated group (n = 4 each). The tumor size was calculated using the following formula: tumor volume = (major axis) × (minor axis) 2 × 0.5. For the JPH203-treated group, the mice were given an intravenous injection of JPH203 (25 mg/kg) once daily, while the control group was given an intravenous injection of the placebo once daily. The tumor size was measured daily with a Vernier caliper. After 15 days, the mice were killed, and the tumor xenografts were resected.
2.13 Statistical analysis
JMP Pro version 15.0.0 (SAS Institute, Cary, NC) was used for all statistical analyses. The unpaired Student's t-test was used for assessing differences between two groups with Bonferroni correction. Statistical significance was set at p-values below 0.05. p-values are indicated by asterisks in the figures: *p < 0.05, **p < 0.01, and ***p < 0.001.
3 RESULTS
4 L-type amino acid transporter 1 expression is upregulated in cabazitaxel-resistant prostate cancer cells and inhibition of L-type amino acid transporter 1 suppresses cell proliferation
First, the sensitivity of cabazitaxel-resistant prostate cancer cell lines to cabazitaxel was compared to that of the normal line. Resistant strains showed similar levels of susceptibility compared to previous studies (Figure 1A).4, 5 We compared the expression of the LAT family and its binding partner, 4F2hc, in cabazitaxel-resistant cell lines and confirmed that LAT1 is predominantly expressed (Figure S4). We compared the LAT1 expression levels between normal PC cell lines and cabazitaxel-resistant cell lines. In both PC-3 and DU145, LAT1 expression was elevated in the cabazitaxel-resistant strains when compared to the normal strains (Figure 1B). We examined the expression of ATF4 in the GCN2 pathway, which is associated with amino acid stress responses.28 ATF4 expression was also significantly elevated in cabazitaxel-resistant strains when compared to the normal strains (Figure 1B,C). In the public dataset, LAT1 expression was not significantly different between the non-cancer and hormone sensitive PC (HSPC) groups but was significantly elevated in the CRPC group when compared to the HSPC group (Figure S5).
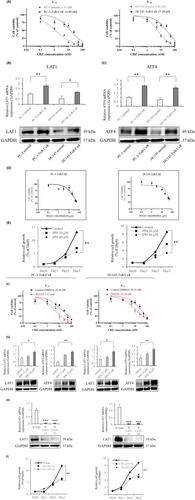
The effect of LAT1 inhibition on cell proliferation was then examined. Administration of JPH203, a specific inhibitor of LAT1, inhibited the uptake of leucine (Figure S4) and the growth of cabazitaxel-resistant strains in a concentration-dependent manner (Figure 1D). The IC50 was 28.33 ± 3.26 μM in PC-3-TxR/CxR and 34.09 ± 4.76 μM in DU145-TxR/CxR. Observations of growth changes every 24 h revealed similar results (Figure 1E). We then tested whether treatment with JPH203 would reduce resistance to cabazitaxel. In both cell lines, treatment with JPH203 decreased the IC50 to cabazitaxel (Figure 1F). Both LAT1 and ATF4 were significantly upregulated 72 h after JPH203 administration (Figure 1G), showing a similar trend to a previous report.28 Similar investigations were conducted using siRNA-based knockdown experiments, and SiLAT1 administration led to a marked downregulation of LAT1 expression (Figure 1H) and caused a remarkable decrease in cell proliferation (Figure 1I). Similar to the administration of JPH203, knockdown of LAT1 decreases the IC50 to cabazitaxel in PC-3 TxR/CxR (Figure S4). The same assay was performed for regular PC-3 and DU145. The IC50 was 11.94 ± 2.19 μM in PC-3 and 19.68 ± 4.01 μM in DU145 (Figures S5 and S6). Administration of JPH203 inhibited cell proliferation in a concentration-dependent manner (Figure S6). Knockdown of LAT1 by siRNA significantly attenuated cell proliferation (Figure S6). We further investigated whether LAT1 affects docetaxel resistance. After confirming docetaxel sensitivity of normal and docetaxel-resistant strains, knockdown of LAT1 resulted in a reduction of docetaxel IC50 (Figure S7). Further, the same assay was performed using a cabazitaxel-resistant strain of 22Rv1 cells (22Rv1-CR) as an AR-positive prostate cancer cell line. Consistent with prior findings, the IC50 of JPH203 was slightly higher in the cabazitaxel-resistant strain compared to the regular 22Rv1 (Figure S7). To investigate whether inhibition of LAT1 reduces the IC50 for cabazitaxel, administration of JPH203 and knockdown of LAT1 each reduced the IC50 compared to the control group (Figure S7).
4.1 Effects of L-type amino acid transporter 1 inhibition on cell migration, invasion, and the expression of related genes
Next, we validated the effects of JPH203 treatment on cell migration, invasion, and the expression of genes involved in epithelial–mesenchymal transition (EMT) in the cabazitaxel-resistant strains. Administration of JPH203 significantly reduced migration in the chamber assay (Figure 2A). The same effect was observed in the wound healing assay (Figure 2B). We subsequently investigated the invasive capacity of the cells, and similar results were obtained (Figure 2C). JPH203 treatment decreased the expression of N-cadherin, Slug, and vimentin in both cell lines (Figure 2D). E-cadherin expression was not observed in PC-3-TxR/CxR. In DU145-TxR/CxR, E-cadherin expression was slightly increased by JPH203 treatment. In addition, a similar assay was performed on a normal strain of PC-3. JPH203 treatment decreased the expression of N-cadherin, Slug, and vimentin and increased the expression of E-cadherin. It also significantly decreased cell migration and invasion (Figure S6).
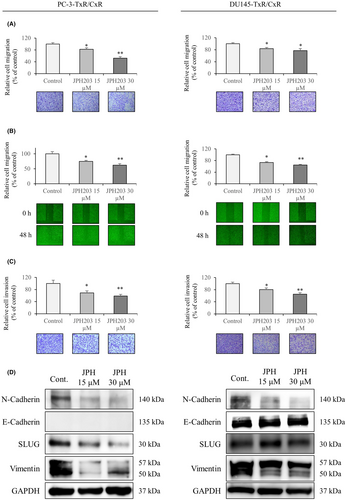
4.2 Identification of kinases that were affected by the inhibition of L-type amino acid transporter 1 and pathway analysis
To explore how the administration of JPH203 affects intracellular phosphorylation, we performed a comprehensive LC–MS analysis using PC-3-TxR/CxR cells. Proteins were extracted from cells in the JPH203-treated and control groups, and finally, 3080 substrate proteins and 10,749 phosphorylated peptides were identified to have been affected. The MA plot for this assay is shown in Figure S8. First, GO and biological process analyses of the variable gene groups were performed. Among the top 10 GO terms that fluctuated were many elements related to the cell cycle (Figure 3A). The same was true for the biological process analysis (Figure 3B). Subsequent analyses included phosphorylation sites using KSEA. The kinase activity of CDK1 and CDK2 was significantly decreased (Figure 3C). In the molecular network analysis, CDK1 and CDK2 were each involved in many molecular pathways (Figure 3D,E). An overview of the molecular network is shown in Figure S9.
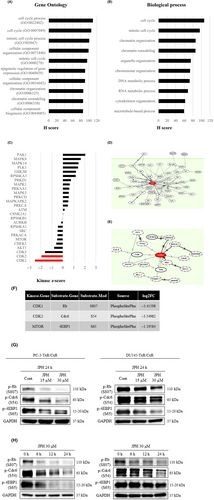
From the kinase-substrate links, the substrates of CDK1, CDK2, and mTOR that were markedly reduced were extracted (Figure 3F). The decreases in phosphorylation were confirmed by western blotting using antibodies recognizing these specific phosphorylation sites. The phosphorylation of cdc6, Rb, and 4EBP1 was decreased in a concentration-dependent manner by treatment with JPH203 (Figure 3G). We also observed a decrease in phosphorylation over time during the 24 h after the administration of JPH203 (Figure 3H). In the analysis of the public database, CDK1 expression was significantly higher in HSPC than in the normal cells and was also significantly higher in CRPC than in HSPC (Figure S5). In contrast, no such trend was observed for CDK2 (Figure S5). When the expression of CDK1 and CDK2 was compared between the normal and cabazitaxel-resistant cell lines of PC-3 and DU145, the expression was significantly higher in the cabazitaxel-resistant line than in the normal line in both PC-3 and DU145 (Figure S10). We subsequently examined whether administration of JPH203 in normal strains decreases phosphorylation of the mTOR pathway. In PC-3 cells, treatment with JPH203 decreased phosphorylation of 4EBP1 (Figure S10). Finally, we investigated whether the downstream phosphorylation signals of LAT1 that we identified in this study are associated with the mTOR pathway. Phosphorylation of 4EBP1 was decreased by JPH203 or by rapamycin, a specific inhibitor of the mTOR pathway. In contrast, phosphorylation of Rb was decreased by JPH203 but not by rapamycin (Figure S10).
4.3 Effects of L-type amino acid transporter 1 inhibition on the cell cycle and apoptosis
Because treatment with JPH203 reduced the CDK1 and CDK2 kinase activity, we investigated the effects of JPH203 treatment on the cell cycle and apoptosis by flow cytometry in cabazitaxel-resistant lines. Administration of JPH203 caused a significant increase of cells in the G0/G1 phase and a significant decrease of cells in the S and G2/M phases (Figure 4A). Furthermore, the percentage of apoptotic cells was significantly higher in the JPH203-treated group than in the control group (Figure 4B). JPH203 treatment increased cleaved PARP expression, suggesting that LAT1 inhibition induces apoptosis in cabazitaxel-resistant PC cells (Figure 4C). We subsequently prepared CDK1 and CDK2 plasmids and SiRNAs to show whether the LAT1 and CDK1 and CDK2 pathways are important for cabazitaxel-resistant prostate cancer. Each plasmid resulted in overexpression, while the SiRNAs nearly abolished expression (Figure 4D).

We then performed a rescue assay using the CDK plasmid. Compared to JPH203 alone, administration of JPH203 and CDK plasmid significantly increased cell proliferation (Figure 4E). Similarly, treatment with plasmids of SiLAT1 and CDK significantly enhanced cell growth compared to treatment with SiLAT1 alone (Figure 4F). Furthermore, administration of JPH203 plus SiCDK1 and SiCDK2 had no significant effect on cell proliferation compared to JPH203 alone (Figure 4G).
We then selected three CDK inhibitors (dinaciclib, flavopiridol, and milciclib), referring to previous reports,15 to explore their growth-inhibitory effects. The combined use of JPH203 and dinaciclib or flavopiridol significantly inhibited cell growth in the two cabazitaxel-resistant lines when compared to JPH203 or the CDK inhibitors alone (Figure S11). In contrast, the combination of JPH203 with milciclib significantly inhibited cell proliferation in DU145-TxR/CxR when compared to milciclib alone, but this was not seen in PC-3-TxR/CxR. Furthermore, there was no significant difference between the combination of JPH203 with milciclib and JPH203 alone in any of the cells (Figure S11). Finally, we performed a database analysis of patients with advanced prostate cancer and found no correlation in expression between Rb (RB1 gene) and the MDR1 gene, which is known to be involved in cabazitaxel resistance (Figure S12). In contrast, there was a negative correlation in expression between RB1 and LAT1 (Figure S12).
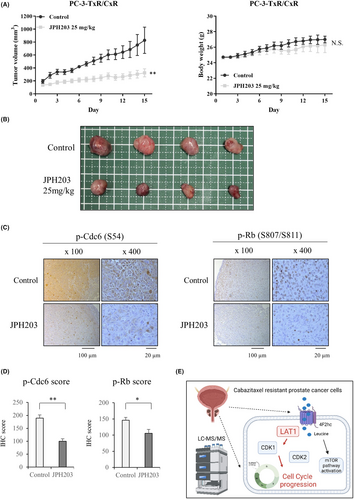
4.4 JPH203 inhibits tumor cell growth in vivo
To assess the growth inhibitory effect of JPH203 in a cabazitaxel-resistant strain in vivo, we implanted PC-3-TxR/CxR cells into C.B-17/Icr-scid/scidJcl mice and treated them with JPH203. JPH203 administration for 2 weeks significantly reduced the volume of the PC-3-TxR/CxR tumors when compared to the control group without causing weight loss (Figure 5A,B). The tumors were then extracted and paraffin-embedded, and IHC assays were performed. Both cdc6 phosphorylation and Rb phosphorylation were decreased in the JPH203 group when compared to the control group (Figure 5C). The IHC scores were also significantly lower in the JPH203 group than in the control group for both cdc6 and Rb phosphorylation (Figure 5D).
5 DISCUSSION
There were two main findings in this study. First, we investigated the effects of the LAT1-specific inhibitor JPH203 on cabazitaxel-resistant PC cells and found that LAT1 expression was upregulated in cabazitaxel-resistant strains and that JPH203 inhibited the growth of cabazitaxel-resistant PC cells both in vitro and in vivo. Furthermore, JPH203 inhibited cell migration, invasion, and the cell cycle and induced apoptosis. Second, we identified CDK1 and CDK2, which are kinases involved in the cell cycle, as downstream targets of LAT1, and the administration of JPH203 significantly decreased the kinase activity of CDK1 and CDK2.
L-type amino acid transporter 1 research in the field of oncology has increased over the past decade, and knowledge has been accumulating.6 The primary role of LAT1 in cancer cells is to supply amino acids necessary for cell proliferation. The mechanism by which the influx of amino acids, especially leucine, into the cell activates mTORC1, which plays an important role in cell proliferation, was recently discovered.29 The leucine taken up via LAT1 binds to sestrin-2, a leucine sensor, and the interaction causes further changes that ultimately lead to mTORC1 activation.30 Other amino acids that have entered the cell have been reported to serve as material for metabolic reprogramming in cancer cells.31 Branched-chain amino acids, including leucine, that are not incorporated into proteins by protein synthesis have recently been reported to be metabolized by multiple enzymes and to ultimately enter the TCA cycle, where they are used for energy and fatty acid synthesis.32 Furthermore, it has recently been reported that the expression of several amino acid transporters, including LAT1, is elevated in CRPC with liver metastases.33 Further functional analyses of amino acid transporters, including LAT1, in the field of oncology are expected in the future.
Previously, 2-aminobicyclo-(2,2,1)-heptane-2-carboxylic acid (BCH), a known nonspecific inhibitor of the LAT family, including LAT1, has been studied for its growth inhibitory effect in PC cell lines and for its inhibitory effect on phosphorylation in the mTOR pathway.34 There have been many studies on the use of BCH, including for other carcinomas, but due to its low specificity for LAT1, it has not been applied clinically. In 2009, JPH203 was created and shown to inhibit the growth of HT-29 human colon cancer cells in vitro.35 A phase I trial of JPH203 was completed in 17 patients with advanced solid tumors, and one patient with biliary tract cancer had a long-term response and minor adverse events.12 We are currently preparing a phase II trial of JPH203 for CRPC patients who have failed standard therapy, including cabazitaxel.
Cyclin-dependent kinases, along with cyclins, are proteins that play important functions in the regulation and progression of the cell cycle.36 Hence, CDKs have been a target of anticancer therapy. Dinaciclib, which is one of the three CDK1 and CDK2 inhibitors that we focused on in this study, has been clinically tested in several carcinomas, including breast cancer.37 Trials of dinaciclib for hematologic tumors are also being conducted not only with dinaciclib alone but also in combination with pembrolizumab and rituximab.38, 39 Furthermore, it has been reported that in PC, combination therapy of PARP with CDK4/6 inhibitors induced apoptosis.40 In the present study, we investigated the combination therapy of JPH203 with CDK1/2 inhibitors in vitro. Table S3 shows the IC50 of each inhibitor to inhibit the activity of CDK1 and CDK2 as kinases.41-43 In this study, combination therapy with dinaciclib or flavopiridol significantly reduced proliferation compared to JPH203 alone. In contrast, combination therapy with milciclib did not significantly reduce proliferation (Figure S11). The reason might be that Milciclib has a nearly 10-fold difference in IC50 for CDK1 and CDK2 and the optimal concentration of milciclib in combination therapy could not be found. The same results were observed in the collaborators’ assay using cholangiocarcinoma cells.15 Further research in this area is needed. CDKs have recently been reported to function not only in the cell cycle but also in epigenetics and DNA repair.44 In PC, it has been reported that the phosphorylation of AR by CDK1 and CDK9 is important in promoting the transcription of AR.45 Further research on the role of CDKs in PC is expected in the future.
In summary, the LAT1-specific inhibitor JPH203 inhibited the growth of cabazitaxel-resistant PC cells in vitro and in vivo. Phosphoproteomics showed that treatment with JPH203 attenuated the kinase activity of CDK1 and CDK2. Furthermore, the administration of JPH203 arrested the cell cycle and induced apoptosis. Combination treatment of JPH203 with CDK inhibitors inhibited cell proliferation in an additive manner. Thus, JPH203 might be an effective therapy for patients with cabazitaxel-resistant CRPC.
AUTHOR CONTRIBUTIONS
Junryo Rii: Conceptualization; data curation; formal analysis; investigation; methodology; project administration; validation; visualization; writing – original draft; writing – review and editing. Shinichi Sakamoto: Conceptualization; funding acquisition; methodology; project administration; resources; supervision; writing – review and editing. Atsushi Mizokami: Methodology; resources; supervision. Minhui Xu: Data curation; methodology; resources. Ayumi Fujimoto: Validation. Shinpei Saito: Data curation. Hidekazu Koike: Data curation; methodology; resources. Takaaki Tamura: Methodology. Takayuki Arai: Funding acquisition. Yasutaka Yamada: Methodology; writing – review and editing. Yusuke Goto: Supervision. Tomokazu Sazuka: Supervision. Yusuke Imamura: Supervision. Kazuhiro Suzuki: Resources; supervision. Yoshikatsu Kanai: Resources; supervision. Naohiko Anzai: Funding acquisition; resources; supervision. Tomohiko Ichikawa: Funding acquisition; project administration; resources; supervision.
ACKNOWLEDGMENTS
The authors thank Dr. Osamu Ohara and Dr. Yusuke Kawashima (Kazusa DNA Research Institute, Kisarazu, Japan) for the phosphoproteomics analysis. The authors also thank Ms. Yoshie Reien, Ms. Hisayo Karahi, Ms. Miyuki Yamaguchi, and Ms. Natsuko Kusama (Experimental Assistants, Chiba University) for their support of this study.
FUNDING INFORMATION
The present work was supported by Grants-in-Aid for Scientific Research of the Ministry of Education, Culture, Sports, Science and Technology of Japan (#20H03813 to TI, #20 K09555 to SS, #20 K09572 to YI, and #20 K18087 to TA).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
ETHICS STATEMENTS
Approval of the research protocol by an Institutional Reviewer Board: N/A.
Informed Consent: N/A.
Registry and the Registration No. of the study/trial: N/A.
Animal Studies: All animal experiments were approved by the Institutional Animal Care and Use Committee of Osaka University.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available in the Gene Expression Omnibus: GSE35988.




