Pharmacokinetic study of the oral fluorouracil antitumor agent S-1 in patients with impaired renal function
Clinical Trial Registration: UMIN000003959
Abstract
Although dose reduction of S-1 is recommended for patients with impaired renal function, dose modification for such patients has not been prospectively evaluated. The aim of the present study was to investigate the pharmacokinetic parameters of 5-fluorouracil, 5-chloro-2,4 dihydroxypyridine and oteracil potassium, and to review the recommended dose modification of S-1 in patients with renal impairment. We classified patients receiving S-1 into 4 groups according to their renal function, as measured using the Japanese estimated glomerular filtration rate (eGFR) equation. The daily S-1 dose was adjusted based on the patient's eGFR and body surface area. Blood samples were collected for pharmacokinetic analysis. A total of 33 patients were enrolled and classified into 4 groups as follows: 10 patients in cohort 1 (eGFR ≥ 80 mL/min/1.73 m2), 10 patients in cohort 2 (eGFR = 50-79 mL/min/1.73 m2), 10 patients in cohort 3 (eGFR = 30-49 mL/min/1.73 m2), and 3 patients in cohort 4 (eGFR < 30 mL/min/1.73 m2). Those in cohorts 3 and 4 treated with an adjusted dose of S-1 showed a similar area under the curve for 5-fluorouracil (941.9 ± 275.6 and 1043.5 ± 224.8 ng/mL, respectively) compared with cohort 2 (1034.9 ± 414.3 ng/mL). Notably, while there was a statistically significant difference between cohort 1 (689.6 ± 208.8 ng/mL) and 2 (P = 0.0474) treated with an equal dose of S-1, there was no significant difference observed in the toxicity profiles of the cohorts. In conclusion, dose adjustment of S-1 in patients with impaired renal function using eGFR is appropriate and safe.
1 BACKGROUND
S-1 (TS-1; Taiho Pharmaceutical; Teysuno, Nordic Group) is a combination drug containing tegafur, a pro-drug of 5-fluorouracil as the active agent, and the 2 biochemical modulators 5-chloro-2,4 dihydroxypyridine (CDHP) and oteracil potassium (Oxo). S-1 is administered as a capsule, granulated medicine or orally disintegrating tablet in a molar ratio of 1:0.4:1 (tegafur: CDHP: Oxo), with each oral form containing 20 or 25 mg tegafur. CDHP inhibits the activity of dihydropyrimidine dehydrogenase (DPD), an enzyme that degrades 5-fluorouracil. Oxo is distributed in the gastrointestinal tract mucosa, preventing the activation of 5-fluorouracil.1-3 S-1 maintains the therapeutic plasma concentration of 5-fluorouracil by inhibiting the activity of DPD, while reducing 5-fluorouracil-induced gastrointestinal toxicity through Oxo.4, 5 It is currently approved for the treatment of gastric, colorectal, head and neck, breast, pancreatic, bile tract, and non–small cell lung cancers in Japan.
Of note, 5-fluorouracil is mainly eliminated by the liver and excreted as expiratory CO2. Therefore, in general, there is no requirement for dose adjustment in patients with renal impairment. In contrast, CDHP is predominantly excreted in urine.4 Hence, lower CDHP clearance in patients with renal impairment leads to greater inhibition of DPD activity, higher plasma concentrations of 5-fluorouracil and an increased incidence of toxicity.5 Therefore, the dose of S-1 is usually determined on the basis of body surface area (BSA) and adjusted according to renal function. A post–marketing survey of S-1 involving 3294 patients with advanced gastric cancer in Japan demonstrated a close relationship between the incidence of grade 3 or worse hematological toxicity and renal function.6 This survey recommended that S-1 doses be reduced in patients with impaired renal function to prevent the occurrence of adverse reactions.6
Although the prescribing information for S-1 recommends a reduction in its dose to manage adverse reactions in patients with impaired renal function, there are no prospective pharmacokinetic and safety studies conducted in this setting. The aim of the present study was to prospectively investigate the pharmacokinetic profiles of 5-fluorouracil and CDHP, and to evaluate the recommended dose modification of S-1 in patients with renal impairment.
2 MATERIAL AND METHODS
2.1 Patient eligibility
Eligibility criteria were as follows: age 20 years or older; histologically or cytologically confirmed malignant solid tumor; S-1 chemotherapy planned as part of clinical practice; Eastern Cooperative Oncology Group performance status 0-2; adequate hematopoietic and hepatic function (absolute neutrophil count ≥ 1500/μL, platelet count ≥ 75 000/μL, hemoglobin ≥ 9.0 g/dL, aspartate aminotransferase and alanine aminotransferase ≤ 1.5 × upper limit of institutional normal level [ULN], and total bilirubin ≤ 1.5 × ULN); and recovery from any adverse events (AE) caused by previous chemotherapy.
Exclusion criteria were as follows: currently receiving treatment with dialysis or estimated glomerular filtration rate (eGFR) < 20 mL/min/1.73 m2; malabsorption such as watery diarrhea, intestinal paralysis or ileus; received treatment with cisplatin and/or S-1 in the previous 6 months; underwent major surgery in the previous 2 weeks; actively receiving treatment with warfarin, phenytoin or flucytosine (ie, potential drug-drug interaction); presence of serious concomitant disorder, including active infection or active peptic ulcer; history of interstitial lung disease; and previous administration of any anticancer agents at least 3 weeks before enrollment in the present study.
2.2 Study design
This prospective study was conducted at 3 institutions in Japan (Kobe University Hospital, Shimane University Hospital and National Cancer Center Hospital).
The primary objective of this study was to evaluate the steady-state pharmacokinetic parameters of 5-fluorouracil and CDHP on day 8 of treatment in patients with various degrees of renal function receiving S-1. The eGFR and BSA were used to adjust the dose of S-1 and the appropriateness of this approach was determined. The secondary objective was to evaluate toxicity, including nausea, vomiting, oral stomatitis, diarrhea and myelosuppression.
The study protocol was approved by the review board of each participating institution. The study was conducted in accordance with the ethical principles stated in the Declaration of Helsinki. Written informed consent was obtained from each participant.
2.3 Treatment and assessment of treatment
We used the Japanese eGFR equation7 to estimate renal function for classification. Historically, GFR has been considered the most reliable index for assessing overall renal function.8 It can be precisely measured using filtration markers such as 51Cr-ethylenediaminetetraacetic acid, 125I-iothalamate, iohexol or inulin.9-11 However, the techniques used for the direct measurement of renal function are complex and time-consuming. Therefore, they are not routinely used in oncology practice. Instead, equations to estimate renal function using serum creatinine (SCr) values have been developed, such as the Cockcroft-Gault formula (CGF),12 and the Modification of Diet in Renal Disease (MDRD) study13 and the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equations.14 In 2008, the Japanese Society of Nephrology established the Japanese eGFR equation.7 The eGFR has been widely accepted as a reliable and simple method for estimating GFR in medical practice in Japan. Although the eGFR equation was developed based on data obtained from Japanese patients with chronic kidney disease (CKD), it offers higher accuracy for predicting GFR than the CGF and MDRD in cancer patients prior to and after treatment with cisplatin.15 The eGFR was calculated using the following formula: eGFR (mL/min/1.73 m2) = 194 × SCr(−1.094) × Age(−0.287) (×0.739 if female). Patients were classified into 4 groups according to their renal function at screening, as follows: cohort 1, normal renal function (eGFR ≥ 80 mL/min/1.73 m2); cohort 2, mild dysfunction (eGFR = 50-79 mL/min/1.73 m2); cohort 3, moderate dysfunction (eGFR = 30-49 mL/min/1.73 m2); and cohort 4, severe dysfunction (eGFR < 30 mL/min/1.73 m2) (Table 1).
| BSA (m2) | Cohort 1; eGFR ≥ 80 | Cohort 2; eGFR = 50-79 | Cohort 3; eGFR = 30-49 | Cohort 4; eGFR < 30 |
|---|---|---|---|---|
| ≤1.25 | 40 mg bid; N = 0 | 40 mg bid; N = 0 | 40 mg bid; N = 0 | 25 mg bid; N = 1 |
| 1.25 ≤ 1.5 | 50 mg bid; N = 6 | 50 mg bid; N = 2 | 50 mg bid; N = 2 | 40 mg bid; N = 0 |
| >1.5 | 60 mg bid; N = 4 | 60 mg bid; N = 8 | 50 mg bid; N = 8 | 40 mg bid; N = 2 |
- The number in each column indicates the actual number of patients enrolled in each cohort.
- BSA, body surface area; eGFR, estimated glomerular filtration rate.
S-1 was administered orally twice daily for more than 14 consecutive days. The dose was adjusted based on the patient's BSA (as stated in the prescribing information in Japan) and modified according to renal function (Table 1). Patients continued to receive S-1 until disease progression, clinical deterioration or the development of intolerable AE that did not improve with supportive care or dose reduction (whichever occurred first).
For the assessment of toxicity and definition of AE, we used the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0 (National Cancer Institute, Bethesda, MD, USA).
2.4 Pharmacokinetic evaluation and analysis
Blood samples were collected on day 8, when CDHP was considered to be at a steady state. This was based on the terminal half-life of 5-fluorouracil and CDHP being approximately 2.9 ± 1.1 and 4.2 ± 1.4 hours, respectively, after 28-day consecutive administration.4 Samples were obtained prior to administration and at 0.5, 1, 2, 4, 6 and 8 hours after administration by drawing 5 mL of blood into heparin-containing tubes. Plasma was separated within 30 minutes through centrifugation at 1500 g for 10 minutes at 4°C and stored at −80°C until analysis. Plasma concentrations of 5-fluorouracil, CDHP, tegafur and Oxo were determined using a liquid and gas chromatography-tandem mass spectrometry assay (FALCO Biosystems) as previously described by Matsushima et al.16 The lower limit of quantification for each compound was as follows: tegafur, 10.0 ng/mL; 5-fluorouracil, 1.0 ng/mL; CDHP, 2.0 ng/mL; and Oxo, 2.0 ng/mL. The pharmacokinetic parameters of tegafur, 5-fluorouracil, CDHP and Oxo were determined using the Phoenix WinNonlin pharmacokinetic program (Pharsight) version 4.01. The area under the curve (AUC) for up to 8 hours (AUC0-8 hours) was calculated using the trapezoidal rule. The linear trapezoidal rule was used for successively increasing concentration values, whereas the logarithmic trapezoidal rule was used for decreasing concentration values.
2.5 Statistical analysis
As in previous pharmacokinetic studies, we considered that 6-8 patients per cohort were sufficient for the evaluation of pharmacokinetics. The target number of patients in each cohort was set at 10. Pharmacokinetic parameters were compared among cohorts using Dunnett's test. Patient characteristics and AE in each cohort were compared using Fisher's exact test. The correlation between the clearance of CDHP or AUC of 5-fluorouracil and eGFR was determined using the Pearson product-moment correlation coefficient. All statistical analyses were performed using the JMP (SAS Institute, version 11.2.0).
3 RESULTS
3.1 Patient characteristics
A total of 33 Japanese patients were enrolled from September 2010 to June 2014 and classified into 4 cohorts according to renal function (Table 1). Patient characteristics are summarized in Table 2 and Table S1. Due to a shortage of suitable patients enrolled in cohort 4, only 3 patients were included in this cohort. In the present study, 25 patients were male and 8 patients were female, with a median age of 68 years (range, 37-85 years). Median BSA was 1.60 m2 (range, 1.24-1.98 m2). There was only 1 patient with BSA < 1.25 m2 in cohort 4. Nine patients had a history of previous treatment with S-1 as postoperative adjuvant chemotherapy. The most frequent type of tumor was gastric cancer (39%).
| Number of patients | Cohort 1 | Cohort 2 | Cohort 3 | Cohort 4 | Total |
|---|---|---|---|---|---|
| 10 | 10 | 10 | 3 | 33 | |
| eGFR (mL/min/1.73 m2) | 91.0 (82.3-118.1) | 69.3 (50-76.8) | 41.0 (34.5-49.1) | 27.4 (26.2-28.5) | 65.7 (26.2-118.1) |
| CLcr (mL/min) | 85.4 (54.2-100.1) | 69.3 (39.1-93.1) | 44.7 (29.7-66.8) | 32.5 (20.1-35.9) | 59 (20.1-100.1) |
| SCr (mg/dL) | 0.65 (0.54-0.76) | 0.83 (0.62-1.15) | 1.21 (0.94-1.61) | 1.94 (1.43-2.04) | 0.89 (0.54-2.04) |
| Height (cm) | 161.6 (153-173) | 162.5 (152.4-170) | 160.7 (148-175.5) | 168.4 (151.4-179) | 161.9 (148-179) |
| Weight (kg) | 49.7 (37-76.4) | 57.3 (39.5-87.4) | 59.1 (38.8-83.2) | 55.3 (35.3-74) | 55.6 (35.3-87.4) |
| BSA (m2) | 1.48 (1.28-1.9) | 1.61 (1.32-1.98) | 1.6 (1.27-1.9) | 1.63 (1.24-1.92) | 1.6 (1.24-1.98) |
- BSA, body surface area; CLcr, clearance of creatinine; eGFR, estimated glomerular filtration rate; SCr, serum creatinine.
3.2 Pharmacokinetics
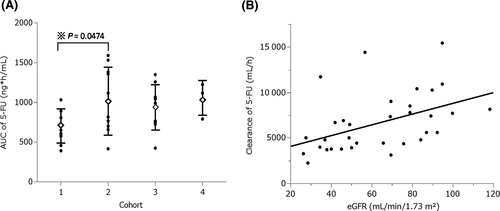
Mean values of pharmacokinetic parameters for CDHP, 5-fluorouracil, tegafur and Oxo on day 8 are presented in Table 3. The plasma concentration-time profiles of 5-fluorouracil, CDHP and tegafur according to renal function are shown in Figure S1. Cohorts 3 and 4 (ie, moderate or severe renal dysfunction) showed a similar plasma concentration of 5-fluorouracil (941.9 ± 275.6 and 1043.5 ± 224.8 ng/mL, respectively) to cohort 2 (1034.9 ± 414.3 ng/mL; Figure 1A). In contrast, patients in cohort 1 (ie, normal renal function) had a significantly lower AUC0-8 hours of 5-fluorouracil than those in cohort 2 (ie, mild renal dysfunction) (Figure 1A, P = 0.0474), despite receiving treatment with an equal dose of S-1. Consequently, a weak negative correlation was observed between the AUC0-8 hours of 5-fluorouracil and eGFR (Figure S2A, r2 = 0.146, P = 0.0280). The correlation between clearance of 5-fluorouracil and eGFR is shown in Figure 1B (r2 = 0.21, P = 0.0079). Clearance of CDHP correlated positively with renal function (Table 3 and Figure S2B, r2 = 0.36, P = 0.0002) and was significantly decreased in cohorts 3 and 4 compared with that reported in cohort 1 (Figure S2C, P = 0.0048 and P = 0.0027, respectively). In addition, clearance of CDHP was also correlated with clearance of creatinine (Figure S2D).
| Cohort | Cmax (ng/mL) | Tmax (h) | AUC (ng*h/mL) | Clearance (mL/h) |
|---|---|---|---|---|
| 1 | ||||
| FT | 4301.2 ± 925.2 | 1.6 ± 1.1 | 27 029.8 ± 8896.8 | 2240 ± 867 |
| 5-FU | 133.1 ± 42.6 | 3.4 ± 1.0 | 689.6 ± 208.8 | 86 398 ± 32 179 |
| CDHP | 263.2 ± 119.4 | 2.4 ± 1.2 | 1188.1 ± 345.9 | 5010 ± 1897 |
| Oxo | 82.0 ± 54.1 | 2.4 ± 1.2 | 376.1 ± 224.8 | 182 246 ± 90 278 |
| 2 | ||||
| FT | 3111.8 ± 815.1 | 1.5 ± 0.8 | 17 907.2 ± 5765.6 | 3560 ± 1210 (P = 0.0187) |
| 5-FU | 206.0 ± 85.6 | 2.6 ± 1.1 | 1034.9 ± 414.3 (P = 0.0474) | 67 508 ± 34 500 (P = 0.3854) |
| CDHP | 383.2 ± 148.9 | 1.9 ± 0.9 | 1691.9 ± 517.0 | 3825 ± 1514 (P = 0.2051) |
| Oxo | 182.5 ± 260.9 | 3.2 ± 1.6 | 690.2 ± 671.0 | 185 011 ± 192 701 (P = 1.000) |
| 3 | ||||
| FT | 3538.2 ± 1169.9 | 2.0 ± 1.2 | 22 514.6 ± 7773.4 | 2382 ± 906 (P = 0.9811) |
| 5-FU | 167.1 ± 40.8 | 3.6 ± 0.8 | 941.9 ± 275.6 (P = 0.1897) | 56 916 ± 24 671 (P = 0.0934) |
| CDHP | 390.0 ± 159.1 | 2.4 ± 1.2 | 1955.9 ± 613 | 2725 ± 1034 (P = 0.0048) |
| Oxo | 50.4 ± 0.40 | 2.8 ± 1.3 | 275.3 ± 189.6 | 310 304 ± 321 360 (P = 0.4348) |
| 4 | ||||
| FT | 2294.7 ± 99.3 | 1.5 ± 0.9 | 13 444.6 ± 2540.5 | 2734 ± 1080 (P = 0.8145) |
| 5-FU | 174.3 ± 32.1 | 3.3 ± 1.1 | 1043.5 ± 224.8 (P = 0.2216) | 35 210 ± 14 218 (P = 0.0395) |
| CDHP | 389.7 ± 30.3 | 2.0 ± 0.0 | 2472.5 ± 163.7 | 1430 ± 413 (P = 0.0027) |
| Oxo | 105.7 ± 68.1 | 2.7 ± 1.2 | 576.8 ± 402.1 | 91 855 ± 69 041 (P = 0.8717) |
- P-value; compared with cohort 1.
- 5-FU, 5-fluorouracil; AUC, area under the curve; CDHP, 5-chloro-2,4 dihydroxypyridine; FT, tegafur; Oxo, oteracil potassium.
3.3 Toxicity
The toxicity profiles during the first 15 days of administration are summarized in Table 4. The frequency and severity of AE were similar among the different cohorts. However, the frequency of decreased platelet count tended to be higher in cohort 3 compared to cohort 1. In addition, there was no difference observed among cohorts in the frequency of S-1 dose suspension or reduction. Toxicities of CTCAE grade 3 and 4 accounted for 20%-30% of all toxicities reported in each cohort.
| CTCAE grade | Cohort 1 | Cohort 2 | Cohort 3 | Cohort 4 | Total | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Any grade | Grade 3, 4 | Any grade | Grade 3, 4 | Any grade | Grade 3, 4 | Any grade | Grade 3, 4 | Any grade | Grade 3, 4 | |
| ANC decreased | 3 (30%) | 0 | 1 (10%) | 0 | 4 (40%) | 0 | 0 | 0 | 8 (24%) | 0 |
| PLT decreased | 1 (10%) | 0 | 0 | 0 | 4 (40%) | 0 | 1 (33%) | 0 | 6 (18%) | 0 |
| Anemia | 1 (10%) | 0 | 2 (20%) | 0 | 1 (10%) | 0 | 0 | 0 | 4 (12%) | 0 |
| AST increased | 2 (20%) | 0 | 1 (10%) | 0 | 0 | 0 | 0 | 0 | 3 (9%) | 0 |
| ALT increased | 2 (20%) | 0 | 1 (10%) | 0 | 1 (10%) | 0 | 0 | 0 | 4 (12%) | 0 |
| SCr increased | 0 | 0 | 1 (10%) | 0 | 2 (20%) | 0 | 0 | 0 | 3 (9%) | 0 |
| Malaise | 3 (30%) | 0 | 3 (30%) | 0 | 4 (40%) | 0 | 1 (33%) | 0 | 11 (33%) | 0 |
| Nausea | 2 (20%) | 0 | 4 (40%) | 1 (10%) | 2 (20%) | 0 | 1 (33%) | 0 | 9 (27%) | 1 (3%) |
| Vomiting | 0 | 0 | 2 (20%) | 0 | 1 (10%) | 0 | 0 | 0 | 3 (9%) | 0 |
| Anorexia | 4 (40%) | 1 (10%) | 4 (40%) | 2 (20%) | 3 (30%) | 0 | 1 (33%) | 0 | 12 (36%) | 3 (9%) |
| Oral mucositis | 0 | 0 | 2 (20%) | 1 (10%) | 1 (10%) | 1 (10%) | 0 | 0 | 3 (9%) | 2 (6%) |
| Diarrhea | 2 (20%) | 0 | 3 (30%) | 0 | 2 (20%) | 0 | 1 (33%) | 0 | 8 (24%) | 0 |
| Skin rash | 1 (10%) | 0 | 2 (20%) | 0 | 3 (30%) | 1 (10%) | 0 | 0 | 6 (18%) | 1 (3%) |
| Others | 3 (30%) | 1 (10%) | 1 (10%) | 0 | 3 (30%) | 1 (10%) | 0 | 0 | 7 (21%) | 2 (6%) |
| All toxicities | 9 (90%) | 2 (20%) | 9 (90%) | 3 (30%) | 10 (100%) | 2 (20%) | 2 (66%) | 0 | 30 (91%) | 8 (24%) |
- ALT, alanine aminotransferase; ANC, absolute neutrophil count; AST, aspartate aminotransferase; PLT, platelet count; SCr, serum creatinine; WBC, white blood cell counts.
4 DISCUSSION
Impairment of renal function leads to decreased clearance of CDHP and, consequently, 5-fluorouracil. This results in increased exposure to 5-fluorouracil. In this study, we investigated whether dose adjustment of S-1 in patients with impaired renal function (using the eGFR as an index) was appropriate with regard to pharmacokinetics and toxicity. The results showed that patients with moderate or severe renal dysfunction (ie, cohorts 3 and 4) treated with a reduced dose of S-1 exhibited similar plasma concentrations of 5-fluorouracil and lower clearance of CDHP as opposed to those with mild renal dysfunction (ie, cohort 2) (Figure 1 and Table 3). This finding indicated that the present strategy for dose adjustment of S-1 based on the BSA and eGFR was pharmacokinetically acceptable and not expected to constitute overtreatment or undertreatment.
In contrast, patients with normal renal function (ie, cohort 1) exhibited significantly lower AUC of 5-fluorouracil than those in cohort 2, despite receiving an equal dosage of S-1. Moreover, no severe toxicities were observed in cohorts 3 and 4. This implies that patients with extremely good renal function are treated with a relatively low dosage, and that dose increment can be considered in these patients. This indicates a limitation in the current dosing strategy of S-1. Similarly, Fujita et al17 suggested that Japanese cancer patients with a large BSA (≥1.5 m2) may be undertreated through the usual BSA-based dosing.
As previously reported,5, 18-20 our study demonstrated a correlation between renal function and clearance of CDHP. Of note, we performed sampling for pharmacokinetic investigation on day 8, when the plasma concentration of CDHP reached a steady state.4 In contrast, previous studies performed sampling for pharmacokinetics on the first day of S-1 administration, prior to CHDP reaching a steady state.18-20 Moreover, previous studies included few patients with moderate or severe renal dysfunction.5, 18, 19 In clinical practice, S-1 is administered twice daily for 14 or 28 days. Thus, we suggest that the pharmacokinetics of S-1 observed in repeated dosing are more meaningful with regard to safety than those observed in single dosing. In this study, we enrolled 33 patients with various degrees of renal function as determined by the eGFR and included a sufficient number of patients in each cohort. Therefore, the present results are important with regard to the pharmacokinetics and safety of S-1 in patients with wide variation in renal function. Booka (2016) investigated pharmacokinetics of 5-fluorouracil, CDHP and tegafur after the single administration of S-1 at 40 mg/m2. Patients with severe renal dysfunction exhibited much higher plasma concentrations of 5-fluorouracil and CDHP compared to patients with normal or mildly impaired renal function. Notably, tegafur showed similar plasma concentrations. Although these investigators developed a similar formula for dosing S-1 and recommended dose reduction in patients with impaired renal function, they did not evaluate toxicities, and prospective validation of the formula is required. In our study, we prospectively evaluated the dosing strategy of S-1 in patients with impaired renal function with regard to pharmacokinetics as well as toxicity.
The eGFR was developed to determine the severity of renal dysfunction in non–cancer patients with CKD,7 rather than in cancer patients or for dose adjustment purposes. However, in the present study, cancer patients with renal dysfunction were adequately treated with S-1 using a dosing strategy based on the eGFR and BSA. There are various approaches to the assessment of renal function. The objective standard is direct measurement using an extraneous substance, which is completely filtered by the glomeruli and does not undergo protein binding, metabolism, secretion or reabsorption at the renal tubular level.8 However, for example, the measurement of GFR using inulin requires repeated blood sampling, substantial consumption of water and repeated punctual urination. These complications have hampered the measurement of GFR using extraneous substances in clinical settings. Other methods, using SCr, have also been developed. However, use of SCr to estimate the GFR is problematic because the level of SCr is affected by various factors, such as muscle mass, nutritional condition, tubular secretion and diet.21 The CGF is a classical method commonly used to estimate renal function.12 However, this method was developed based on data obtained from Caucasian populations. Moreover, because the SCr was measured using the Jaffe method rather than the enzymatic method or isotope dilution mass spectrometry, the renal function may have been overestimated. For these reasons, the National Institutes of Health issued a recommendation for creatinine standardization.22 The MDRD13 and CKD-EPI14 equations are currently used to estimate GFR in United States. Similarly, in Japan, the eGFR equation was developed for patients with CKD, offering accurate estimations of the GFR in cancer patients even during chemotherapy with cisplatin.15, 23
In addition, there was no significant difference in the frequency or severity of AE among the cohorts and the toxicity profile in this study was similar to those previously reported (Table 4).
Our study has several limitations. First, only 3 patients with severe renal impairment were included in cohort 4. Second, it was not possible to prospectively assess the efficacy of S-1 in patients with renal dysfunction due to the inclusion of patients with various types of cancer. Third, it was not possible to assess the intra-day and inter-day variation in our study. Finally, polymorphisms of CYP2A6 (*4A, *7 and *9), which play a role in the biotransformation of tegafur to 5-fluorouracil, were not assessed.
In conclusion, we performed a prospective pharmacokinetic study of S-1 in patients with different levels of renal function. The results showed that patients with lower renal function maintained adequate plasma concentrations of 5-fluorouracil. Furthermore, there were no significant differences observed among the cohorts in the occurrence of AE. Therefore, we propose that the dose adjustment of S-1 used in this study for patients with impaired renal function is useful in clinical practice.
ACKNOWLEDGMENTS
This study was supported by the Cancer Research and Development Expenditure of the Ministry of Health, Labor and Welfare.
CONFLICT OF INTEREST
Yutaka Fujiwara received research grants from Abbvie, BMS, Chugai, Daiichi-Sankyo, Incyte, Merck Serono and MSD. Naomi Kiyota received honoraria from BMS and Ono Pharmaceutical Company. Kenji Tamura received honoraria from Pfizer. Noboru Yamamoto received honoraria from Eli Lilly, Ono Pharmaceutical Company, AstraZeneca, Pfizer and BMS. All remaining authors have no conflict of interest to declare.




