Dietary glycemic index, glycemic load, and endometrial cancer risk: The Japan Public Health Center-based Prospective Study
Financial support
National Cancer Center Research and Development Fund; Ministry of Health, Labour and Welfare of Japan.
Abstract
Evidence supporting the association of glycemic index (GI) and glycemic load (GL) with the risk of endometrial cancer is controversial and reports from Asia were limited. Therefore, we aimed to investigate the association in Japanese women. We evaluated 52 460 women in the Japan Public Health Center-based Prospective Study aged 45-74 years who responded to the 5-year follow-up survey. GI and GL were calculated from a validated food frequency questionnaire, and the participants were divided into three groups by GI and GL. The hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated with the Cox proportional hazard model adjusted for potential confounding factors. As a result, within 15.5 years of follow-up, 166 new cases of endometrial cancer were identified. Compared with the lowest GI and GL tertile groups, the HR of the risk of endometrial cancer in the highest GI tertile group was 0.80 (95% CI, 0.53-1.20; Ptrend = .33), and that of the highest GL tertile group was 0.79 (95% CI, 0.52-1.19; Ptrend = .82). The results were unchanged after stratification by body mass index, coffee consumption, and history of diabetes. In conclusion, we did not find any significant association between GI and GL with the risk of endometrial cancer. Further research is required to clarify the association.
1 INTRODUCTION
Endometrial cancer is one of the most prevalent cancers globally, especially in developed countries.1, 2 Age-adjusted incidence rates range from around 15 per 100 000 women in North America and parts of Europe, to less than 5 per 100 000 in most of Africa and Asia.3 However, the age-adjusted incidence rate in Japan was 14.7 in 20174 and has markedly increased over the past 30 years,5 mainly due to changes in women’s lifestyles. For example, the rates of obesity, low physical activity, diabetes mellitus, or opportunities for estrogen exposure (eg early menarche and late menopause), which are known risk factors for endometrial cancer, have been increasing.1, 3, 6, 7
The glycemic load (GL) has been recently reported to be a possible risk factor for endometrial cancer.8 The glycemic index (GI) is an indicator that quantifies the degree of blood sugar increase under the same amount of carbohydrates consumed. It shows the speed of rising blood sugar levels with each food consumed. GL is an indicator with the added a quantitative factor of carbohydrate consumption with GI. Consumption of high GI or GL foods promotes insulin secretion. Insulin and insulin-like growth factors accelerate cell division and inhibit apoptosis. Therefore, there is a possibility that GI and GL are associated with the risk of endometrial cancer.2, 6, 9
Previous studies on the association between GI and GL with the risk of endometrial cancer were mainly undertaken in countries with a high incidence of endometrial cancer such as the United States and those in Europe.9 In a previous meta-analysis, the pooled relative risk (RR) between GI and endometrial cancer for the highest versus the lowest category was 1.15 (95% confidence interval [CI], 0.95-1.40) and that of GL was 1.21 (95% CI, 1.09-1.33),10 and the World Cancer Research Fund reported that GL was a probable risk factor of endometrial cancer.7 However, reports from large-scale prospective studies in Asia have been limited. Furthermore, only one case-control study from China suggested a positive association,11 and thus additional evidence on the association of GI and GL with endometrial cancer from Asian countries is needed.
Japanese dietary habits differ from those in Western countries; accordingly, the foods affecting GI and GL are different. Japanese GI and GL are derived mainly from rice, but those from Western countries include various foods such as cereals or bread.12, 13 Additionally, the body mass index (BMI) of Japanese individuals tends to be lower than that in Western countries. Thus, the association between GI and GL with the risk of endometrial cancer in Asians could be different from those among people in non-Asian countries.9, 10 Evaluating the association among the Japanese population might help further understand the etiologic role of GI and GL in the development of endometrial cancer, particularly in Asians. Thus, this study aimed to investigate the association between GI and GL with the risk of endometrial cancer in Japanese women in the Japan Public Health Center-based Prospective Study (JPHC Study).
2 MATERIALS AND METHODS
2.1 Study design and participants
This was a prospective study of women in the JPHC Study aged 45-74 years. The JPHC Study consists of two cohort studies, cohort I and cohort II. Cohort I started in 1990 and included five areas, namely, Akita, Iwate, Nagano, Tokyo, and Okinawa-Chubu. Cohort II started in 1993 and included six areas, namely, Niigata, Ibaraki, Kochi, Nagasaki, Osaka, and Okinawa-Miyako. The Tokyo residents were excluded from Cohort I because their cancer data were not available. The details of this study protocol have been described previously.14
In this study, we set a 5-year follow-up survey as a baseline because we have more detailed data of the Food Frequency Questionnaire (FFQ) than that of the baseline survey, and included 52 460 women who responded to the questionnaire. Among them, those who reported a history of endometrial cancer in the questionnaire (n = 654), were diagnosed with endometrial cancer before the 5-year follow-up survey (n = 14), or had missing or extreme energy intake (n = 3055) (upper or lower 2.5 percentile) were excluded. Finally, 48 737 women were included in the analysis.
2.2 Dietary and other variable assessment
The amount and frequency of food item intake for each participant were investigated using a validated FFQ.15, 16 This FFQ includes 138 food items and consists of eating frequency (never, 1-3 times/mo, 1-2 times/wk, 3-4 times/wk, 5-6 times/wk, 1 time/d, 2-3 times/d, 4-6 times/d, over 7 times/d) and portion size (less than half, same, 1.5-fold, or over). Food intake was estimated by multiplication of frequency and portion size. In addition, we obtained information about lifestyle habits and other confounding factors from the 5-year survey questionnaire and FFQ.
2.3 Exposure
GI and GL were estimated using the same method as in a previous study.17 In brief, the GI for individual food items were derived from publications, from the 2008 International Tables of Glycemic Index and Glycemic Load Values, and some published Japanese studies.18-20 GI and GL were calculated using the following formula:  ;
;
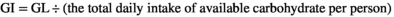 .
.
2.4 Follow-up and identification of cases
All participants were followed up from the date of completion of the 5-year follow-up survey questionnaire until December 31, 2013 except for those in the Osaka area, whose follow-up finished by the end of 2012. During this period, 7793 (16.0%) participants died, 3964 (8.1%) moved out of the study area, and 30 (0.1%) were lost to follow-up. We identified new cases of endometrial cancer by active patient notification from local hospitals and population-based cancer registries in the study areas. Cancer cases were coded according to the International Classification of Diseases for Oncology, Third Edition (ICD-O-3). Endometrial cancer was identified using the ICD-O-3 code C54. The proportion of cases registered by death certificates was 1.8%.
2.5 Statistical analysis
GI and GL were set as the exposure variables and the incidence of endometrial cancer as the outcome. GI and GL were energy-adjusted using the residual method and were divided into tertiles. The hazard ratio (HR) was estimated with the lowest group set as a reference. The HR and 95% CI were calculated using the Cox proportional hazard model adjusted for confounding factors of GI and GL. As potential confounding factors, we included age (years), area (10 public health center areas), BMI (<21, 21-23.9, 24-26.9, ≥27 kg/m2), age at menarche (≤13, 14-15, ≥16 years), number of deliveries (0, 1-2, ≥3), menopause status and age at menopause (premenopause, postmenopause [≤49, 50-54, ≥55 years]), use of exogenous female hormones (yes, no), smoking status (never, ever, <20, or ≥20 cigarettes/d), alcohol intake (no-drinker, occasional drinker, regular drinker ≤150, or >150 g of ethanol per week), leisure-time physical activity (none, ≤2, or ≥3 d/wk), coffee consumption (none, 1-4 cups/wk, 5-7 cups/wk, ≥2 cups/d), total energy (quartile), and history of diabetes mellitus (yes, no). Four models were constructed; model 1 was adjusted for age and area (10 public health center areas), model 2 was all potential confounding factors, model 3 excluded cases identified within ≤3 years (n = 23) of follow-up in model 2, and model 4 was the same as model 2 but excluded the history of diabetes mellitus. In total, the number of women with missing data were: 1315 on BMI; 2304 on leisure-time physical activity; 3178 on smoking; 1411 on alcohol drinking; 2776 on use of exogenous female hormones; 1578 on menopausal status; 6583 on menarche age; and 2525 on coffee intake. We used multivariate normal imputation with the SAS PROC MI procedure to impute missing data for covariates, and we carried out 10 rounds of imputations.21, 22 Then we combined each imputed dataset with the SAS PROC MIANALYZE procedure.21, 22 All statistical analyses were undertaken using SAS 9.3 (SAS Institute). All P values were two-sided, and P < .05 was considered statistically significant.
3 RESULTS
During 753 995.8 person-years and 15.47 years (mean of follow-up period), we identified 166 new cases of endometrial cancer. Table 1 shows the characteristics of participants according to GI and GL tertiles. Particularly, there was a clear tendency that the highest GI group was older and had lower physical activity, lower alcohol intake, higher menarche age, a higher proportion of diabetes mellitus, and lower coffee consumption. Similarly, the highest GL group also tended to have lower physical activity and alcohol intake, but the group also had lower menarche age and a lower proportion of participants with diabetes mellitus.
| Energy-adjusted GI tertiles (T) (n = 48 737) | Energy-adjusted GL tertiles (T) (n = 48 737) | |||||
|---|---|---|---|---|---|---|
| T1 | T2 | T3 | T1 | T2 | T3 | |
| No. of participants | 16 245 | 16 246 | 16 246 | 16 245 | 16 246 | 16 246 |
| GI (mean ± SD)a | 55.1 ± 3.7 | 61.1 ± 1.0 | 65.4 ± 2.1 | 57.3 ± 5.2 | 60.9 ± 3.3 | 63.6 ± 3.4 |
| GL (mean ± SD)a | 125.0 ± 23.6 | 144.7 ± 18.6 | 159.4 ± 24.2 | 114.9 ± 16.6 | 143.7 ± 5.7 | 170.5 ± 15.2 |
| Age at 5-year follow-up survey (y) | 55.5 ± 7.7 | 57.0 ± 7.8 | 58.4 ± 8.0 | 56.2 ± 7.8 | 56.9 ± 7.9 | 57.9 ± 8.0 |
| Body mass index (kg/m2)b | 23.3 ± 3.1 | 23.5 ± 3.2 | 23.6 ± 3.3 | 23.5 ± 3.2 | 23.5 ± 3.2 | 23.5 ± 3.2 |
| Leisure-time physical activity | ||||||
| None | 60.8 | 65.2 | 68.7 | 60.0 | 64.3 | 70.4 |
| ≤2 d/wk | 23.3 | 20.2 | 16.1 | 22.9 | 20.1 | 15.9 |
| ≥3 d/wk | 12.6 | 10.8 | 8.2 | 12.9 | 10.9 | 7.9 |
| Unknown/missing data | 3.3 | 3.8 | 7.1 | 4.3 | 4.0 | 5.9 |
| Smoking status (%) | ||||||
| Never | 85.7 | 89.3 | 87.0 | 85.1 | 88.9 | 87.9 |
| Ever | 1.1 | 1.0 | 1.0 | 1.4 | 0.8 | 0.9 |
| Current (<20 cigarettes/d) | 4.5 | 2.5 | 3.0 | 4.5 | 2.8 | 2.6 |
| Current (≥20 cigarettes/d) | 2.7 | 1.3 | 1.5 | 2.6 | 1.6 | 1.3 |
| Unknown/missing data | 6.0 | 5.9 | 7.6 | 6.4 | 5.9 | 7.3 |
| Alcohol intake | ||||||
| Nondrinker | 73.4 | 79.7 | 81.0 | 72.5 | 79.0 | 82.6 |
| Occupational drinker | 8.3 | 6.4 | 5.2 | 7.0 | 6.9 | 6.1 |
| Regular drinker (≤150 g ethanol/wk) | 12.7 | 9.2 | 7.0 | 12.3 | 9.8 | 6.7 |
| Regular drinker (>150 g ethanol/wk) | 3.3 | 2.3 | 2.9 | 5.7 | 1.8 | 1.0 |
| Unknown/missing data | 2.4 | 2.4 | 3.9 | 2.5 | 2.53 | 3.7 |
| Age at menarche (%) | ||||||
| ≤13 y | 26.8 | 23.6 | 19.2 | 23.5 | 24.1 | 22.0 |
| 14-15 y | 39.0 | 40.5 | 37.9 | 38.1 | 40.1 | 39.2 |
| ≥16 y | 19.6 | 23.5 | 29.5 | 23.7 | 23.5 | 25.4 |
| Unknown/missing data | 14.6 | 12.5 | 13.4 | 14.8 | 12.3 | 13.5 |
| No. of deliveries (%) | ||||||
| None | 6.0 | 5.3 | 4.8 | 5.8 | 5.1 | 5.2 |
| 1-2 | 38.1 | 36.3 | 31.1 | 34.5 | 36.4 | 34.6 |
| ≥3 | 38.4 | 43.1 | 46.3 | 41.3 | 43.0 | 43.5 |
| Unknown/missing data | 17.5 | 15.3 | 17.8 | 18.4 | 15.5 | 16.7 |
| Menopause status and age at menopause at the 5-year follow-up survey (%) | ||||||
| Premenopause | 26.6 | 20.9 | 17.5 | 24.1 | 21.8 | 19.2 |
| Postmenopause from an unknown age | 1.5 | 1.6 | 1.9 | 1.6 | 1.6 | 1.7 |
| Postmenopause from age ≤49 y | 32.9 | 35.2 | 36.2 | 33.6 | 34.6 | 36.0 |
| Postmenopause from age 50-54 y | 33.2 | 36.0 | 35.1 | 33.8 | 35.6 | 34.8 |
| Postmenopause from age ≥55 y | 3.5 | 3.9 | 4.4 | 3.9 | 3.7 | 4.3 |
| Unknown/missing data | 2.3 | 2.4 | 5.0 | 3.0 | 2.7 | 4.0 |
| Exogenous hormone use (%) | ||||||
| Yes | 3.0 | 2.6 | 2.4 | 3.0 | 2.5 | 2.4 |
| No | 92.7 | 92.9 | 89.4 | 91.8 | 92.5 | 90.8 |
| Unknown/missing data | 4.3 | 4.5 | 8.3 | 5.2 | 5.0 | 6.7 |
| History of diabetes mellitus (%) | 3.2 | 3.7 | 4.3 | 4.0 | 3.7 | 3.5 |
| Coffee consumption (%) | ||||||
| None | 15.3 | 25.9 | 41.0 | 21.6 | 26.7 | 34.0 |
| 1-4 cups/wk | 22.6 | 33.6 | 31.3 | 26.3 | 30.7 | 30.5 |
| 5-7 cups/wk | 25.3 | 25.5 | 14.7 | 25.3 | 22.6 | 17.6 |
| ≥2 cups/d | 34.2 | 11.5 | 3.5 | 22.4 | 15.9 | 10.8 |
| Unknown/missing data | 2.7 | 3.5 | 9.4 | 4.4 | 4.1 | 7.1 |
| Dietary intake, median (IQR) | ||||||
| Energy (kcal/d) | 1797(1450-2243) | 1810(1499-2194) | 1693(1375-2101) | 1797(1437-2254) | 1798(1500-2166) | 1708(1381-2126) |
| Carbohydrate (g/d)c | 236.3(213.4-259.5) | 249.6(230.6-269.2) | 256.9(235.1-277.7) | 215.8(197.8-229.4) | 247.4(237.7-257.7) | 277.4(265.1-292.3) |
| Cereal(g/d)c | 405.5(338.7-474.7) | 487.1(434.3-543.2) | 551.9(494.7-618.6) | 389.3(330.8-439.1) | 488.0(447.6-530.2) | 577.7(526.2-639.2) |
| Rice (g/d)c | 263.8(178.6-312.9) | 367.6(294.4-412.8) | 428.1(371.0-476.4) | 260.2(175.7-306.3) | 364.3(295.6-411.4) | 433.3(386.0-481.0) |
| Noodle (g/d)c | 77.2(42.9-132.3) | 69.1(41.0-111.9) | 55.9(31.6-89.4) | 64.1(35.2-110.6) | 67.6(40.4-109.8) | 66.7(38.3-110.4) |
| Potato (g/d)c | 23.9(13.8-38.8) | 24.3(14.4-39.1) | 21.2(11.3-36.8) | 22.4(12.6-36.7) | 24.1(14.3-39.2) | 22.9(12.6-39.2) |
| Bread (g/d)c | 18.6(6.6-44.1) | 15.1(5.5-36.8) | 11.6(4.2-30.7) | 16.0(5.3-41.3) | 14.6(5.3-36.5) | 14.2(5.1-35.0) |
| Fat (g/d)c | 57.8(50.1-66.0) | 52.3(46.0-58.9) | 48.8(41.0-56.5) | 64.0(58.8-70.8) | 53.3(49.7-57.0) | 42.8(37.6-47.2) |
| Protein (g/d)c | 68.1(61.9-75.1) | 66.2(60.8-72.1) | 63.6(57.8-70.1) | 72.3(66.1-79.0) | 66.9(62.2-71.8) | 60.2(55.4-64.7) |
| Meat (g/d)c | 46.9(29.0-70.9) | 46.8(29.3-68.4) | 47.6(27.7-72.4) | 63.8(40.9-94.5) | 48.8(32.1-68.7) | 33.9(20.4-50.3) |
| Fish (g/d)c | 76.1(51.8-106.9) | 77.4(53.6-106.0) | 72.6(48.0-104.2) | 85.7(56.8-121.5) | 79.1(55.1-107.5) | 64.2(43.7-88.8) |
| Vegetable (g/d)c | 9.9(6.7-14.3) | 9.0(6.2-12.8) | 7.7(4.9-11.3) | 9.5(6.3-13.8) | 9.1(6.3-13.0) | 8.0(5.1-11.7) |
| Fruit (g/d)c | 244.1(150.2-376.9) | 213.8(138.6-308.6) | 149.0(86.2-223.4) | 174.3(101.3-271.8) | 202.7(131.2-294.9) | 216.8(130.0-333.5) |
| Fiber (g/d)c | 13.9(11.0-17.0) | 13.0(10.7-15.7) | 11.4(9.2-13.9) | 12.5(9.8-15.8) | 12.9(10.6-15.7) | 12.6(10.2-15.4) |
Note
- Values are presented as mean ± SD or number (%).
- Abbreviation: IQR, interquartile range.
- a Energy-adjusted values calculated using the residual method.
- b A total of 1315 women had missing data on body mass index.
- c Energy-adjusted intake with the residual method.
Table 2 shows the HRs and 95% CIs of the risk of endometrial cancer according to GI and GL. Neither GI nor GL were significantly associated with endometrial cancer after adjustment for confounding factors (model 2). In model 2, with the lowest tertile groups of GI and GL as the reference, the HR of the highest GI group was 0.80 (95% CI, 0.53-1.20; Ptrend = .33) and that of the highest GL group was 0.79 (95% CI, 0.52-1.19; Ptrend = .82). After excluding cases (n = 23) that were identified within 3 years of follow-up (model 3), the associations were not changed. Furthermore, after excluding the history of diabetes mellitus from adjusted variables (model 4), the associations were not changed. Additionally, when we divided GI and GL into quartiles or quintiles, the associations remained the same. In the sensitivity analysis, the associations were still not statistically significant after stratification by BMI, coffee consumption, and types of endometrial cancer or after exclusion of cases with diabetes mellitus (Tables 3 and S1).
| All | Tertiles (T) | Ptrend | |||
|---|---|---|---|---|---|
| Lowest (T1) | Middle (T2) | Highest (T3) | |||
| GIa | ≤59.2 | 59.2-62.8 | 62.8≤ | ||
| Person-years | 753 995.8 | 248 182.2 | 253 687.8 | 252 125.8 | |
| No. of participants | 48 737 | 16 245 | 16 246 | 16 246 | |
| No. of cases | 166 | 55 | 58 | 53 | |
| Model 1 HR (95% CI)b | 1 (Reference) | 1.01 (0.70-1.46) | 0.93 (0.63-1.36) | .78 | |
| Model 2 HR (95% CI)c | 1 (Reference) | 0.91 (0.62-1.33) | 0.80 (0.53-1.20) | .33 | |
| Model 3 HR (95% CI)d | 1 (Reference) | 0.83 (0.56-1.24) | 0.65 (0.41-1.02) | .10 | |
| Model 4 HR (95% CI)e | 1 (Reference) | 0.91 (0.62-1.33) | 0.79 (0.53-1.20) | .33 | |
| GLa | ≤133.5 | 133.5-153.7 | 153.7≤ | ||
| Person-years | 753 995.8 | 248 903.4 | 252 918.9 | 252 173.5 | |
| No. of participants | 48 737 | 16 245 | 16 246 | 16 246 | |
| No. of cases | 166 | 54 | 63 | 49 | |
| Model 1 HR (95% CI)b | 1 (Reference) | 1.10 (0.76-1.58) | 0.82 (0.56-1.22) | .89 | |
| Model 2 HR (95% CI)c | 1 (Reference) | 1.07 (0.74-1.57) | 0.79 (0.52-1.19) | .82 | |
| Model 3 HR (95% CI)d | 1 (Reference) | 1.06 (0.73-1.58) | 0.72 (0.47-1.12) | .62 | |
| Model 4 HR (95% CI)e | 1 (Reference) | 1.07 (0.73-1.55) | 0.78 (0.52-1.18) | .78 | |
- a Energy-adjusted values calculated using the residual method.
- b Adjusted for age (y) and area (10 public health center areas in Japan).
- c Adjusted for age (y), area (10 public health center areas), body mass index (<21, 21-23.9, 24-26.9, ≥27 kg/m2), age at menarche (≤13, 14-15, ≥16 y), number of deliveries (0, 1-2, ≥3), menopause status, and age at menopause (premenopause, postmenopause [≤49, 50-54, ≥55 y]), use of exogenous female hormones (yes, no), smoking status (never, ever, <20 cigarettes/d, ≥20 cigarettes/d), alcohol intake (non-drinker, occasional drinker, regular drinker ≤150 or >150 g of ethanol per week), leisure-time physical activity (none, ≤2 d/wk, ≥3 d/wk), coffee consumption (none, 1-4 cups/wk, 5-7 cups/wk, ≥2 cups/d), total energy (quartile), and diabetes mellitus (yes, no).
- d Cases identified within ≤3 y (n = 23) of follow-up in model 2 were excluded.
- e The same as model 2 but excluded history of diabetes mellitus.
| All | Tertiles (T) (n = 48 737) | Ptrend | |||
|---|---|---|---|---|---|
| Lowest (T1) HR (95% CI) | Middle (T2) HR (95% CI) | Highest (T3) HR (95% CI) | |||
| GIa | ≤59.2 | 59.2-62.8 | 62.8≤ | ||
| Stratified by body mass index | |||||
| <25 kg/m2 (no. of cases) | 106 | 38 | 34 | 34 | |
| Model 1 HR (95% CI)b | 1 (Reference) | 0.79 (0.49-1.28) | 0.78 (0.47-1.31) | .24 | |
| ≥25 kg/m2 (no. of cases) | 60 | 16 | 23 | 18 | |
| Model 1 HR (95% CI)b | 1 (Reference) | 1.15 (0.60-2.22) | 0.81 (0.40-1.68) | .94 | |
| Stratified by coffee consumption | |||||
| <4 cups/wk (no. of cases) | 97 | 25 | 34 | 38 | |
| Model 2 HR (95% CI)c | 1 (Reference) | 0.82 (0.49-1.39) | 0.74 (0.44-1.24) | .25 | |
| ≥5 cups/wk (no. of cases) | 61 | 30 | 22 | 9 | |
| Model 2 HR (95% CI)c | 1 (Reference) | 1.12 (0.64-1.96) | 0.90 (0.42-1.91) | .90 | |
| Excluding participants with a history of diabetes | |||||
| (no. of cases) | 156 | 52 | 55 | 49 | |
| Model 3 HR (95% CI)d | 1 (Reference) | 0.90 (0.61-1.34) | 0.76 (0.50-1.17) | .29 | |
| GLa | ≤133.5 | 133.5-153.7 | 153.7≤ | ||
| Stratified by body mass index | |||||
| <25 kg/m2 (no. of cases) | 106 | 34 | 39 | 33 | |
| Model 1 HR (95% CI)b | 1 (Reference) | 1.02 (0.64-1.63) | 0.83 (0.50-1.36) | .66 | |
| ≥25 kg/m2 (no. of cases) | 57 | 19 | 23 | 15 | |
| Model 1 HR (95% CI)b | 1 (Reference) | 1.25 (0.67-2.35) | 0.77 (0.38-1.56) | .76 | |
| Stratified by coffee consumption | |||||
| <4 cups/wk (no. of cases) | 97 | 30 | 40 | 27 | |
| Model 2 HR (95% CI)c | 1 (Reference) | 1.11 (0.68-1.80) | 0.67 (0.39-1.14) | .54 | |
| ≥5 cups/wk (no. of cases) | 61 | 23 | 22 | 16 | |
| Model 2 HR (95% CI)c | 1 (Reference) | 1.10 (0.61-2.00) | 0.97 (0.50-1.88) | .72 | |
| Excluding participants with a history of diabetes | |||||
| No. of cases | 156 | 50 | 60 | 46 | |
| Model 3 HR (95% CI)d | 1 (Reference) | 1.10 (0.75-1.62) | 0.78 (0.51-1.19) | .68 | |
- a Energy-adjusted values calculated using the residual method.
- b Adjusted for age (y), area (10 public health center areas in Japan), age at menarche (≤13, 14-15, ≥16 y), number of deliveries (0, 1-2, ≥3), menopause status, and age at menopause (premenopause, postmenopause [≤49, 50-54, ≥55 y]), use of exogenous female hormones (yes, no), smoking status (never, ever, <20 cigarettes/d, ≥20 cigarettes/d), alcohol intake (non-drinker, occasional drinker, regular drinker ≤150 g or >150 g of ethanol per week), leisure-time physical activity (none, ≤2 d/wk, ≥3 d/wk), coffee consumption (none, 1-4 cups/wk, 5-7 cups/wk, ≥2 cups/d), total energy (quartile), and diabetes mellitus (yes, no).
- c The same as model 1 but further adjusted for body mass index (<21, 21-23.9, 24-26.9, ≥27 kg/m2) and excluded coffee consumption.
- d The same as model 1 but further adjusted for body mass index (<21, 21-23.9, 24-26.9, ≥27 kg/m2) and excluded history of diabetes mellitus.
4 DISCUSSION
Evidence on the association of GI and GL with the risk of endometrial cancer in Asians is limited. In this prospective study with a long follow-up period, we found null associations among GI, GL, and the risk of endometrial cancer. The results remained unchanged in the stratified analysis, including stratification for BMI.
In contrast with our findings, previous meta-analyses in North American and European countries10, 23 suggested a significant association between GL and the risk of endometrial cancer. The subtotal RR among prospective cohort studies was 1.22 (95% CI, 1.09-1.37).10 Such difference could be due to the difference in the proportion of obese participants, with Japan having a lower proportion of obese individuals (BMI ≥ 30) than North American and European countries. A total of 38.2% of US adults are obese, whereas only 3.7% of Japanese adults are.24 This is further supported by the difference in the results of stratified analysis by BMI in some prospective studies.25, 26 For example, Larsson et al found that GL was positively associated with the risk of endometrial cancer among overweight and inactive women (the RR for the highest vs lowest GL was 2.99 [95% CI, 1.17-7.67; Ptrend = .02]).25 In contrast, we did not find such an association. This indicates that differences in the proportion of obese participants might affect the difference in the results between previous studies in North America and Europe10, 23, 25, 26 and ours.
The difference in dietary factors that contributed to GI and GL might be the other reason for the inconsistency between the previous meta-analysis and our results. As shown in Table S2, the GL of Japanese study participants is mainly derived from rice as their staple food, with 53% of GL in our study derived from white rice. This is consistent with previous studies from Japan that reported that over 50% of GL was from white rice.12, 13, 27 Conversely, the GI and GL of non-Asians are derived from various foods such as cereals and bread.12, 13, 27 In the United States, GL is derived from sweetened beverages (12.14%), bread (11.44%), starchy side dishes (8.62%), or cereals (7.43%).28 Even in Japan, the GL of Okinawa was relativity lower than that of other areas (data not shown); however, the association was not changed. The Japanese, who mainly eat white rice (GI = 76) as a staple food, could have a lower variation in their source of GI and GL than non-Asians who consume more varied types of sugar- and carbohydrate-containing food such as cereals (GI = 81) and bread (GI = 75).18 Therefore, because of the small variation in GI and GL in Japanese individuals due to their dietary habits, it might be difficult to find an association between GL and the risk of endometrial cancer.
Two pathways have been suggested as potential mechanisms that increase the risk of developing endometrial cancer. First, insulin resistance from obesity decreases blood glucose tolerance, increases insulin secretion during meals,29 and leads to an increase in bioavailable estradiol in the blood by lowering the sex hormone-binding protein.6 Second, bioavailable estradiol is also increased by obesity because, in mast cells, androgen is converted into estradiol by aromatase.1 This study included a low proportion of obese participants, and thus the effects of the above pathways might be limited. Even after stratification for factors that could affect these pathways (eg BMI, coffee intake,30 and cancer type [especially type 1]1, 31), the results did not change.
The strengths of this study include a long follow-up period, a high response rate (83.7%), a high follow-up rate (91.8%) from the questionnaire, and reliable cancer information from cancer registry. However, this study also has some limitations. First, although 166 new cases of endometrial cancer were identified, the number was relatively smaller than that in studies from Europe and North America.25, 26 This is because the incidence of endometrial cancer in Japan is lower than in Europe and North America.3, 4 Further pooled analysis including a larger number of cases is required to more accurately identify the association of GI and GL and the risk of endometrial cancer. Second, information on food intake, which was used for calculating GI and GL, was collected using a self-reported questionnaire; hence, there might be a measurement error. However, this measurement error might not have a profound impact because GI and GL are mainly derived from rice, and a reporting error of this item is unlikely because it is the main staple food of the Japanese.12, 13, 27 Third, the FFQ in our study was not especially designed to calculate the dietary GI and GL, and a study for the validity including a direct comparison with dietary records was not conducted. However, the association between GL and diabetes mellitus has already been reported previously,32 and the validity of the FFQs for assessing the nutritional components of GI and GL has already been confirmed.15, 16 Our GI and GL values reflect valid value to some extent according to the already published papers from our prospective studies.17, 32, 33 Finally, even after adjusting for confounding factors to the fullest extent possible, there was a possibility that unmeasured residual confounding factors existed. Given the increasing importance of dietary factors in preventing endometrial cancer, further research should be undertaken to confirm the association in Asian countries.
In conclusion, GI and GL were not significantly associated with the risk of endometrial cancer in Japanese participants from a long follow-up prospective study.
ACKNOWLEDGMENTS
We thank all the staff members for their contributions and efforts in the survey. The members of the JPHC Study Group are listed at https://epi.ncc.go.jp/en/jphc/781/index.html. This study was supported by the National Cancer Center Research and Development Fund (since 2011) and a Grant-in-Aid for Cancer Research from the Ministry of Health, Labour and Welfare of Japan (1989-2010).
DISCLOSURE
The authors declare no conflict of interest.
ETHICAL APPROVAL AND CONSENT TO PARTICIPATE
Participants were informed of the purpose of the study and were considered to have agreed to the study by answering the questionnaire. This study was approved by the Institutional Review Board at the National Cancer Center (approval number: 2015-085). All the work conformed to the provisions of the Declaration of Helsinki.
Open Research
DATA AVAILABILITY STATEMENT
Data described in the manuscript, codebook, and analytic code will be made available upon request pending approval (for information on how to submit an application for gaining access to JPHC data/or biospecimens, please follow the instructions at http://epi.ncc.go.jp/en/jphc/805/8155.html).




