CXC chemokine ligand-10 promotes the accumulation of monocyte-like myeloid-derived suppressor cells by activating p38 MAPK signaling under tumor conditions
Yingying Sun and Yan Mo contribute equally to this study.
Abstract
CXC chemokine ligand-10 (CXCL10) is a small (10 kDa) secretory protein in the CXC subfamily of cytokines. CXCL10 has been reported to play an important role in antitumor immunity as a chemotactic factor. Tumor development is always accompanied by the formation of an immunosuppressive tumor microenvironment, and the role of CXCL10 in tumor immunosuppression remains unclear. Here, we reported that CXCL10 expression was significantly upregulated in mice with melanoma, and tumor cells secreted large amounts of CXCL10. Myeloid-derived suppressor cells (MDSCs) are an important part of the immunosuppressive tumor microenvironment. Our results showed that CXCL10 promoted the proliferation of monocyte-like (mo)-MDSCs by activating the p38 MAPK signaling pathway through CXCR3, which led to the abnormal accumulation of mo-MDSCs under tumor conditions. This finding provides a new understanding of the mechanism by which a tumor-induced immunosuppressive microenvironment forms and suggests that CXCL10 could be a potential intervention target for slowing tumor progression.
Abbreviations
-
- CTL
-
- cytotoxic T lymphocyte
-
- CXCL10
-
- CXC chemokine ligand-10
-
- CXCR3
-
- chemokine receptor 3
-
- G-MDSC
-
- granulocyte-like MDSC
-
- IFN-γ
-
- γ-interferon
-
- iNOS
-
- inducible nitric oxide synthase
-
- MDSC
-
- myeloid-derived suppressor cell
-
- mo-MDSC
-
- monocyte-like MDSC
-
- NK
-
- natural killer
1 INTRODUCTION
Tumor development is always accompanied by the formation of an immunosuppressive microenvironment, and MDSCs are important components of the microenvironment.1-4 Myeloid-derived suppressor cells consist of two major populations: mo-MDSCs and G-MDSCs. In mice, MDSCs are characterized by coexpression of the myeloid markers Gr1 and CD11b, and the two subsets can be further identified based on the expression of Ly6G and Ly6C (mo-MDSCs are CD11b+Ly6ChiLy6G− and G-MDSCs are CD11b+Ly6CloLy6G+).5-8 Myeloid-derived suppressor cells mainly inhibit immune effector cells (such as T cells and NK cells) from exerting immune functions. The mechanisms of the immunosuppressive effects of the two MDSC subtypes are different: mo-MDSCs mainly upregulate the expression of iNOS, whereas G-MDSCs mainly upregulate the expression of Arginase 1.9, 10 It has been reported that MDSCs play a critical role in preventing anticancer immunity and promoting tumor development.11-14 Therefore, the factors and mechanisms by which tumors promote the abnormal accumulation of MDSCs are worth exploring.
CXC chemokine ligand-10 (interferon-γ-inducible protein, IP10) is a member of the non-ELR CXC chemokine subfamily and is mainly secreted by monocytes, endothelial cells, fibroblasts, and cancer cells in response to IFN-γ.15, 16 CXCL10 plays multiple roles in tumors and immunity.17 It has been reported that the interaction between CXCL10 and CXCR3 plays an important role in immune cell migration, differentiation, and activation.18, 19 The immune response occurs through the recruitment of cytotoxic T lymphocytes, NK cells, NKT cells, and macrophages through the CXCL10/CXCR3 axis.20, 21 In contrast, cancer cells have a propensity to metastasize mainly through tumor-derived ligand activity and the CXCR3 receptor.22 Our previous work showed that the melanoma cell line B16F10 could secrete large amounts of CXCL10,23 and its specific role in immunosuppression remains unclear.
Mitogen-activated protein kinase cascades are key signaling pathways that regulate a wide variety of cellular processes, including proliferation, differentiation, apoptosis, and stress responses.24 The MAPK family is composed of several subfamilies: ERK, p38 MAPK, and JNK.25 Here, we reported that CXCL10 promoted the proliferation of mo-MDSCs through p38 MAPK signaling under tumor conditions, which suggests that inhibiting the expression of CXCL10 or the activation of p38 MAPK signaling could decrease mo-MDSC generation and improve host immunosurveillance.
2 MATERIALS AND METHODS
2.1 Mice and cell lines
C57BL/6J mice (8–10 weeks old) and CXCR3−/− mice on a C57BL/6J background were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. B16F10 cells (1 × 106) were implanted subcutaneously in female mice (n = 4–6 mice/group). Tumor growth was measured using calipers, and tumor volume was calculated as follows: V = (length × width2) × 0.5. All animal procedures and studies were carried out in accordance with the Institutional Animal Care and Use Committee guidelines. Mouse melanoma B16F10 cells, mouse embryonic fibroblasts and human embryonic kidney HEK-293 T cells were purchased from ATCC. All cells were grown in DMEM supplemented with 10% heat-inactivated FBS (HyClone) and 1% penicillin/streptomycin. All cells were routinely validated for lack of Mycoplasma infection with LookOut Mycoplasma PCR Detection Kit (Sigma) and used within 10 passages.
2.2 Reagents and Abs
Recombinant murine CXCL10 (250-16) was purchased from PeproTech. Directly conjugated anti-mouse mAbs CD11b-FITC (M1/70), CD45-PE/Cy7 (30-F11), Ly6G-APC/Cy7 (1A8), and Ly6C-PE (AL-21) were from BD Pharmingen. Annexin V-APC apoptosis analysis kit (AO2001-11P-G) was obtained from Sungene. The MDSC isolation kit was from Miltenyi Biotec. Anti-Erk (Ser473, #4696), anti-p-Erk (#5726), anti-JNK (#9252), anti-p-JNK (Thr183/Tyr185, #9255), anti-p38 (#8690), and anti-p-p38 (Thr180/Tyr182, #4511) Abs were purchased from Cell Signaling Technology. SB203580 (HY-10256) were purchased from Med Chem Express.
2.3 Cell isolation
Bone marrow cells were isolated by flushing the femurs and tibias with PBS supplemented with 2% FBS. Single cell suspensions were passed through a 70-μm mesh followed by erythrocytes removal using ammonium chloride lysis buffer. The mo-MDSCs and G-MDSCs were sorted with a Myeloid Derived Suppressor Cell Isolation Kit using AutoMACS sorter (Miltenyi Biotec) according to the manufacturer's instructions.
2.4 Flow cytometry
Peripheral blood was treated with ammonium chloride lysis buffer to eliminate erythrocytes, and the isolated cells were stained with fluorochrome-conjugated Abs at 4°C for 30 min. Tumor tissues were digested with collagenase IV at 4°C for 1 h. Single cell suspensions were passed through a 70-μm mesh followed by erythrocyte removal using ammonium chloride lysis buffer. Following tumor tissues processing, single-cell suspensions were resuspended in Fc (fragment crystallizable) Receptor blocked (CD16/32, BD #553141) on ice and then incubated with Ab master mix. The cells were further fixed using 10% formaldehyde (Sigma Aldrich) for 10 min and permeabilized using 0.1% Triton X-100 (Sigma Aldrich) for 10 min, then stained with Ki-67 for 1 h (Percp-cy5.5-conjugated; BioLegend). For apoptosis analysis, cells were stained with surface marker followed by annexin V staining in binding buffer (BioLegend). The treated cells were tested by flow cytometry using FACSCanto II (BD Biosciences), and the data were analyzed with FACSDiva software (BD Biosciences) and FlowJo 7.6.1 software.
2.5 Enzyme-linked immunosorbent assay
The level of CXCL10 was determined using ELISA kits (eBioscience) according to the manufacturer's instructions.
2.6 Construction of lentiviral expression plasmid and gene transfection
Synthesized shRNA sequences were cloned into pLVTHM vector, and the constructed plasmids or shControl plasmid were transfected into HEK-293 T cells, together with the packaging plasmid psPAX2 and the envelope plasmid pMD2.G (both from Addgene) using Lipofectamine 2000 reagent (Invitrogen). The collected supernatant was concentrated and intrapleurally injected into 2-week tumor-bearing mice four times every other day. The relative sequences used are listed in Table S1. To knock down CXCL10, the collected supernatant and 5 μg/ml polybrene (Sigma) were used to infect the B16F10 cells. Stable cell lines infected with CXCL10 shRNA (shCXCL10) or control shRNA (shControl) were separated by flow cytometry sorting. To knock down CXCL10 in tumor-bearing mice, the collected supernatant was concentrated and intrapleurally injected into mice four times every other day.
2.7 RNA extraction and quantitative PCR
Total RNA was extracted with TRIzol (Invitrogen), and the cDNA was synthesized with reverse transcriptase (Thermo Fisher Scientific).The cDNA was then used as a template for semiquantitative or quantitative PCR analysis with Power SYBR Green PCR Master Mix (Thermo Fisher Scientific) using the StepOne Plus Real Time PCR System (Applied Biosystems). The primer sequences that were used are listed in Table S2.
2.8 Transwell analysis
Sorted mo-MDSCs (5 × 104) were loaded on the upper wells, and the chemokine CXCL10 was placed in the lower wells. Based on the size of the cells, a 5-μm pore Transwell chamber was used. The migrated cells were collected in the lower chamber and calculated after incubation at 37°C with 5% CO2 for 3 h.
2.9 Cell proliferation assay
The effect of CXCL10 on mo-MDSC proliferation was estimated by the CCK-8 assay (Bimake). Approximately 104 cells were seeded in 96-well plates with 100 μl medium in each well. After 24 h of cultivation, each well was incubated with 10 μl CCK-8 solution for 2 h away from light before measuring the absorbance at 450 nm.
2.10 Western blot analysis
Cells were harvested and solubilized in RIPA buffer containing protease and phosphatase inhibitors. Ten micrograms of protein was electrophoresed on Bio-Rad precast gradient gels and electroblotted onto PVDF membranes. Proteins were detected by incubation with 1:1000 dilutions of primary Abs, washed, and incubated with goat anti-rabbit-HRP Abs or goat anti-mouse-HRP Abs and detected after incubation with a chemiluminescent substrate.
2.11 Statistics
Statistical and graphical analyses were undertaken using GraphPad Prism. Results are presented as means ± SEM. The t-test was applied using GraphPad Prism version 6 software. Differences were considered to be statistically significant when p values were less than 0.05 (*p < 0.05, **p < 0.01, and ***p < 0.001). All experiments were independently repeated at least three times.
3 RESULTS
3.1 CXCL10 promotes abnormal accumulation of mo-MDSCs under tumor conditions
Myeloid-derived suppressor cells abnormally accumulate under tumor conditions, and our previous work showed that B16F10 melanoma cells secreted large amounts of CXCL10.23 To explore the relationship between CXCL10 and MDSCs, B16F10 melanoma cells were injected subcutaneously into mice. The proportions of MDSCs in the peripheral blood was examined by flow cytometry (Figure 1A). The results showed that mo-MDSCs and G-MDSCs abnormally accumulated in the peripheral blood of mice under tumor conditions (Figure 1B,C). Then the expression of CXCL10 under tumor conditions was analyzed, and the results showed that CXCL10 levels in the tumor-conditioned medium and serum of tumor-bearing mice were significantly upregulated (Figure 1D,E). These results indicate that the proportions of MDSCs positively correlated with the level of CXCL10 under tumor conditions. We then generated two independent shRNAs to stably decrease CXCL10 expression in mice (Figure 1F). After shRNA transfection in vivo, the proportion of mo-MDSCs decreased, while the proportion of G-MDSCs did not change significantly (Figure 1G,H). These results indicate that CXCL10 promotes the abnormal accumulation of mo-MDSCs under tumor conditions.
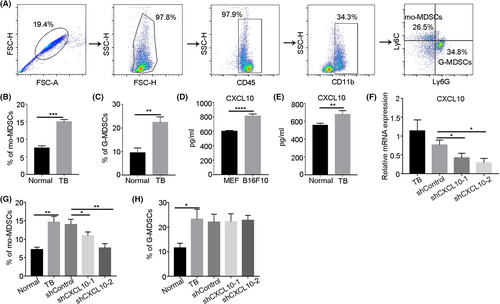
3.2 CXCL10 derived from melanoma cells contributes to the abnormal accumulation of mo-MDSCs and tumor progression
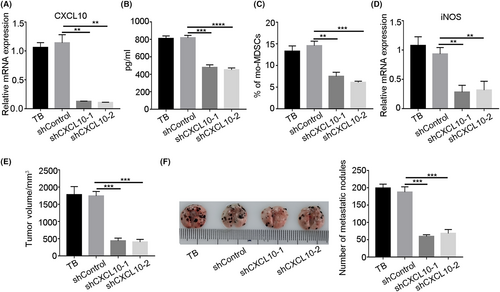
To determine the effect of CXCL10 secreted by the tumor on the accumulation of mo-MDSCs, we constructed a tumor cell line with CXCL10 interference. The knockdown efficiency of CXCL10 in B16F10 cells was first examined (Figure 2A,B). Then B16F10 cells with CXCL10 knockdown were injected subcutaneously into mice. After 3 weeks, the proportion of mo-MDSCs was examined, and the results showed that the proportion of mo-MDSCs in the peripheral blood of tumor-bearing mice decreased significantly after CXCL10 was knocked down in B16F10 cells (Figure 2C), and the expression of iNOS in mo-MDSCs was also significantly downregulated (Figure 2D). We also found that CXCL10 knockdown in B16F10 cells significantly inhibited the proportion of mo-MDSCs in lung (Figure S1), and then inhibited tumor growth and metastasis (Figure 2E,F). These results indicate that tumor-derived CXCL10 contributes to the abnormal accumulation of mo-MDSCs and tumor progression.
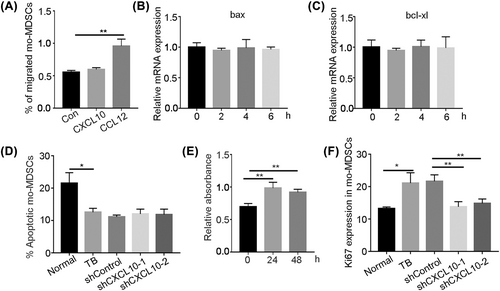
3.3 CXCL10 promotes proliferation of mo-MDSCs in bone marrow of tumor-bearing mice
To explore how CXCL10 affects the abnormal accumulation of mo-MDSCs under tumor conditions, we first examined the chemotactic effect of CXCL10 on mo-MDSCs. A Transwell assay was carried out to examine whether CXCL10 recruits mo-MDSCs, and the results showed that CXCL10 did not recruit mo-MDSCs (Figure 3A). We then examined the effect of CXCL10 on apoptosis in mo-MDSCs. The results showed that after bone marrow-derived mo-MDSCs were stimulated with CXCL10 for 2 h, the expression of the apoptotic gene bax and the antiapoptotic gene bcl-xl was not changed (Figure 3B,C). Annexin V staining of peripheral blood mo-MDSCs after CXCL10 knockdown was examined by flow cytometry, and the results showed that annexin V levels were not affected (Figure 3D). These results suggest that CXCL10 does not affect apoptosis of mo-MDSCs. The effect of CXCL10 on the proliferation of mo-MDSCs was also examined in vitro by CCK-8 assays. The results showed that CXCL10 could significantly promote the proliferation of mo-MDSCs (Figure 3E). We next examined Ki-67 expression in bone marrow-derived mo-MDSCs by flow cytometry, and the results showed that the Ki-67 expression was significantly downregulated after CXCL10 knockdown (Figure 3F). These results indicate that CXCL10 promotes the proliferation of mo-MDSCs in the bone marrow of tumor-bearing mice.
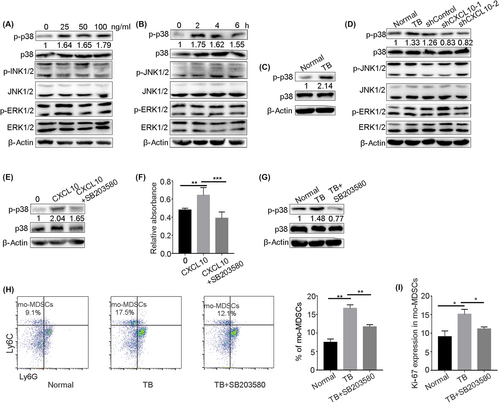
3.4 CXCL10 can activate p38 MAPK signaling in mo-MDSCs
We examined the mechanism by which CXCL10 affects the proliferation of mo-MDSCs under tumor conditions. The MAPK pathway is an evolutionarily conserved pathway that links extracellular signals to the machinery that controls fundamental cellular processes such as growth, proliferation, differentiation, migration, and apoptosis.26, 27 We found that p38 MAPK signaling was activated after bone marrow-derived mo-MDSCs were stimulated with CXCL10 in vitro, and the phosphorylation levels of ERK1/2 and JNK1/2, which are two components of the MAPK pathway, were not affected by CXCL10 stimulation (Figure 4A,B). Then we examined the phosphorylation level of p38 in mo-MDSCs in bone marrow. The results showed that the phosphorylation level of p38 in the mo-MDSCs of tumor-bearing mice was significantly upregulated (Figure 4C). We also found that the phosphorylation level of p38 in mo-MDSCs from tumor-bearing mice was decreased after CXCL10 knockdown (Figure 4D). These results suggest that CXCL10 might regulate the proliferation of mo-MDSCs by activating p38 MAPK signaling under tumor conditions. To further confirm these results, we added a p38 inhibitor (SB203580) to bone marrow-derived mo-MDSCs stimulated with CXCL10 in vitro. The results showed that the phosphorylation level of p38 was obviously downregulated (Figure 4E), and the proliferation of mo-MDSCs was significantly inhibited by the p38 inhibitor (Figure 4F). We then intraperitoneally injected the p38 inhibitor into tumor-bearing mice and examined the effect on the proportion of mo-MDSCs. The results showed that inhibiting p38 phosphorylation (Figure 4G) reduced the proportion and proliferation of mo-MDSCs in bone marrow (Figure 4H,I). These results indicate that CXCL10 promoted the proliferation of mo-MDSCs by activating p38 signaling, which led to the accumulation of mo-MDSCs.
3.5 CXCR3 deficiency decreases accumulation of mo-MDSCs
Chemokine receptor 3 is expressed by various effector T lymphocytes, monocytes, macrophages, and NK cells. The CXCL10–CXCR3 axis was previously shown to be involved in the recruitment of immune cells.28 We examined the expression of CXCR3 on the surface of mo-MDSCs under tumor conditions, and the results showed that mo-MDSCs expressed CXCR3 (Figure 5A). Then we examined whether CXCL10 promotes the accumulation of mo-MDSCs through CXCR3. The results showed that the proportion of mo-MDSCs in peripheral blood were significantly decreased in CXCR3−/− tumor-bearing mice (Figure 5B). We sorted mo-MDSCs from the bone marrow of CXCR3−/− mice, stimulated the cells with CXCL10 in vitro, and examined the proliferation of mo-MDSCs. The results showed that, compared with that of cells from normal mice, the proliferation of mo-MDSCs from CXCR3−/− mice was significantly decreased after incubation with CXCL10 (Figure 5C). Next, we examined Ki-67 expression in mo-MDSCs isolated from the bone marrow of CXCR3−/− mice. The results showed that, compared with that in normal tumor-bearing mice, Ki-67 expression in mo-MDSCs from CXCR3−/− tumor-bearing mice was decreased (Figure 5D). The expression of p38 and p-p38 in mo-MDSCs in the bone marrow was decreased in CXCR3−/− tumor-bearing mice compared with normal tumor-bearing mice (Figure 5E), and the expression of p38 and p-p38 were decreased in bone marrow-derived mo-MDSCs from CXCR3−/− mice compared with normal mice after incubation with CXCL10 (Figure 5F). These results suggest that CXCL10 promotes the accumulation of mo-MDSCs by activating the p38 signaling pathway through CXCR3.

4 DISCUSSION
Tumor cells release tumor-derived soluble factors that can alter myelopoiesis.29 We showed here that the immunosuppressive environment created by tumors is closely related to CXCL10. CXCL10, which is also known as IP10, is strongly induced by IFN.30 In many kind of tumors, high levels of CXCL10 are produced.31 There have been various reports on the effect of CXCL10 on tumor development. CXCL10 can promote tumor growth and metastasis through autocrine signaling in tumor cells. It has been reported that CXCL10 promotes invasion-related properties in human colorectal carcinoma cells.32 Oncogenic CXCL10 signaling drives metastasis and poor clinical outcomes in melanoma and colon and renal cell carcinomas.33 CXCL10/CXCR3 signaling is also associated with paracrine interactions that regulate leukocyte trafficking and promote antitumor immunity. It has been reported that CXCL10 stimulates immune cells through Th1 polarization and activation.34, 35 Serum CXCL10 levels reportedly correlated with the proportions of circulating lymphocytes in head and neck cancer treated with radiation therapy.36 In our study, we found that the expression of CXCL10 in tumor-bearing mice significantly increased with tumor progression, and the expression of CXCL10 was positively correlated with the proportion of mo-MDSCs. In tumor-bearing mice, mo-MDSCs are highly immunosuppressive, and the expansion of mo-MDSCs is a critical step associated with immunosuppression.29, 37 Further experiments showed that CXCL10 knockdown could decrease the proliferation of mo-MDSCs under tumor conditions. These results indicate that CXCL10 is essential for the accumulation of mo-MDSCs under tumor conditions.
The MAPK signaling pathway regulates a wide variety of cellular processes, including proliferation, differentiation, apoptosis, and stress responses. The role of the MAPK signaling pathway in tumor immunity is still controversial. Upregulation of the MAPK pathway could polarize macrophages from the M2 phenotype to the M1 phenotype and inhibit the suppressive effects of lipopolysaccharide-induced MDSCs.38 It has also been reported that interleukin-6 promotes the proliferation and immunosuppressive effects of MDSCs through the MAPK signaling pathway.39 In bladder cancer, tumor cells induced MDSC accumulation and expansion through the CXCL2/CXCR2 signaling pathway and increased phosphorylation of p38, ERK, and p65.40 In our study, CXCL10-dependent activation of the p38 signaling pathway but not ERK or JNK in mo-MDSCs was related to cell proliferation under tumor conditions, which suggests that inhibiting the activation of p38 MAPK signaling could decrease mo-MDSC generation and improve the immunosuppressive tumor microenvironment. We also found that CXCR3, which is the receptor of CXCL10, was not involved in the mobilization of mo-MDSCs, which was consistent with previous reports.41 Notably, we found that CXCR3 plays a key role as a receptor of CXCL10 in promoting the proliferation and accumulation of mo-MDSCs through p38 MAPK signaling.
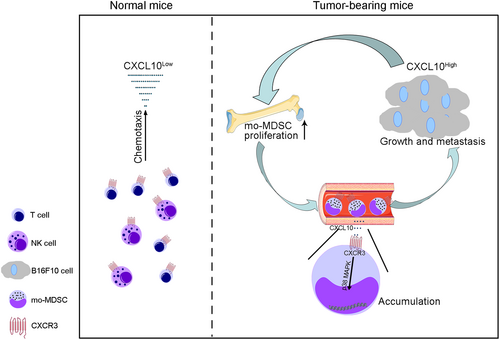
In summary, our study revealed an important role of CXCL10 in the abnormal accumulation of mo-MDSCs under tumor conditions and that the CXCL10–CXCR3 axis could promote the proliferation and accumulation of mo-MDSCs through p38 MAPK signaling (Figure 6), which could provide a strategy for combating the immunosuppressive microenvironment.
AUTHOR CONTRIBUTIONS
Conception and design: Y.S., X.Z. Development of methodology: Y.S., Y.M. Acquisition of data: Y.S., Y.M., S.J. Statistical analysis: Y.S., S.J. C.S. Writing, review, and/or revision of the manuscript: Y.S., S.J., C.S., Y.M., Y.F., X.Z.
ACKNOWLEDGMENT
This work was supported by grants from the National Natural Science Foundation of China (Grant Nos. 31870888, 32170916, and 81670095).
FUNDING INFORMATION
National Natural Science Foundation of China, Grant/Award Number: 31870888, 32170916, 81670095.
DISCLOSURE
The authors declare that they have no conflict of interest.
ETHICS STATEMENT
Animal studies: All animal procedures and studies were conducted in accordance with the Institutional Animal Care and Use Committee guidelines.




