Growth differentiation factor 15 induces cisplatin resistance through upregulation of xCT expression and glutathione synthesis in gastric cancer
Abstract
Gastric cancer is a common cancer worldwide, particularly in East Asia. Chemotherapy is used in adjuvant or palliative therapies for gastric cancer. However, subsequent chemoresistance often develops. Growth differentiation factor 15 (GDF15) links to several cancers, but its effect on chemoresistance in gastric cancer remains unclear. Here, we analyzed clinical samples from genetic databases and included patients with gastric cancer. We dissected the regulatory mechanism underlying GDF15-mediated resistance of cisplatin in human gastric cancer cells. We showed that GDF15 serum levels might be a valuable biomarker for predicting prognosis in gastric cancer. The expressions of GDF15 and its receptor glial cell-derived neurotrophic factor family receptor a-like (GFRAL) in gastric tumors are important for malignant progression. Moreover, GDF15 expression is increased in gastric cancer cells with cisplatin resistance, resulting from elevated intracellular glutathione (GSH) and antioxidant activities. Upregulated GDF15 could increase intracellular GSH content by activating the GFRAL-GCN2-eIF2α-ATF4 signaling, enhancing cystine-uptake transporter xCT expression, and contributing biosynthesis of GSH in human gastric cancer cells. In conclusion, our results indicate that GDF15 could induce chemoresistance by upregulating xCT expression and GSH biosynthesis in human gastric cancer cells. Targeting GDF15 could be a promising treatment method for gastric cancer progression.
Abbreviations
-
- AARE
-
- amino acid response element
-
- ARE
-
- antioxidant-responsive element
-
- ATF4
-
- activating transcription factor 4
-
- BSO
-
- buthionine sulfoximine
-
- CisR
-
- cisplatin-resistant
-
- DCF
-
- dichlorodihydro-fluorescein
-
- DFS
-
- disease-free survival
-
- eIF2α
-
- eukaryotic initiation factor 2α
-
- GCN2
-
- general control nonderepressible 2
-
- GDF15
-
- growth differentiation factor 15
-
- GEPIA
-
- Gene Expression Profiling Interactive Analysis
-
- GFRAL
-
- glial cell-derived neurotrophic factor family receptor a-like
-
- GSH
-
- glutathione
-
- HER2
-
- human epidermal growth factor receptor 2
-
- HRI
-
- heme-regulated eIF2α kinase
-
- IHC
-
- immunohistochemistry
-
- NAC
-
- N-acetylcysteine
-
- OS
-
- overall survival
-
- PERK
-
- PKR-like endoplasmic reticulum kinase
-
- PFS
-
- progression-free survival
-
- PI
-
- propidium iodide
-
- PKR
-
- protein kinase R
-
- RET
-
- rearranged during transfection
-
- rhGDF15
-
- recombinant human GDF15
-
- ROS
-
- reactive oxygen species
-
- SSA
-
- sulfasalazine
-
- STAT3
-
- signal transducer and activator of transcription 3
-
- TGF-β
-
- transforming growth factor-β
1 INTRODUCTION
Gastric cancer is still a common cancer, even though its global incidence has gradually declined.1-3 The role of chemotherapy in patients with early- or late-stage gastric cancer was identified, which has clinical benefits on disease recurrence and cancer-mediated discomfort signs.4 Although its clinical benefit in gastric cancer is acceptable, gastric cancer often progresses after several months of treatment.5
Several pathologic conditions such as cardiovascular, brain, and kidney diseases, cancer, inflammation, and obesity often upregulate GDF15.6 Glial cell-derived neurotrophic factor family receptor a-like has recently been revealed as an important GDF15 receptor. Growth differentiation factor 15 binding to GFRAL and activating RET is important for nutrient homeostasis in animal models.7-9 High GDF15 levels in tumors compared to normal tissues could be observed in several cancers.10 In gastric cancer, GDF15 expression is abundant in cancer tissues and cell lines and increased in metastatic and less differentiated tumor tissues.11 However, whether GDF15 is involved in chemoresistance of gastric cancer remains unclear. Platinum agents are the main chemotherapeutic agents for patients with gastric cancer.12-15 Molecular mechanisms regarding cisplatin resistance have been widely investigated.16, 17 Glutathione is responsible for cisplatin resistance by inactivating intracellular cisplatin.18
We previously revealed that increased intracellular GSH through upregulated cystine-uptake transporter xCT contributes to chemoresistance under stress conditions in human gastric cancer cells.19, 20 As cellular stress can trigger GDF15 expression, we proposed that the upregulated GDF15 in gastric cancer cells might regulate their sensitivity to cisplatin. Herein, we evaluated the GDF15-GFRAL axis in malignant progression of gastric cancer and dissected the mechanism underlying GDF15-enhanced chemoresistance of human gastric cancer cells.
2 MATERIALS AND METHODS
2.1 Differential expression and survival analyses in clinical databases
Gene Expression Profiling Interactive Analysis was used to analyze the gene expression levels of GDF15 and GFRAL between normal and tumor tissues of gastric cancer patients (http://gepia.cancer-pku.cn/).21 The OS and PFS of gastric cancer patients expressing different levels of GDF15 were determined using the Kaplan–Meier plotter with the GDF15 gene (Affy ID 221577_x_at) and GSE15459 dataset (https://kmplot.com/analysis/).22
2.2 Patient samples
A total of 100 patients with gastric cancer who received curative surgery were included. Patients who underwent palliative surgery or who had unresectable tumors were excluded. The operated specimens were fixed and wax embedded immediately after the procedure. The definition of patient characteristics and subsequent clinical follow-up were modified from a previous study.23
2.3 Immunohistochemistry analyses
Initially, clinical samples from 100 patients with gastric cancer were analyzed by IHC staining using a previous method.24 Because some tumor tissues were absent from the collected slices, we evaluated GDF15 and GFRAL expressions in 87 and 92 tumor slices, respectively. The GDF15 (sc-377195; Santa Cruz Biotechnology) and GFRAL (PA5-24545; Invitrogen, Thermo Fisher Scientific) Abs were used, and the expression of GDF15 or GFRAL in tissue samples was assessed with a semiquantitative system. The IHC analysis was independently performed by a pathologist (Li AFY). SPSS software (version 20.0; SPSS Inc.) and R software (The R Project for Statistical Computing) were used to analyze the statistical calculations and undertake the Kaplan–Meier survival analysis.
2.4 Serum and culture medium GDF15 analyses
The GDF15 levels of available serum samples derived from gastric cancer patients (n = 97) and from cell culture medium (12 h and normalized by cell number) were analyzed using a GDF15 ELISA (ELH-GDF15; Raybiotech) according to the user manual.
2.5 Cell culture and reagents
AGS, NUGC-3, and TSGH9201 human gastric cancer cells and human embryonic kidney 293 T (HEK293T) cells were cultured in a 5% CO2 and 37°C incubator. The gastric cancer cells were cultured with a medium RPMI-1640 (Gibco) containing 10% FBS (Gibco) and 1% penicillin–streptomycin (Biological Industries). HEK293T cells were maintained in DMEM (Gibco) containing 10% FBS, 1% antibiotics, 1% nonessential amino acids, and 1% L-glutamine (Biological Industries). Cisplatin (P-4394), SSA, BSO, and NAC were obtained from Sigma-Aldrich (Merck). The GDF15 neutralizing Ab (MAB957) and recombinant human GDF15 (Escherichia coli-expressed) protein (#9279-GD-050) were obtained from R&D Systems. The purified mouse IgG2b κ isotype control Ab (400302) was obtained from BioLegend. SPP86 was purchased from Tocris Bioscience (Bio-Techne). SB-431542 was purchased from Cayman Chemical.
2.6 RNA extraction and quantitative real-time PCR
TRIzol reagent (Invitrogen) was used to extract cellular RNA. RNA (5 μg) was used for reverse transcription to cDNA with RevertAid reverse transcriptase (Invitrogen). The StepOne System (Thermo Fisher Scientific) and KAPA SYBR FAST (Roche, Merck) were used to undertake real-time PCR as previously reported.23 The oligonucleotide primer sequences were: GAPDH-f: 5′-CCGTCTAGAAAAACCTGCC-3′; GAPDH-r: 5′-GCCAAATTCGTTGTCATACC-3′; GDF15-f: 5′-GTGTTGCTGGTGCTCTCGTG-3′; GDF15-r: 5′-CGGTGTTCGAATCTTCCCAG-3′; xCT-f: 5′-TCATTGGAGCAGGAATCTTCA-3′; and xCT-r: 5′-TTCAGCATAAGACAAAGCTCCA-3′.
2.7 Western blot analysis
Protein samples were prepared using a radioimmunoprecipitation assay lysis buffer.23 Samples were further heated and analyzed with SDS-PAGE. After transferring and blocking, targeted proteins were detected with specific primary/secondary Abs and ECL reagents using GE Healthcare luminescence fluorescence image capture system (AI680). The MultiGauge image software (Fujifilm) was used for quantification. The PERK (sc-32577) Ab was purchased from Santa Cruz Biotechnology. The p-GCN2 (ab75836) and p-PKR (phospho T446, ab32036) Abs were purchased from Abcam. Eukaryotic initiation factor 2α (AHO0802), p-eIF2α (44-728G), GFRAL (PA5-24545), and α-tubulin (A11126) Abs were obtained from Invitrogen. Antibodies against GCN2 (#65981), PKR (#12297), PERK (#5683), xCT (#12691), Akt (#4685), p-Akt T308 (#2965), p-Akt S473 (#9271), STAT3 (#9139), p-STAT3 (#9145), ERK1/2 (#9101), and p-ERK1/2 (#9102) were purchased from Cell Signaling Technology. Heme-regulated eIF2α kinase Ab was purchased from MyBiosource (MBS2538144). Activating transcription factor 4 (10835-1-AP) Ab was obtained from Proteintech.
2.8 Small interfering RNA
The mixed siRNA solution was prepared according to a previous study.23 Mixed siRNA solutions (ON-TARGET plus SMART Pool human siRNAs: scramble siRNAs, D-001810-01-05; GDF15, L-019875-00-0005; GFRAL, L-032083-02-0005; PKR, L-003527-00-0005; HRI, L-005007-00-0005; and GCN2, L-005314-00-0005) were added to the cell culture dish for 48–72 h (Dharmacon, GE Healthcare).
2.9 Sulforhodamine B assay for cell growth curve and drug sensitivity
At the end of experiments, cells were fixed by trichloroacetic acid (Sigma-Aldrich), stained by sulforhodamine B (Sigma-Aldrich), dissolved with Tris Base (10 mM; J.T. Baker), and determined at optical density 510 nm with Sunrise absorbance microplate reader (Tecan) as described previously.23
2.10 Intracellular ROS and mitochondrial ROS levels
After treatment, cells were stained with DCF (Molecular Probes, Thermo Fisher Scientific) diacetate or MitoSOX Red (Molecular Probes) according to a previous study.23 The fluorescence intensity of DCF and MitoSOX Red was determined by FACSCalibur flow cytometry (Becton Dickinson). Cells (≥10,000) were evaluated by CellQuest software (Becton Dickinson).
2.11 Propidium iodide exclusion assay
After treatment, cells were harvested and stained with PI solution (Sigma-Aldrich). The PI fluorescence intensity was determined using a flow cytometer according to a previous study.25 Cells (≥10,000) were collected and the proportion of cells with PI exclusion was evaluated using CellQuest software.
2.12 Transwell migration assay
Cells were seeded onto Transwell inserts in a serum-free medium. Transwell inserts were incubated using a 24-well plate with serum-containing medium. The inserts were then fixed with methanol and visualized with Liu's A and B (Tonyar Biotech) according to a previous study.23 A total of four view fields in each group were analyzed under a microscope with 100–200× magnification and evaluated with ImageJ software (NIH).
2.13 Overexpression of GDF15
The plasmid mixture of pcDNA plasmid carrying the ORF of GDF15 (pcDNA-GDF15) or empty vector (pcDNA) and Lipofectamine 3000 transfection reagent (Invitrogen) was added to the cells with an antibiotic-free medium for 12 h according to the manufacturer's instructions. Subsequently, the cells were cultured with fresh medium for 48 h.
2.14 Reporter luciferase activity assay
The cells were transfected with a mixture of plasmid and Turbofect transfection reagent (Thermo Fisher Scientific). According to a previous study,19 enhanced green fluorescent protein was cotransfected to normalize the luciferase activity. The pGL3 vectors (Promega) carrying the different (WT, ARE-mutant, and AARE-mutant) xCT luciferase reporter promoters were constructed in a previous study.19 The reporter activity was analyzed using the Bright-Glo Luciferase Assay System (Promega) according to the manufacturer's instructions.
2.15 Glutathione level assay
Determination of reduced and total GSH levels was carried out by a GSH Colorimetric Assay Kit (ab239709; Abcam) according to a previous study.20
2.16 Statistical analyses
Data are presented as mean ± SEM. A p value of less than 0.05 was regarded as a significant difference. Statistical analysis was carried out with SigmaPlot (Systat) and graphs were plotted with Prism software (GraphPad).
3 RESULTS
3.1 GDF15 gene expression is elevated in gastric tumors and high serum GDF15 level is correlated with poor prognosis in gastric cancer patients
Using the GEPIA online database, we found that the gene expression level of GDF15 in tumor tissues was significantly higher than that in normal tissues of patients with gastric cancer (Figure 1A). In addition, the Kaplan–Meier plotter showed that high GDF15 gene expression had significantly lower OS and PFS rates in patients with gastric cancer than in those with low GDF15 gene expression (Figure 1B).
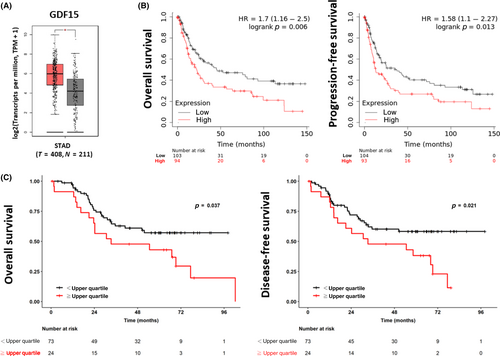
We further used IHC stain to determine the protein expression of GDF15 in gastric tumor tissues and found that GDF15 was highly expressed in male patients (Table 1). However, high GDF15 protein expression did not interfere with the OS or DFS rates (Table 1). To evaluate whether circulated GDF15 can predict the prognosis in gastric cancer, we determined the serum level of GDF15 using ELISA. The upper quartile of serum GDF15 level in this study was used to define the high and low levels. We found that high serum GDF15 levels were predominantly observed in male patients or those aged ≥65 years (Table 2). Low serum GDF15 levels were more frequently observed in gastric cancer patients with positive nodal involvement and increased depth of cancer invasion (Table 2). The gastric cancer patients with high serum GDF15 levels had lower OS and DFS rates compared to patients with low serum GDF15 levels (Figure 1C and Table 2). These results suggest that serum GDF15 levels might be a reliable prognostic marker for gastric cancer.
| Variable | Low expression (0, 1) | High expression (2, 3) | p value |
|---|---|---|---|
| (n = 49) | (n = 38) | ||
| Age (years) | |||
| <65 | 21 | 19 | 0.507 |
| ≧65 | 28 | 19 | |
| Sex | |||
| Male | 32 | 33 | 0.022* |
| Female | 17 | 5 | |
| Tumor size (cm) | |||
| <4 | 8 | 7 | 0.889 |
| 4–8 | 30 | 24 | |
| >8 | 11 | 7 | |
| Lesion location | |||
| Upper | 5 | 7 | 0.068 |
| Middle | 26 | 11 | |
| Lower | 18 | 18 | |
| Whole stomach | 0 | 2 | |
| Differentiated cell grade | |||
| Poor | 28 | 21 | 0.861 |
| Moderate | 21 | 17 | |
| Borrmann gross appearance | |||
| Superficial type | 4 | 1 | 0.546 |
| Type 1 and 2 | 17 | 14 | |
| Type 3 and 4 | 28 | 23 | |
| Type of stromal reaction | |||
| Medullary | 4 | 4 | 0.276 |
| Intermediate | 30 | 28 | |
| Scirrhous | 15 | 6 | |
| Histology of Lauren | |||
| Intestinal | 23 | 21 | 0.537 |
| Diffuse | 25 | 17 | |
| Mixed | 1 | 0 | |
| Ming's classification | |||
| Expanding | 7 | 4 | 0.601 |
| Infiltrating | 42 | 34 | |
| Nodal involvement | |||
| Negative | 10 | 10 | 0.516 |
| Positive | 39 | 28 | |
| Lymphovascular invasion | |||
| No | 14 | 12 | 0.761 |
| Yes | 35 | 26 | |
| Field of cancer invasion | |||
| Mucosa | 12 | 9 | 0.961 |
| Submucosa | 27 | 22 | |
| Proper muscle | 10 | 7 | |
| TNM stage | |||
| I | 6 | 6 | 0.846 |
| II | 15 | 10 | |
| III | 28 | 22 | |
| Chemotherapy | |||
| No | 24 | 22 | 0.409 |
| Yes | 25 | 16 | |
| 5-Year overall survival rate (%) | 53.1 | 54.3 | 0.617 |
| 5-Year disease-free survival rate (%) | 50.4 | 55.0 | 0.497 |
- Note: Immunohistochemistry (IHC) 0, <5%; IHC 1, 5%–25%; IHC 2, 26%–75%; IHC 3, >75% positive cells.
- * p < 0.05, statistically significant.
| Variable | Low GDF15 conc. | High GDF15 conc. | p-Value |
|---|---|---|---|
| (<upper quartile) | (≥upper quartile) | ||
| (n = 73) | (n = 24) | ||
| Age (years) | |||
| <65 | 42 | 4 | 0.001* |
| ≥65 | 31 | 20 | |
| Sex | |||
| Male | 51 | 22 | 0.032* |
| Female | 22 | 2 | |
| Tumor size (cm) | |||
| <4 | 12 | 5 | 0.500 |
| 4–8 | 49 | 13 | |
| >8 | 12 | 6 | |
| Lesion location | |||
| Upper | 16 | 4 | 0.410 |
| Middle | 33 | 8 | |
| Lower | 23 | 12 | |
| Whole stomach | 1 | 0 | |
| Differentiated cell grade | |||
| Poor | 44 | 11 | 0.215 |
| Moderate | 29 | 13 | |
| Borrmann gross appearance | |||
| Superficial type | 3 | 3 | 0.115 |
| Type 1 and 2 | 24 | 11 | |
| Type 3 and 4 | 46 | 10 | |
| Type of stromal reaction | |||
| Medullary | 5 | 4 | 0.057 |
| Intermediate | 46 | 18 | |
| Scirrhous | 22 | 2 | |
| Histology of Lauren | |||
| Intestinal | 34 | 14 | 0.541 |
| Diffuse | 38 | 10 | |
| Mixed | 1 | 0 | |
| Ming's classification | |||
| Expanding | 8 | 4 | 0.461 |
| Infiltrating | 65 | 20 | |
| Nodal involvement | |||
| Negative | 14 | 10 | 0.027* |
| Positive | 59 | 14 | |
| Lymphovascular invasion | |||
| No | 22 | 8 | 0.769 |
| Yes | 51 | 16 | |
| Field of cancer invasion | |||
| Mucosa | 14 | 10 | 0.028* |
| Submucosa | 43 | 11 | |
| Proper muscle | 16 | 2 | |
| Serosa | 0 | 1 | |
| TNM stage | |||
| I | 8 | 7 | 0.099 |
| II | 21 | 5 | |
| III | 44 | 12 | |
| Chemotherapy | |||
| No | 36 | 16 | 0.139 |
| Yes | 37 | 8 | |
| 5-Year overall survival rate (%) | 57.2 | 43.0 | 0.037* |
| 5-Year disease-free survival rate (%) | 58.2 | 38.3 | 0.021* |
- * p < 0.05, statistically significant.
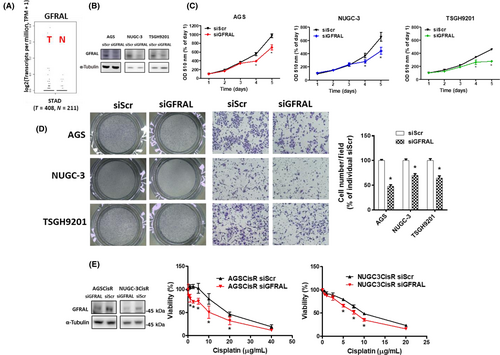
3.2 Expression of GDF15 is essential for cell proliferation and migration
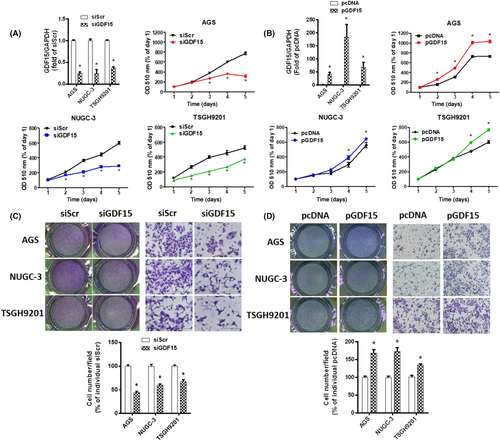
To clarify the role of GDF15 in gastric cancer, siRNA against GDF15 and pcDNA-GDF15 were used. The siGDF15 could ameliorate cell proliferation in human gastric cancer cells (Figure 2A). In contrast, GDF15 overexpression enhanced the cell growth (Figure 2B). Moreover, knockdown and overexpression of GDF15 repressed and increased cell migration, respectively (Figure 2C,D). These results suggest that GDF15 expression is essential for cell proliferation and migration in human gastric cancer cells.
3.3 Growth differentiation factor 15 is abundant in cisplatin-resistant gastric cancer cells and responsible for cisplatin resistance
To determine the GDF15-mediated chemoresistance, we compared the GDF15 expression between previously established CisR human gastric cancer cells19, 20 and their parental cells. The gene expression of GDF15 in CisR cells was significantly higher than that in parental cells (Figure 3A). Similarly, GDF15 protein expression and secreted GDF15 levels were significantly increased in CisR cells compared to parental cells (Figure 3B,C). Moreover, knockdown of GDF15 significantly decreased cisplatin resistance in CisR cells (Figure 3D). Using the PI exclusion assay, we further validated that the knockdown of GDF15 can increase cisplatin sensitivity in CisR cells (Figure 3E).
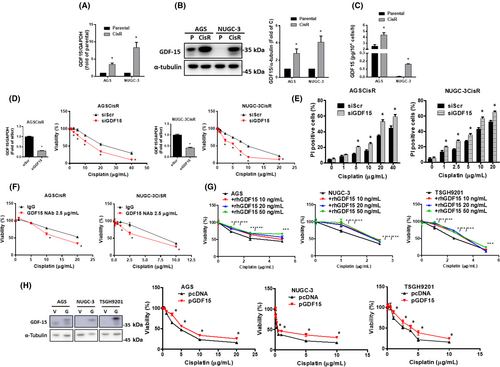
Based on the fact that GDF15 is a secreted growth factor that acts through autocrine or paracrine signaling, we treated CisR cells with GDF15 neutralizing Ab and found that it can increase cisplatin sensitivity (Figure 3F). In contrast, treatment with rhGDF15 reduced cisplatin sensitivity in parental cells (Figure 3G). Moreover, overexpression of GDF15 reduced cisplatin sensitivity in parental cells (Figure 3H). High GDF15 could decrease cisplatin sensitivity in human gastric cancer cells.
3.4 Growth differentiation factor 15 upregulated xCT expression through eIF2α-ATF4 signaling and increased GSH levels contribute to cisplatin resistance
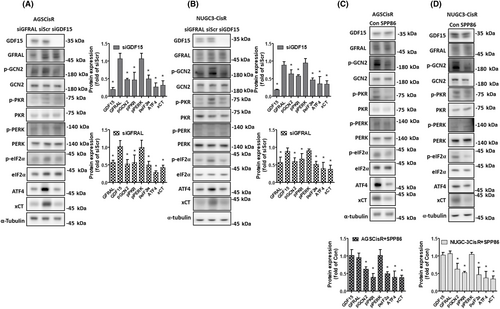
Intracellular GSH has been reported to inactivate cisplatin and contribute to resistance of cisplatin.18 We previously found that CisR human gastric cancer cells contained higher intracellular GSH levels than parental cells.19, 20 To evaluate whether GDF15 can modulate intracellular GSH levels, we determined the intracellular total and reduced form of GSH levels in CisR cells following GDF15 knockdown. Knockdown of GDF15 decreased the total and reduced GSH levels in CisR cells (Figure 4A,B). Moreover, consistent results were observed in CisR cells following GFRAL knockdown (Figure 4A,B). Knockdown of GDF15 significantly increased the intracellular and mitochondrial ROS levels in CisR cells under cisplatin treatment (Figure 4C,D). These results suggest that increased GDF15 in CisR cells can modulate intracellular GSH and ROS levels. We previously revealed that the cystine-uptake transporter xCT-increased intracellular GSH levels was crucial for cisplatin resistance in human gastric cancer cells.19, 20 Knockdown of GDF15 decreased the gene and protein expressions of xCT in CisR cells (Figure 4E,F). The elevated GDF15 might upregulate xCT and increase intracellular GSH levels, which downregulates cisplatin sensitivity in human gastric cancer cells.
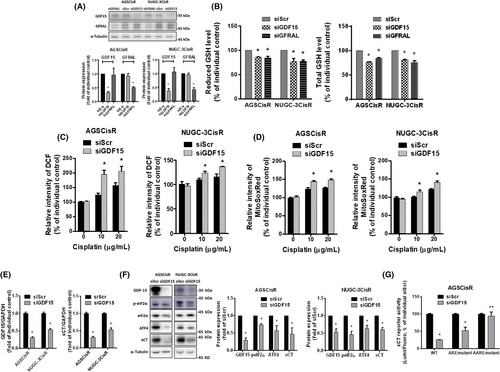
Transcription factors ATF4 and NF-E2-related factor 2 (Nrf2) can upregulate xCT gene expression.26 Figure 4F also shows that knockdown of GDF15 reduced ATF4 expression and the phosphorylation level of eIF2α in CisR cells. To determine the regulation of xCT, we transfected AGSCisR cells with pGL3 luciferase reporter vectors carrying the different types (WT, ARE-mutant, or AARE-mutant) of xCT promoters (Figure S1A). Knockdown of GDF15 significantly reduced the promoter activities of ARE-mutant and WT xCT but did not affect the activity of AARE-mutant xCT promoter (Figure 4G). These results suggest that GDF15 might upregulate xCT expression through the eIF2α-ATF4 pathway and increase intracellular GSH levels in CisR cells.
To validate the GDF15 on upregulation of eIF2α-ATF4-xCT and intracellular GSH levels, we transfected with pcDNA-GDF15 to overexpress GDF15 in parental cells. Overexpression of GDF15 increased the gene and protein expression of xCT (Figure 5A,B) and upregulated the eIF2α-ATF4 pathway (Figure 5B). Overexpression of GDF15 also significantly increased the activities of WT and ARE-mutant xCT promoters, but mildly increased the activity of AARE-mutant xCT promoter in HEK293T cells (Figure 5C). Additionally, SSA (an xCT inhibitor27) and BSO (a GSH synthesis inhibitor28) can reverse GDF15 overexpression-decreased cisplatin sensitivity (Figure 5D). These results indicate that GDF15 overexpression upregulates the eIF2α-ATF4-xCT pathway and increases intracellular GSH levels, which could downregulate cisplatin sensitivity in gastric cancer cells.

Growth differentiation factor 15 belongs to a novel TGF-β superfamily of cytokines29, 30 and triggers cellular response through the Smads pathway.31, 32 Some lines of evidence showed that GDF15 might contribute to chemo- or radioresistance through the Smads pathway.33-35 A previous study mentioned that recombinant GDF15 might be contaminated by TGF-β and the effect and signaling transduction of GDF15 must be interpreted cautiously.36 In our study, we found that both GDF15 overexpression and rhGDF15 could induce cisplatin resistance (Figure 3G,H). In addition, GDF15 neutralizing Ab might sensitize the cisplatin-resistant gastric cancer cells to cisplatin (Figure 3F). We further used SB-431542 (an inhibitor of the TGF-β family type 1 receptors/ALK5, ALK4, and ALK7 that preferentially inhibit Smad2/337) to dissect the role of the Smads pathway in the GDF15-mediated cisplatin resistance. However, the cisplatin sensitivity of the AGSCisR cells was not changed by SB-431542 (Figure S1B,C). These results suggest that the Smads pathway in GDF15-mediated cisplatin resistance might be minimal.
3.5 Growth differentiation factor 15-GFRAL-enhanced eIF2α-ATF4-xCT pathway and cisplatin resistance are mainly mediated by ROS-activated GCN2
Four eIF2 kinases (GCN2, HRI, PERK, and PKR) are responsible for the phosphorylation of eIF2α.38-41 To elucidate the upstream regulator of eIF2α in CisR cells, we determined whether siGDF15 and siGFRAL affected the phosphorylation levels of GCN2, PKR, and PERK. Knockdown of GDF15 or GFRAL decreased the phosphorylation levels of GCN2 and PKR, as well as the eIF2α phosphorylation and the expression of ATF4 and xCT in CisR cells (Figure 6A,B). Additionally, we used SPP86 (an inhibitor of RET, the GFRAL downstream target42, 43) to evaluate the GDF15-GFRAL-RET downstream regulation. Similar to thyroid cancer cell lines,42 the treated dose of SPP86 could effectively inhibit the RET-ERK and Akt signaling (Figure S1D). SPP86 could suppress the GCN2, PKR, and eIF2α phosphorylation and the ATF4 and xCT protein expressions in CisR cells (Figure 6C,D). Our results indicated that GCN2 and PKR could be crucial for the GDF15/GFRAL-mediated eIF2α-ATF4-xCT signaling in CisR cells.
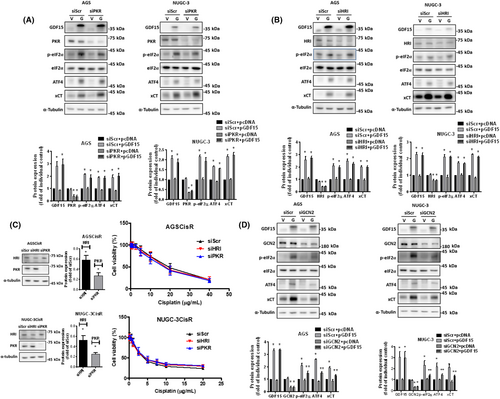
Although the levels of PKR phosphorylation can be downregulated in CisR cells after knockdown of GDF15 or GFRAL and treatment with SPP86 (Figure 6), knockdown of PKR did not reverse the GDF15 overexpression-activated eIF2α phosphorylation or protein expressions of ATF4 and xCT in parental cells (Figure 7A). Moreover, siRNA against HRI could not reverse the GDF15 overexpression-activated eIF2α phosphorylation or the ATF4 and xCT protein expressions (Figure 7B). Furthermore, knockdown of HRI and PKR did not sensitize CisR cells to cisplatin (Figure 7C). Importantly, the ATF4 and xCT protein expression and phosphorylation of eIF2α were suppressed by knockdown of GCN2 in GDF15-overexpressed cells (Figure 7D). Our result revealed that GDF15 activates GFRAL-GCN2-xCT signaling and decreases cisplatin sensitivity in human gastric cancer cells.
General control nonderepressible 2 could not only be activated under amino acid deprivation, but also be triggered by UV irradiation, ATP-competitive kinase inhibitors, and oxidative stress.19, 41, 44-47 Moreover, GDF15 overexpression might increase the oxidative stress.48, 49 In order to dissect the role of ROS in the GDF15-regulated GCN2, we assessed the intracellular ROS levels under GDF15 overexpression. We found that GDF15 overexpression might elevate the intracellular and mitochondrial ROS levels (Figure S1E,F). Furthermore, NAC (a ROS scavenger50) could mitigate the GDF15 overexpression-elevated GCN2 phosphorylation (Figure S1G). These results suggest that ROS might be a critical mediator for GCN2 activation under GDF15 overexpression.
3.6 Glial cell-derived neurotrophic factor family receptor a-like required for GDF15-mediated cancer progression of gastric cancer cells
As a GDF15 receptor, GFRAL is important for nutritional homeostasis.7-9 Although it has been identified in the nervous system and in some cancer cells, the exact role of GFRAL in gastric cancer remains unknown.51, 52 The GEPIA online database showed that the gene expression of GFRAL was not significantly different between the tumor and normal counterparts of gastric cancer patients (Figure 8A). To further validate the role of GFRAL in patients with gastric cancer, we determined the GFRAL protein expression with IHC stain and characterized their clinical importance. Table 3 shows that the GFRAL protein was highly expressed in malignant gross appearance, such as Borrmann type 3 and 4; however, GFRAL expression was downregulated in advanced malignant cell grade, worse Lauren's histology, and increased nodal involvement. In addition, gastric cancer patients with high GFRAL protein expression did not affect the clinical outcomes (Table 3).
| Variable | Low expression (0, 1) | High expression (2, 3) | p value |
|---|---|---|---|
| (n = 42) | (n = 50) | ||
| Age (years) | |||
| <65 | 20 | 21 | 0.589 |
| ≥65 | 22 | 29 | |
| Sex | |||
| Male | 31 | 38 | 0.809 |
| Female | 11 | 12 | |
| Tumor size (cm) | |||
| <4 | 6 | 8 | 0.484 |
| 4–8 | 24 | 33 | |
| >8 | 12 | 9 | |
| Lesion location | |||
| Upper | 8 | 8 | 0.200 |
| Middle | 19 | 18 | |
| Lower | 13 | 24 | |
| Whole stomach | 2 | 0 | |
| Differentiated cell grade | |||
| Poor | 29 | 22 | 0.016* |
| Moderate | 13 | 28 | |
| Borrmann gross appearance | |||
| Superficial type | 4 | 2 | 0.017* |
| Type 1 and 2 | 21 | 13 | |
| Type 3 and 4 | 17 | 35 | |
| Type of stromal reaction | |||
| Medullary | 5 | 4 | 0.688 |
| Intermediate | 26 | 35 | |
| Scirrhous | 11 | 11 | |
| Histology of Lauren | |||
| Intestinal | 14 | 33 | 0.006* |
| Diffuse | 27 | 17 | |
| Mixed | 1 | 0 | |
| Ming's classification | |||
| Expanding | 3 | 8 | 0.192 |
| Infiltrating | 39 | 42 | |
| Nodal involve | |||
| Negative | 4 | 17 | 0.005* |
| Positive | 38 | 33 | |
| Lymphovascular invasion | |||
| No | 9 | 8 | 0.192 |
| Yes | 33 | 42 | |
| Field of cancer invasion | |||
| Mucosa | 8 | 13 | 0.611 |
| Submucosa | 24 | 28 | |
| Proper muscle | 9 | 9 | |
| Serosa | 1 | 0 | |
| TNM stage | |||
| I | 2 | 10 | 0.086 |
| II | 14 | 12 | |
| III | 26 | 28 | |
| Chemotherapy | |||
| No | 19 | 29 | 0.222 |
| Yes | 23 | 21 | |
| 5-Year overall survival rate (%) | 53.2 | 51.1 | 0.952 |
| 5-Year disease-free survival rate (%) | 49.3 | 51.5 | 0.735 |
- Note: Immunohistochemistry (IHC) 0, <5%; IHC 1, 5%–25%; IHC 2, 26%–75%; IHC 3, >75% positive cells.
- * p < 0.05, statistically significant.
To characterize the importance of GFRAL, GFRAL siRNA was used in human gastric cancer cells. Similar to the effects of GDF15 knockdown, knockdown of GFRAL mitigated cell proliferation and decreased migration (Figures 8B–D). Importantly, knockdown of GFRAL sensitized CisR cells to cisplatin (Figure 8E). These results suggest that GDF15 and its receptor, GFRAL, are essential for gastric cancer malignant progression.
4 DISCUSSION
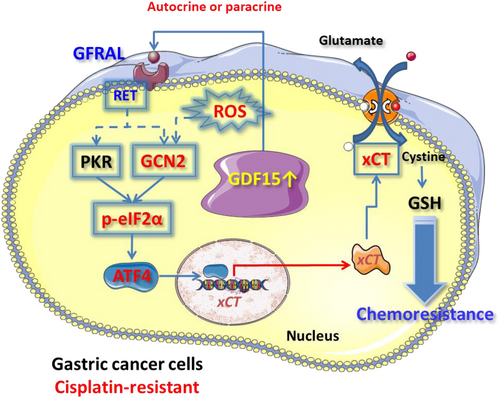
Our results suggest that serum GDF15 could be a reliable biomarker for gastric cancer progression. We further validated the GDF15-GFRAL axis in the malignant phenotype of gastric cancer cells. Moreover, GDF15 contributes to the reduction of cisplatin sensitivity in human gastric cancer cells through intracellular GSH-mediated inactivation of cisplatin. Our results revealed that GDF15 could enhance cisplatin resistance by activating the GFRAL-GCN2-eIF2α-ATF4-xCT pathway and intracellular GSH biosynthesis. In addition, ROS might be an important mediator between GDF15 and GCN2 (Figure 9).
Recent evidence showed that tumor grading is related to GDF15 and GFRAL expression, and Lauren's classification is related to GFRAL expression in gastric cancer patients.53 Inconsistently, we revealed that GDF15 and GFRAL protein expressions might not be good prognostic factors for patients with gastric cancer (Tables 1 and 3). We propose that the discordant results might originate from the characteristics of the patients with gastric cancer, which include earlier stage cancer patients receiving curative surgery, and limited patient numbers in our study. In the early stage, GDF15 might contribute to apoptosis and cell cycle arrest in cancer cells and antiangiogenesis.54 However, the level of GDF15 expression might be elevated with malignant transformation and this could be further increased by antitumor therapies.54 Additionally, GDF15 can be associated with cancer progression through other GDF15 systemic effects, such as the urokinase-type plasminogen activator system in the late stage.54, 55
A number of molecular mechanisms, such as Akt/glycogen synthase kinase-3β/β-catenin in hepatocellular carcinoma cells, transforming growth factor-β-Smad2/3-mediated epithelial–mesenchymal transition in colorectal cancer cells, Akt/cyclic AMP-responsive element-binding protein 1 in pancreas cancer cells, actin/focal adhesion kinase/transforming protein RhoA (rhoA)/early growth response protein 1/interleukin-6 in androgen-sensitive prostate cancer cells, actin/rhoA/Fascin/alpha-parvin in breast cancer cells, and particularly interesting new Cys-His protein 1/MMP13/rhoA in glioblastoma cells, for GDF15-mediated cancer progression had been proposed.56 In gastric cancer, GDF15 participates in the cancer progression of gastric cancer cells that overexpress HER2 through the HER2-AKT and ERK-1/2 pathway.57 Moreover, GDF15 might contribute to cancer progression of gastric cancer cells by inducing tumor cell invasion through the upregulation of the urokinase-type plasminogen activator through the ERK-1/2-dependent pathway.55 Furthermore, GDF15 might enhance cell proliferation, migration, and invasion through STAT3 activation in gastric cancer cells.58 In our experimental model, we also observed that GDF15 might activate the STAT3, ERK, and Akt pathways (Figure S1H). The GDF15-activated STAT3, ERK, and Akt pathways might be involved in cancer progression of gastric cancer cells.
High GDF15 expression might be used to predict the response to first-line chemotherapy in epithelial ovarian cancers.59, 60 Several GDF15-mediated chemoresistance mechanisms have been proposed, such as the PI3K/Akt pathway in colon cancer cells, the p27 pathway in ovarian cancer cells, p38 MAPK-dependent-GDF15 expression in pancreatic cancer cells, and the nuclear factor-κB-dependent pathway in epithelial ovarian cancer cells.35, 61-63 Growth differentiation factor 15-related microenvironment remodeling might contribute to chemoresistance through cancer-associated fibroblasts in acute myeloid leukemia cells and GDF15-producing tumor-associated macrophages in gastric/colorectal cancer cells.64-66 Our results reveal a new regulatory mechanism by which GDF15 enhances cisplatin resistance through xCT-elevated intracellular GSH levels. The regulatory mechanism could be a druggable target against chemoresistant gastric cancer.
The linkage between GDF15-GFRAL and GCN2 signaling is not fully understood. We proposed that ROS might play an important role in the GDF15-mediated GCN2 activation (Figure S1E–G). RET is a tyrosine kinase, and its activation results in dimerization of RET receptors and autophosphorylation of several tyrosine residues of the intracellular region.67 The phosphorylation of the intracellular domain of RET could activate subsequent downstream signaling pathways such as Src homology phosphotyrosyl phosphatase 2, STAT3, phosphoinositide phospholipase Cγ, Src, PI3K/Akt, RAS/ERK, c-Jun N-terminal kinases, and MAPK.68 In human hepatoma cells, the MAPK/ERK kinase-ERK pathway is essential for activation of the GCN2-eIF2α under histidine deprivation.69 The inhibition of the MAPK/ERK kinase-ERK pathway by PD98059 or U0126 could reverse the elevated eIF2α-ATF4 pathway by histidine deprivation. Another study also reported that ERK2 regulates the GCN2/eIF2α/ATF4 pathway and activates the death machinery under severe metabolism stress.70 Moreover, Figure S1H shows that GDF15 might activate the ERK pathway. Therefore, the ERK pathway might be a potential mechanism linking between RET and GCN2. The detailed mechanism underlying GDF15 activates GCN2 warrants further study.
Elevated intracellular GSH not only increases treatment resistance but also promotes tumor progression.71 Cysteine is essential for GSH synthesis and can be taken up into cells through system xc-, which is composed of xCT and 4F2 heavy chains.72 The xCT has been implicated in cancer progression, such as the fact that some specific cancer types might be reliable for xCT, eliciting the progression of cancer stem cells, and cross-talk between xCT and tumor immunity.73 Hence, xCT inhibitors could be a potential therapeutic strategy against cancer.74 Our findings suggest that xCT inhibitors might be beneficial for treating patients with gastric cancer with high GDF15 expression. In our previous study, impaired mitochondrial function-mediated ROS was shown to decrease cisplatin sensitivity through eIF2α-ATF4 signaling in human gastric cancer cells.19 Moreover, ATF4 is essential for necroptosis and ferroptosis under cystine deprivation.75 Recently, decreased GDF15 levels were shown to enhance erastin-induced ferroptosis through downregulation of xCT expression.76 Herein, our findings further suggest that GDF15-mediated xCT and cisplatin resistance are mainly regulated by GCN2-eIF2α-ATF4. In conclusion, targeting GDF15-GFRAL-GCN2-eIF2α-ATF4-xCT signaling could be a promising strategy for the treatment of cancer.
AUTHOR CONTRIBUTIONS
Conceptualization: S.F. Wang, K.H. Huang, and H.C. Lee. Methodology: Anna F.Y. Li. Validation: K.H. Huang. Formal analysis: S.F. Wang, T.Y. Liu, and Anna F.Y. Li. Resources: W.L. Fang, C.F. Chen, T.S. Yeh, G.Y. Hung, K.H. Huang, and H.C. Lee. Data curation: S.F. Wang and K.H. Huang. Original draft: S.F. Wang. Edited: H.C. Lee. Visualization: S.F. Wang and H.C. Lee. Supervised: Y.L. Chang and H.C. Lee. Administrated project: S.F. Wang and K.H. Huang. Funding: S.F. Wang.
ACKNOWLEDGMENTS
We thank Shu-Hui Li, Chih-Yu Tseng, and Ting-Yu Liu for their technical assistance.
FUNDING INFORMATION
This study was granted by Taipei Veterans General Hospital, Taipei, Taiwan (V111B-007 and V112C-047), National Science and Technology Council, Taiwan (110-2320-B-075-007), and the Melissa Lee Cancer Foundation (MLCF-V111_A11103, MLCF-V111_B11106, and MLCF_V112_A11206).
ETHICS STATEMENTS
Approval of the research protocol by an institutional review board: The Institutional Review Board, Taipei Veterans General Hospital approved the current study (TPEVGH IRB No. 2021-01-006BC).
Informed consent: Patient informed consent was obtained before surgery.
Registry and registration no. of the study/trial: N/A.
Animal studies: N/A.
CONFLICT OF INTEREST STATEMENT
The authors have no conflict of interest.




