Immunostimulatory oncolytic activity of coxsackievirus A11 in human malignant pleural mesothelioma
Abstract
Malignant pleural mesothelioma (MPM) is an aggressive solid cancer with a poor prognosis, whereas coxsackievirus A11 (CVA11) is a potential oncolytic virus for cancer treatment. We here investigated the oncolytic activity of CVA11 with human MPM cell lines. CVA11 infection was cytotoxic in all six MPM cell lines examined and showed no or minimal cytotoxicity toward normal human normal cell lines. MPM cells with a higher surface level of intercellular adhesion molecule-1 (ICAM-1) expression tended to be more susceptible to CVA11-induced cytotoxicity, and a neutralizing antibody to ICAM-1 attenuated such cytotoxicity. CVA11 infection activated signaling by Akt and extracellular signal-regulated kinase (ERK) pathways, and inhibitors of such signaling also abrogated CVA11-mediated cytotoxicity. Furthermore, CVA11 infection-triggered multiple modes of tumor cell death including apoptosis, pyroptosis, and necroptosis, and such death was accompanied by the release or exposure of the proinflammatory cytokine interleukin-1β and damage-associated molecular patterns such as calreticulin, high-mobility group box-1, annexin A1, and heat shock protein 70, which are hallmarks of immunogenic cell death. Notably, in vivo treatment of human MPM xenografts with intratumoral CVA11 injection resulted in significant suppression of tumor growth in SCID mice, and all mice infected with CVA11 showed no significant change in body weight. Our findings collectively suggest that the oncolytic activity of CVA11 for MPM is dependent on ICAM-1 as a virus receptor, as well as on Akt and ERK signaling, and that oncolytic virotherapy with CVA11 is a promising treatment modality with immunostimulatory activity for human MPM.
Abbreviations
-
- CRT
-
- calreticulin
-
- CVA11
-
- coxsackievirus A11
-
- DAF
-
- decay-accelerating factor
-
- DAMP
-
- damage-associated molecular pattern
-
- HMGB1
-
- high-mobility group box 1
-
- HSP70
-
- heat shock protein 70
-
- ICAM-1
-
- intercellular adhesion molecule-1
-
- IL
-
- interleukin
-
- MLKL
-
- mixed lineage kinase domain like
-
- MPM
-
- malignant pleural mesothelioma
-
- PARP
-
- poly(ADP-ribose) polymerase
-
- PD-1
-
- programmed cell death-1
-
- PD-L1
-
- programmed cell death-ligand 1
-
- PD-L2
-
- programmed cell death-ligand 2
-
- RIPK1
-
- receptor-interacting protein kinase 1
1 INTRODUCTION
Malignant pleural mesothelioma is an aggressive cancer with a dismal prognosis. The incidence of the disease appears to have peaked in Europe and the USA but is expected to increase in developing countries.1 Surgery, radiation therapy, and chemotherapy have been the mainstay of treatment for MPM in the past and, recently, with the advent of immune checkpoint inhibitors (ICIs), the efficacy of immunotherapy has been proven.2, 3 As standard therapy, first-line treatment with nivolumab and ipilimumab and second- and later-line treatment with nivolumab monotherapy were approved in Japan in 2021. However, these available treatments for MPM have had limited efficacy, with there thus being an urgent need for new therapeutic strategies. Oncolytic virotherapy is a rapidly emerging therapeutic approach, as evidenced by the recent approval and initial success of talimogene laherparepvec (T-VEC) for the treatment of individuals with advanced melanoma.4 Oncolytic viruses represent a new class of therapeutic agents with a dual mechanism of action dependent on their preferential replication in, and consequent direct killing of, malignant cells, as well as the induction of secondary systemic antitumor immunity.
Using RNA viruses as oncolytic agents appears to be safer than using DNA viruses because most single-stranded RNA viruses replicate in the cytoplasm of host cells and lack the potential genotoxicity associated with integration of the viral genome into host DNA.5 In particular, enteroviruses, a diverse group of small RNA viruses belonging to the Picornaviridae family, have been proposed as candidates for oncolytic virotherapy that induce immunogenic cell death (ICD) of host cells as evidenced by the release or exposure of DAMPs.6, 7 Therapeutic advantages to using enteroviruses include their rapid induction of cytolytic changes, the absence of oncogenes from their genomes, and the relative ease with which they can be manipulated by reverse genetics approaches. Importantly, most enteroviruses other than poliovirus are prevalent and usually associated with asymptomatic infections or mild disease.8
We previously showed that the Enterovirus strain of CVA11 exerts marked oncolytic activity in human colon cancer cell lines both in vitro and in vivo, without inducing body weight loss or mortality in mice.7 As far as we have searched, there has been no previous report on whether CVA11 is cytotoxic to human normal cells, and there has been no evidence of serious disease caused by CVA11 infection, with aseptic meningitis being the most common but relatively rare condition associated with such infection,9 suggestive of a low pathogenicity of CVA11. To develop novel virotherapy for MPM with an improved safety profile, we here investigated the oncolytic activity of CVA11 with a panel of human MPM cell lines, characterized the modalities of cell death elicited by CVA11 infection, and examined the potential effect of the virus on tumor immunogenicity.
2 MATERIALS AND METHODS
2.1 Mice
Four-week-old female CB-17/Icr-scid/scidJcl mice were purchased from CLEA Japan, Inc. All animal experiments (approval number: A22-213) were carried out under the Kyushu University Guidelines for Animal Experiments and the Japanese government's Law 105 Notification 6.
2.2 Cell lines
Human MPM cell lines H2052, H2452, and MSTO, the human normal mesothelial cell line MET-5A, and human non–small-cell lung cancer cell line H1299 were obtained from American Type Culture Collection. The human MPM cell lines ACC-MESO-1, ACC-MESO-4,10 and HMMME were obtained from the RIKEN Cell Bank. The normal human epidermal keratinocyte cell line HaCaT was kindly provided by T. Yokomizo (Department of Molecular and Cellular Biochemistry, Faculty of Medicine, Juntendo University, Japan). All cell lines were expanded for at least one passage before storage at −80°C until use. Thawed HMMME, HaCaT, MeT-5A, and the other cells were resuspended in Ham's F-12 medium, DMEM, Tissue Culture Medium-199, and RPMI-1640 medium, respectively. Ham's F-12 medium and the other media were supplemented with 15% and 10% FBS, respectively. All media were supplemented with 1% penicillin–streptomycin. All cell lines were confirmed to be free of Mycoplasma contamination using a MycoAlert Mycoplasma Detection Kit (Lonza).
2.3 Production of Enterovirus
CVA11 (prototype Belgium-1 strain) was kindly provided by K. Tani (Laboratory of ALA Advanced Medical Research, Institute for Quantitative Biosciences, The University of Tokyo) and was propagated in H1299 cells. The median tissue culture infectious dose (TCID50) per milliliter was determined using a HeLa cell monolayer, as previously described.11
2.4 Crystal violet staining
Cells cultured in 24-well plates were gently washed twice with PBS, fixed for 10 min with 0.5% glutaraldehyde in PBS, washed again with PBS, and stained with 0.1% crystal violet (Sigma-Aldrich) in a solution of 2% ethanol in distilled water. The cells were then washed twice with distilled water, air dried, and photographed under a BZ-X800 digital microscope (Keyence). The area of staining in each culture well was quantitated using BZ-H4A software (Keyence).
2.5 Flow cytometric analysis
Cells were stained with phycoerythrin-conjugated antibodies to human DAF (BD Biosciences, Franklin Lakes, NJ), fluorescein isothiocyanate (FITC)-conjugated antibodies to human ICAM-1 (Biolegend), allophycocyanin-conjugated antibodies to human CD274 (B7-H1, PD-L1; Biolegend), phycoerythrin (PE)-conjugated antibodies to human CD273 (B7-DC, PD-L2; Biolegend), primary antibodies to human calreticulin (Abcam), or Alexa Fluor 647-conjugated secondary antibodies (Abcam) for examination of surface expression of the corresponding antigens. For detection of apoptosis, cells were stained with FITC-labeled annexin V (Biolegend) at 0.45 μg/ml and with propidium iodide (PI) (Biolegend) at 5 μg/ml.12 Data were obtained with a FACSverse instrument (BD Biosciences), and analyzed using FlowJo software version 10.5.3 (FlowJo, LLC).
2.6 MTS assay of cell viability
The MTS assay was performed using a CellTiter 96 AQueous One Solution Cell Proliferation Assay Kit (Promega).
2.7 Inhibitor treatment
Cells were exposed for 1 h before CVA11 infection to the PI3K inhibitor LY294002 (Santa Cruz Biotechnology), the MEK inhibitor PD0325901 (Wako), the pan-caspase inhibitor Z-VAD-FMK (R&D Systems), the caspase-3 inhibitor Z-DEVD-FMK (Selleck, Houston, TX), the caspase-8 inhibitor Z-IETD-FMK (Selleck), the caspase-1 inhibitor Ac-YVAD-CMK (Bachem), the caspase-12 inhibitor Z-ATAD-FMK (R&D Systems), or the RIPK1 inhibitor necrostatin-1 (Chemscene).
2.8 Neutralization
Cells were exposed for 1 h before CVA11 infection to anti-human CD54 monoclonal antibody clone HCD54 (Biolegend).
2.9 ELISAs
The concentrations of HMGB1, HSP70, annexin A1, and IL-1β in culture supernatants were measured using an HMGB1 ELISA Kit Exp (Shino-Test), an HSP70 ELISA Kit (Proteintech), a Human Annexin A1 ELISA Kit (Abcam), and a Human IL-1β ELISA Kit (Abcam), respectively.
2.10 Immunoblot analysis
Cell lysates were fractionated using SDS-PAGE and subjected to immunoblot analysis, as described previously.13 Primary antibodies included those to S473-phosphorylated Akt, to Akt, to T202/Y204-phosphorylated ERK1/2, to ERK1/2, to S358-phosphorylated MLKL, to MLKL, to cleaved caspase-3, to PARP, and to β-actin (all from Cell Signaling Technology).
2.11 Statistical analysis
Statistical analysis was performed using GraphPad Prism 8.4.3 software (GraphPad Software). Data are presented as means + SD values as indicated and were compared with the unpaired t-test. A p-value of <0.05 was considered statistically significant. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
3 RESULTS
3.1 Cytotoxicity of CVA11 infection in human MPM cell lines
To evaluate the potential oncolytic activity of CVA11 infection for MPM, we exposed various human MPM cell lines, a normal human mesothelial cell line (MeT-5A), and a normal human skin epidermal keratinocyte cell line (HaCaT) to CVA11 at a multiplicity of infection (MOI) of 0.1, 1, or 10 and measured cell viability after 72 h by staining with crystal violet. Infection with CVA11 manifested MOI-dependent oncolytic activity in all six MPM cell lines examined, whereas cytotoxicity was less in MeT-5A cells and absent in HaCaT cells (Figure 1A; Figure S1A). Quantitative analysis with the MTS cell viability assay confirmed, for entry of CVA11, the oncolytic effect of CVA11 infection in all six cell lines, and proved that CVA11 had less or no cytotoxicity compared with normal cell lines (Figure 1B; Figure S1B).
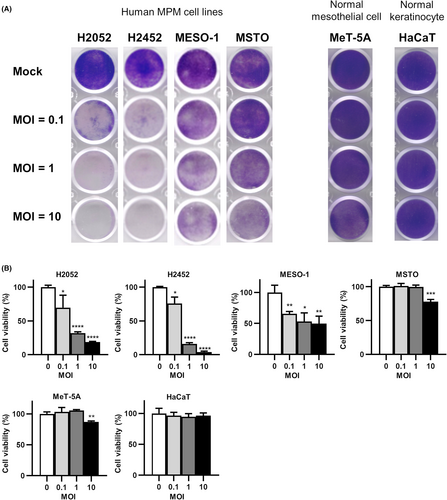
3.2 ICAM-1 as a putative cell surface receptor for CVA11 infection in human MPM cell lines
The receptor for entry of CVA11 into host cells has not been identified definitively. We investigated the relationship between the surface expression level of intercellular adhesion molecule-1 (ICAM-1) or DAF, both of which are candidate receptors for CVA11 infection,14, 15 and CVA11-mediated cytotoxicity in human MPM cell lines. The surface expression levels of ICAM-1 and DAF varied among the six human MPM cell lines examined (Figure 2A; Figure S2A). We found that cell lines with a higher ICAM-1 level tended to be more susceptible to CVA11-mediated cytotoxicity (Table 1). Furthermore, crystal violet staining revealed that neutralizing antibodies to human ICAM-1 markedly attenuated CVA11-dependent cytotoxicity in all six MPM cell lines (Figure 2B; Figure S2B). Together, these results thus implicated ICAM-1 as a primary receptor for CVA11 entry into host cells.
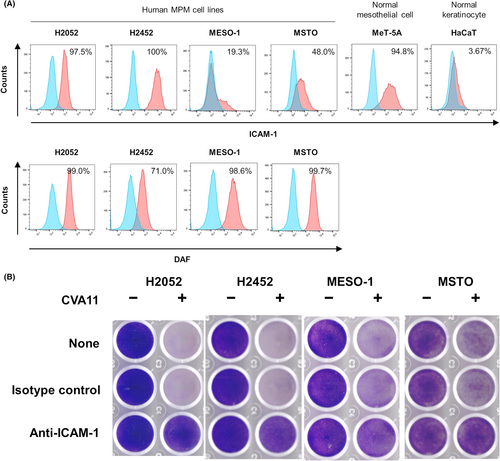
| Cell line | CVA11-mediated cytotoxicity | Surface expression (%) | |||
|---|---|---|---|---|---|
| MOI = 0.1 | MOI = 1 | MOI = 10 | ICAM-1 | DAF | |
| H2052 | ++ | +++ | +++ | 97.5 | 99.0 |
| H2452 | − | +++ | +++ | 100 | 71.0 |
| MESO-1 | + | ++ | ++ | 19.3 | 98.6 |
| MSTO | − | − | + | 48.0 | 99.7 |
- Note: CVA11-mediated cytotoxicity was determined 3 days post-infection by crystal violet staining and was classified as − (0%–25% decrease in staining intensity), + (26%–50%), ++ (51%–75%), or +++ (76%–100%). The surface expression of ICAM-1 and DAF was quantified by flow cytometry for the same batches of cells assessed for CVA11 cytotoxicity.
3.3 Contribution of MEK/ERK and PI3K/Akt signaling pathways to CVA11-mediated cytotoxicity in human MPM cell lines
The replication and cytotoxicity of enteroviruses are dependent on the ERK kinase (MEK)/extracellular signal-regulated kinase (ERK) and phosphatidylinositol 3-kinase (PI3K)/Akt signaling pathways.16-18 We therefore investigated whether CVA11 infection induced the phosphorylation (activation) of ERK and Akt in human MPM cell lines. CVA11 infection induced marked phosphorylation of ERK and Akt in all four MPM cell lines examined (Figure 3A). We next examined the effects of the MEK inhibitor PD0325901 and the PI3K inhibitor LY294002 on CVA11-mediated cytotoxicity in H2052 cells. Both PD0325901 and LY294002 significantly inhibited the cytotoxicity of CVA11 infection in these cells and also reduced the viral titer of CVA11 released from H2052 cells at 36 h post-infection (Figure 3B; Figure S3), revealing that activation of both MEK/ERK and PI3K/Akt pathways was associated with replication of CVA11 in these MPM cells.
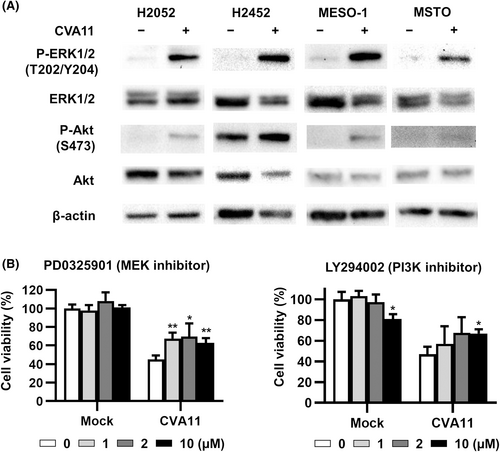
3.4 Contribution of apoptosis, pyroptosis, and necroptosis to CVA11-mediated cytotoxicity in human MPM cell lines
Oncolytic viruses have been found to kill cancer cells through the induction of various types of cell death, including apoptosis, necroptosis, and pyroptosis.19 We evaluated whether CVA11 infection might induce these modes of cell death in human MPM cell lines. Flow cytometric analysis revealed that all three human MPM cell lines examined manifested a substantial proportion of both early apoptotic (annexin V+PI−) and late apoptotic (annexin V+PI+) cells after infection with CVA11 (MOI = 10) (Figure 4A). In addition, immunoblot analysis showed that the abundance of cleaved forms of caspase-3 and PARP, both of which are hallmarks of apoptosis, was increased in CVA11-infected H2052 cells (Figure 4B). Furthermore, an enzyme-linked immunosorbent assay (ELISA) showed that infection of human MPM cells with CVA11 induced a significant increase in the extracellular level of the proinflammatory cytokine interleukin (IL)-1β, a hallmark of pyroptosis (Figure 4C).
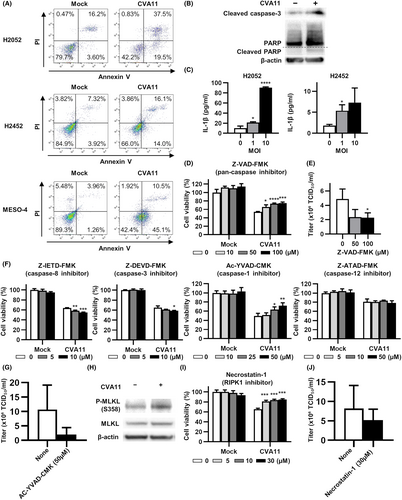
We next evaluated the effect of Z-VAD-FMK, a pan-caspase inhibitor, on CVA11-mediated cytotoxicity in H2052 cells. Z-VAD-FMK attenuated the cytotoxicity of CVA11 in a concentration-dependent manner, reducing the viral titer of CVA11 released into the culture supernatant (Figure 4D,E). To identify caspases that contributed to CVA11-mediated cytotoxicity, we further evaluated the effects of the caspase-3 inhibitor Z-DEVD-FMK and the caspase-8 inhibitor Z-IETD-FMK, both of which prevent apoptosis; the caspase-1 inhibitor Ac-YVAD-CMK, which prevents pyroptosis; and the caspase-12 inhibitor Z-ATAD-FMK, which prevents endoplasmic reticulum (ER) stress-induced apoptosis. Among these agents, only the caspase-1 inhibitor Ac-YVAD-CMK attenuated CVA11-mediated cytotoxicity in a concentration-dependent manner, and reduced the titer of CVA11 released into the culture supernatant (Figure 4F,G).
To evaluate whether CVA11 infection also induced necroptosis in human MPM cells, we examined the possible effect of such infection on the phosphorylation of MLKL in H2052 cells. CVA11 infection indeed induced MLKL phosphorylation in these cells (Figure 4H). We therefore assessed the effect of necrostatin-1, a RIPK1 inhibitor that prevents necroptosis, on CVA11-mediated cytotoxicity. Necrostatin-1 attenuated the cytotoxicity of CVA11 infection in H2052 cells with a reduction in the CVA11 titer in the culture supernatant (Figure 4I,J). Together, these various data thus indicated that CVA11 induces multiple modes of cell death, including apoptosis, pyroptosis, and necroptosis in human MPM cells.
3.5 CVA11 infection elicits ICD as evidenced by DAMP induction in human MPM cell lines
ICD is a type of regulated cell death in tumor cells that activates secondary adaptive immune responses specific for tumor-associated antigens.20 ICD has been defined by exposure on the cell surface or the release of a group of molecules known as DAMPs and include calreticulin, HMGB1, annexin A1, and HSP70. These molecules are components of the innate immune response and are exposed on, or released from, damaged or dying cells due to trauma or infection with a pathogen.21-23 We investigated whether CVA11 infection induces ICD in MPM cells. Flow cytometry showed that infection of human MPM cell lines with CVA11 (MOI = 10) induced marked exposure of calreticulin on the cell surface (Figure 5A). Furthermore, ELISAs revealed that CVA11 infection induced significant release of HMGB1, annexin A1, or HSP70 in an MOI-dependent manner from all five human MPM cell lines examined (Figures 5B–D).
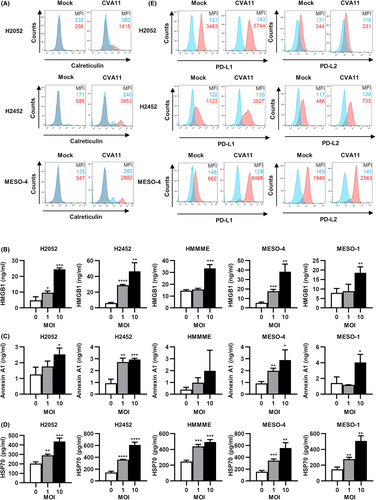
Programmed cell death-ligand 1 has been implicated in the suppression of the adaptive immune system.24 We therefore investigated the effect of CVA11 infection on the surface expression of PD-L1 and the related molecule PD-L2 in human MPM cell lines. Infection with CVA11 resulted in a marked increase in surface expression of PD-L1 but not of PD-L2 in H2052, H2452, or MESO-4 cells (Figure 5E).
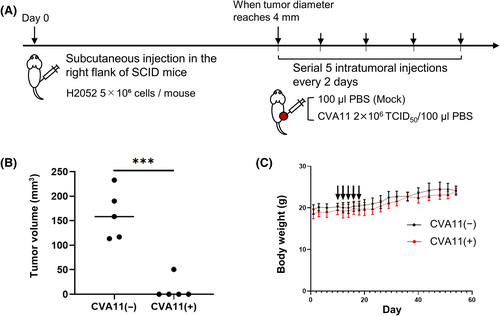
3.6 Serial intratumoral administrations of CVA11 result in robust tumor regression in xenograft models of human MPM cells
We determined the in vivo oncolytic effect and tolerability of five consecutive intratumoral CVA11 injections on severe combined immunodeficiency (SCID) mice bearing pre-established subcutaneous H2052 MPM xenografts (4 mm in diameter) (Figure 6A). The mice treated with consecutive injections of CVA11 showed a significant regression in tumor volume compared with the untreated mice on day 54 (p < 0.001; Figure 6B), and we observed complete tumor regression in four of the five mice (80%) treated with CVA11, whereas none of the untreated mice rejected the tumor. In addition, none of the mice treated with CVA11 showed significant weight loss (Figure 6C), nor died from side effects.
4 DISCUSSION
We have here revealed robust cytotoxicity of CVA11 infection in numerous human MPM cell lines, even at an MOI as low as 0.1, suggesting that tumor cells of mesothelial origin are highly susceptible to such infection. Furthermore, we found that intratumoral CVA11 administration into the subcutaneous xenografts elicited significant suppression of tumor growth without serious side effects in the CVA11-treated mice. The reason for the marked in vivo antitumor activity of CVA11 in which 80% of mice manifested complete regression could stem from the abundant expression of ICAM-1 in H2052 cells, suggesting that expression levels of ICAM-1 in tumors might be a predictive biomarker for efficacy of CVA11 treatment.
Coxsackieviruses of the same Enterovirus cluster as CVA11 have been found to target ICAM-1 as their primary receptor for attachment and internalization, as well as DAF as a coreceptor for a secondary site of virus attachment.25 Our results now suggest that ICAM-1 might also serve as a primary receptor for CVA11 entry into cancer cells, with neutralizing antibodies to human ICAM-1 being shown to inhibit CVA11-mediated cytotoxicity in human MPM cells. Among the human MPM cell lines we used, MESO-1 and MSTO are non-epithelial, and the others are epithelial according to the product information of the suppliers. Figure 1 and Figure S1 show that CVA11 infection tends to be less cytotoxic to normal human cells and non-epithelial human cancer cells, whereas it shows high cytotoxicity in epithelial cancer cells. The different sensitivity between human epithelial and non-epithelial mesothelioma cell lines to CVA11 infection may be partially explained by the ICAM-1 expression levels on cellular membranes (Figure 2; Figure S2). ICAM-1 has previously been implicated in the progression, prognosis, and metastasis of solid tumors26-28 as well as been shown to express at a high level on human malignant mesothelioma cells.29 These various observations provide a plausible basis for the use of CVA11 treatment of individuals with advanced MPM expressing ICAM-1 at a high level.
The MEK/ERK and PI3K/Akt signaling pathways play key roles in the regulation of cell proliferation, growth, differentiation, and survival.30, 31 These pathways are activated in many solid tumors, including MPM.32-34 We have now found that CVA11 infection increased the activity of both ERK and Akt and that inhibitors of MEK and PI3K attenuated CVA11-mediated cytotoxicity in human MPM cells. These inhibitors also attenuated the release of CVA11 virions from infected MPM cells, highlighting an interplay of CVA11 replication with cellular components that might be exploited to improve the antitumor efficacy of CVA11 infection. Furthermore, the systemic administration of MEK or PI3K inhibitors might block undesired CVA11 replication and thereby mitigate adverse events in the clinical setting.
We found that CVA11 infection induced the death of human MPM cells via apoptosis, pyroptosis, and necroptosis. A caspase-1 inhibitor was proven to significantly attenuate CVA11-mediated cytotoxicity and viral replication, whereas inhibitors of caspases 3, 8, or 12 had no such effects. Caspase-1 was previously shown to mediate not only pyroptotic, but also apoptotic, cell death.35 Our results thus suggest that caspase-1 activity is a key mediator of CVA11-dependent cytotoxicity and viral replication in human MPM cells, and that release of the proinflammatory cytokine IL-1β associated with such cytotoxicity might promote innate cellular immune responses to MPM tumors. We also found that CVA11 infection increased the phosphorylation of MLKL and that a RIPK1 inhibitor significantly attenuated CVA11-mediated cytotoxicity and viral replication, implicating necroptosis in the CVA11-induced death of human MPM cells. Treatment with inhibitors of caspase-3 or caspase-8 actually enhanced CVA11-mediated cytotoxicity in human MPM cells. Given that inhibition of caspase-3 or caspase-8 has previously been shown to abrogate apoptosis but to promote necroptosis,36, 37 our results might be explained by the promotion of necroptosis by the caspase-3 and caspase-8 inhibitors in human MPM cells. The simultaneous induction of apoptosis, pyroptosis, and necroptosis in MPM cells may prevent the development of resistance to CVA11 infection due to the acquisition of mutations that block any one pathway,38 leading to robust inflammatory cell death and activation of the immune system. Indeed, the induction of apoptosis, pyroptosis, or necroptosis in cancer cells has attracted recent attention as a potential new treatment strategy for cancer.39-41 The induction of multiple modes of cancer cell death accompanied by proinflammatory cytokine release thus makes CVA11 a promising oncolytic agent capable of eradicating tumor cells resistant to standard anticancer treatments.
In addition to direct cytolysis, another important component of the sustained therapeutic effect of oncolytic viruses is their ability to elicit secondary antitumor immune responses.42, 43 Such secondary antitumor immunity is likely to depend on both the quality and quantity of inflammatory DAMPs induced within targeted tumors.16 It is therefore important to determine whether oncolytic viruses can elicit ICD.44 We found that CVA11 infection induced immunogenic changes, including calreticulin exposure and the release of HMGB1, annexin A1, and HSP70 in human MPM cells. These immunostimulatory properties of CVA11 may prime the generation of adaptive immunity that synergizes with its direct oncolytic activity.
A high level of PD-L1 expression in tumors has been associated with a better clinical outcome of PD-1 blockade therapy.45, 46 The abundance of PD-L1 at the tumor cell surface has also been found to be increased by infection with oncolytic viruses,47-49 and combination therapy with an oncolytic virus and an immune checkpoint inhibitor has proven to be effective for human head and neck squamous cell carcinoma.50 We have now shown that CVA11 infection upregulated the surface expression of PD-L1 in human MPM cell lines, suggesting that CVA11 can remodel the tumor microenvironment and that the combination of CVA11 treatment with antibodies to PD-1 or to PD-L1 might overcome the resistance to immune checkpoint therapy and promote the recruitment of cytotoxic T cells specific for tumor-associated antigens to tumor beds.
With regard to safety, clinical data have revealed that CVA11 is rarely detected in the cerebrospinal fluid of infected adults or children,51 suggesting that it can be considered a nonpathogenic virus. Indeed, as we previously showed with human colon cancer cell xenografts in nude mice,7 serial intratumoral administration of CVA11 into human MPM xenografts in SCID mice did not demonstrate loss of body weight, indicative of its high tolerability. However, further preclinical studies, including biochemical and pathological analyses in mice and nonhuman primates, are needed to confirm the safety of CVA11.
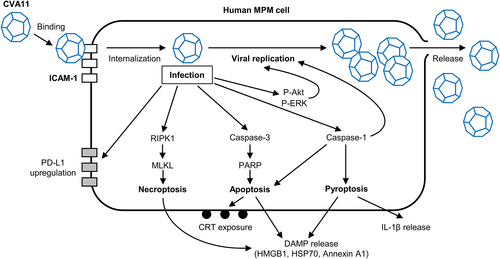
We propose a model for the life cycle of CVA11 and its relationship to the killing of human MPM cells by the induction of various types of regulated cell death and the associated exposure or release of immunostimulatory DAMPs (Figure 7). Our results suggest that CVA11 is a promising oncolytic virus for the treatment of individuals with MPM. Its cytotoxicity for human MPM cells and ability to elicit multiple modes of cell death and induce immunostimulatory DAMPs provide a robust basis for future preclinical and clinical development.
ACKNOWLEDGMENTS
We are grateful for providing HaCaT cells from T. Yokomizo (Juntendo University). We thank the Center for Clinical and Translational Research of Kyushu University for support.
FUNDING INFORMATION
This work was supported by grants from the Translational Research Network Program of the Japan Agency for Medical Research and Development (AMED) (grant number A238).
CONFLICT OF INTEREST
This work was supported by the Translational Research Network Program of AMED (grant number A238); Hiroyuki Inoue receives a remuneration from Jocavio Inc. as an adviser, owns shares of stock in Jocavio Inc., and holds a patent on CVA11 [PCT/UP2O13A061686, PCT/JP2013/061686]; The other authors have no conflict of interest; all authors had full access to all of the data in the study and had final responsibility for the decision to submit for publication.
ETHICS STATEMENT
Approval of the research protocol by an Institutional Reviewer Board: N/A.
ANIMAL STUDIES
All animal experiments (approval number: A22-213) were carried out under the Kyushu University Guidelines for Animal Experiments and the Japanese government's Law 105 Notification 6.




