FOXO1 inactivation induces cisplatin resistance in bladder cancer
Ide and Goto contributed equally to this work.
Graphical Abstract
We found that FOXO1-shRNA sublines or FOXO1-positive cells co–treated with a FOXO1 inhibitor were significantly more resistant to cisplatin treatment at pharmacological concentrations, compared with respective control sublines or those with mock treatment. Western blot demonstrated considerable increases in the expression levels of a phosphorylated inactive form of FOXO1 (p-FOXO1) in cisplatin-resistant sublines established by long-term culture with low/increasing doses of cisplatin, compared with respective controls. Immunohistochemistry in surgical specimens from patients with muscle-invasive bladder cancer undergoing cisplatin-based neoadjuvant therapy further showed a strong trend to associate between p-FOXO1 positivity and unfavorable response to chemotherapy.
Dear Editor,
Urinary bladder cancer has been one of the most commonly diagnosed malignancies and is often a highly aggressive disease. Since the late 1980s, cisplatin (CDDP)-based combination chemotherapy has been the standard of care in patients with muscle-invasive bladder cancer in a neoadjuvant or adjuvant setting, and in those with metastatic disease.1 Importantly, the mortality rate of bladder cancer patients has not significantly improved in the past several decades.2 Consequently, the development of chemosensitization strategies constitutes a goal with critical clinical implications.
Emerging evidence suggests that androgen receptor (AR) and estrogen receptor (ER)-β signals promote urothelial cancer progression.3, 4 We have additionally demonstrated that AR5 and ERβ6 activation is associated with resistance to CDDP treatment in bladder cancer cells. The rates of AR and ERβ immunoreactivity in bladder cancer specimens from patients subsequently undergoing neoadjuvant chemotherapy were also found to be higher in those from non–responders, compared with responders. Meanwhile, our recent study, using preclinical models for urothelial cancer, indicated that FOXO1, a transcription factor known to be targeted for phosphorylation through several protein kinases on specific sites (eg Ser256 by AKT), was inactivated in tumor cells through the AR/ERβ pathways as their downstream target and functioned as a tumor suppressor.7 Specifically, the expression of AR/ERβ vs FOXO1 or its phosphorylated inactive form (p-FOXO1) was inversely or positively, respectively, correlated. In the present study, we further assessed the role of FOXO1 in modulating chemosensitivity in bladder cancer.
Detailed information for experimental procedures is described in Appendix S1. We first compared the cytotoxic effects of CDDP in three sets of control vs FOXO1 knockdown bladder cancer sublines expressing shRNA or siRNA (Figure S1). Cell viability assay showed dose-dependent growth inhibition by CDDP, and FOXO1-shRNA/siRNA sublines were significantly more resistant to CDDP treatment at 1.5-12.5 µmol/L, compared with respective control sublines (Figure 1A). CDDP cytotoxicity was also compared in FOXO1-positive cells with and without treatment of a FOXO1 inhibitor, AS1842856. Consistent with the findings in FOXO1 knockdown cells, the inhibitory effect of CDDP was considerably diminished when AS1842856 was co–treated (Figure 1B). In these assays, induction of cell growth by FOXO1 inhibition, irrespective of CDDP, was excluded through comparison with those of respective sublines or lines with respective treatments without CDDP. TUNEL assay was then performed to assess the impact of FOXO1 knockdown on CDDP-induced apoptosis. Correspondingly, there were significant decreases in apoptosis in FOXO1-shRNA/siRNA sublines, compared with respective control sublines (Figure 1C). These data indicate an association between FOXO1 activity and CDDP sensitivity in bladder cancer cells.
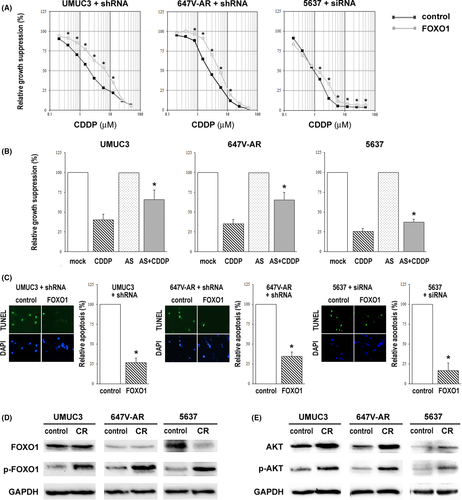
In our previous studies5, 8 we established CDDP-resistant (CR) sublines from UMUC3, 647V-AR and 5637 by long-term culture with low/increasing doses of CDDP. UMUC3-CR,5 647V-AR-CR5 and 5637-CR (Figure S2) cells were shown to be significantly more resistant to CDDP treatment at its pharmacological concentrations (eg 1.3-8.4 µmol/L). Using these sublines, we compared the expression levels of FOXO1 and p-FOXO1 (Ser256). Western blot showed no significant increase (AR-positive/ERβ-positive UMUC3/647V-AR, where the basal level was relatively low)7 or a considerable decrease (AR-negative/ERβ-positive 5637, where it was relatively high)7 in FOXO1 expression in CR sublines (Figure 1D). More interestingly, there were considerable increases in p-FOXO1 expression in all three CR sublines, compared with respective controls. In these control/CR sublines, we further assessed the expression of AKT/p-AKT through which FOXO1 could be phosphorylated and inactivated. Correspondingly, AKT and p-AKT expression were upregulated in CR sublines (Figure 1E). Indeed, AKT activation has also been implicated in chemoresistance in various types of malignancies.9 These data support the association of FOXO1 inactivation with CDDP resistance in bladder cancer cells.
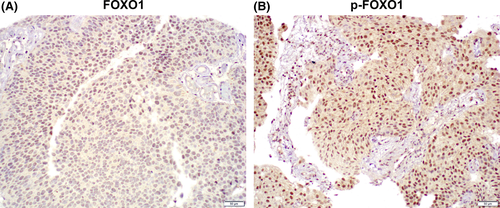
Finally, we immunohistochemically stained for FOXO1 and p-FOXO1 in a set of tissue microarray consisting of muscle-invasive bladder cancer specimens from 46 patients who had subsequently received CDDP-based neoadjuvant chemotherapy. Positive signals of FOXO1 and p-FOXO1 were detected predominantly in the nucleus of urothelial cancer cells (Figure 2). Predominant nuclear localization of FOXO1/p-FOXO1 was confirmed by immunofluorescence in 5637 cells (Figure S3). Overall, FOXO1 and p-FOXO1 were expressed in 5 (10.9%) and 26 (56.5%) tumors, respectively (Table 1). We then compared the levels of FOXO1 and p-FOXO1 expression in tumors from responders (n = 18) vs non–responders (n = 28) to the neoadjuvant chemotherapy. FOXO1 or p-FOXO1 was immunoreactive in 3 (16.7%) or 7 (38.9%) responders vs 2 (7.1%) or 19 (67.9%) non–responders, respectively. Thus, p-FOXO1 positivity tended to associate with chemoresistance (P = 0.053). In addition, when the status of AR5 and ERβ6 immunoreactivity previously determined in tissue samples where the entire current cohort was included (AR-negative: n = 32; AR-positive: n = 14; ERβ-negative: n = 22; ERβ-positive: n = 24) was considered, the association of chemosensitivity with p-FOXO1/AR (ie p-FOXO1-negative/AR-negative vs p-FOXO1-positive/AR-positive) (P = 0.039; Table S1) or p-FOXO1/ERβ (ie p-FOXO1-negative/ERβ-negative vs p-FOXO1-positive/ERβ-positive) (P = 0.010; Table S2) expression was statistically significant. These data further support that FOXO1 inactivation is associated with CDDP resistance in patients with bladder cancer.
| n | FOXO1 | p-FOXO1 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Expression levels | P valuea | Expression levels | P valuea | ||||||
| 0 | 1+ | 0 | 1+ | 2+ | 1+/2+ | ||||
| Responders | 18 | 15 (83.3%) | 3 (16.7%) | 0.331 | 11 (61.1%) | 3 (16.7%) | 4 (22.2%) | 7 (38.9%) | 0.053 |
| Non–responders | 28 | 26 (92.9%) | 2 (7.1%) | 9 (32.1%) | 14 (50.0%) | 5 (17.9%) | 19 (67.9%) | ||
- a Negative (0) vs positive (1+ or 1+/2+).
Although resistance to CDDP-based chemotherapy is commonly seen in urothelial cancer patients, its underlying mechanisms remain poorly understood. Nonetheless, other FOXO family members, such as FOXO3a, have been implicated in CDDP resistance.10 The present study demonstrated strong associations between FOXO1 inactivation via its shRNA/siRNA expression or inhibitor treatment and reduced sensitivity to CDDP, presumably through inhibiting apoptosis induced by CDDP in bladder cancer cells as well as between the elevated expression of phosphorylated inactive p-FOXO1 in bladder cancer lines or tissue samples and CDDP resistance. While recent immunohistochemical studies using surgical specimens have indicated that the status of FOXO17, 11, 12 or p-FOXO17 expression predicts the recurrence and/or progression of bladder tumors, p-FOXO1 expression is here suggested to serve as a predictor of chemoresistance, especially along with AR/ERβ expression, in patients with muscle-invasive disease. These findings indicate a new role for FOXO1, as a tumor suppressor, in modulating chemosensitivity, in addition to preventing urothelial tumorigenesis and tumor growth. Specifically, FOXO1 inactivation is likely to induce CDDP resistance in bladder cancer cells. Accordingly, FOXO1 activation has the potential to be a means of chemosensitization, especially in patients with p-FOXO1-positive tumors. Further studies are required to determine exactly how FOXO1 signals modulate chemosensitivity in bladder cancer.
DISCLOSURE
The authors have no conflict of interest to disclose.





