Modulation of the tumor microenvironment by Epstein-Barr virus latent membrane protein 1 in nasopharyngeal carcinoma
Abstract
Latent membrane protein 1 (LMP1) is a primary oncogene encoded by the Epstein-Barr virus, and various portions of LMP1 are detected in nasopharyngeal carcinoma (NPC) tumor cells. LMP1 has been extensively studied since the discovery of its transforming property in 1985. LMP1 promotes cancer cell growth during NPC development and facilitates the interaction of cancer cells with surrounding stromal cells for invasion, angiogenesis, and immune modulation. LMP1 is detected in 100% of pre-invasive NPC tumors and in approximately 50% of advanced NPC tumors. Moreover, a small population of LMP1-expressing cells in advanced NPC tumor tissue is proposed to orchestrate NPC tumor tissue maintenance and development through cancer stem cells and progenitor cells. Recent studies suggest that LMP1 activity shifts according to tumor development stage, but it still has a pivotal role during all stages of NPC development.
1 INTRODUCTION
Epstein-Barr virus (EBV) was discovered in Burkitt's lymphoma tumor cells in 1964 and is a causative agent of various human cancers.1, 2 Most of these cancers arise in the lymphatic organ, especially B lymphocyte-origin tumors.3, 4 Nasopharyngeal carcinoma (NPC), an EBV-associated malignant disease, is unique in that it is an epithelial origin tumor and expresses EBV primary oncogene latent membrane protein 1 (LMP1) at the premalignant stage.2, 5 Normal pharyngeal epithelial cells are a proliferating site for EBV, and EBV infection results in lysis of the infected cell. Thus, switching from lytic EBV infection to a latent one in the epithelium is an initial step in NPC carcinogenesis.6 Inactivation of the p16 tumor suppressor and overexpression of cyclin D1, commonly detected in the premalignant and dysplastic nasopharyngeal epithelium, may enable the transition to an EBV latent infection in the nasopharyngeal epithelium.7, 8 Of the 3 types of latent infection, NPC is categorized as a type II infection, which is associated with the expression of 3 virus-encoded proteins (EBV nuclear antigen 1, LMP1, and LMP2) and some virus-encoded RNAs, EBV-encoded small RNAs (EBERs) and BamHI A rightward transcripts (BARTs). Among these EBV gene products, LMP1 has a major role in NPC carcinogenesis (Table 1).3, 9 In addition, the importance of the tumor microenvironment is becoming clear with recent progress in the cancer research field.
| EBV latency | EBV gene transcription | Infected cell types and tumors |
|---|---|---|
| Type 0 | EBERs | Memory B cell |
| Type I | EBERs, EBNA1, BARTs | Burkitt's lymphoma |
| Type II | EBERs, EBNA1, BARTs, LMP1, LMP2 | Nasopharyngeal carcinoma, gastric cancer,a Hodgkin's lymphoma, NK/T lymphoma |
| Type III | EBERs, EBNA1, EBNA-LP, EBNA2, EBNA3A-C, BARTs, LMP1, LMP2 | Lymphoblastoid cell (infectious mononucleosis), post-transplant lymphoproliferative disease, patients with immunosuppression |
- BART, BamHI A rightward transcript; EBER, EBV-encoded small RNA; EBNA, EBV nuclear antigen; EBNA-LP, EBV nuclear antigen leader protein.
- In this review, we tentatively categorized in latency type II.
- a Expression of latent membrane protein 1 (LMP1) in gastric cancer and EBV latency of gastric cancer is controversial.
Here, we review the pathogenic roles of LMP1 in the tumor microenvironment during the development of NPC.
2 LATENT MEMBRANE PROTEIN 1 EXPRESSION IN NPC
Latent membrane protein 1 was recognized as a primary transforming gene product of EBV after its transforming property in rodent fibroblasts was reported.10 More than 30 years of LMP1 research revealed its multipotency to modulate various cell signal transduction pathways, leading to the proliferation and simultaneous suppression of apoptotic programs in LMP1-expressing cells.11-13 It is involved in not only cell transformation, but also invasion, metastasis upregulation, cytokine production, immune modulation, and tumor angiogenesis.
Although LMP1 protein was detected in only 20%-40% of NPC samples in a series of previous reports, more sensitive approaches using immunohistochemical staining have revealed LMP1 expression in almost 100% of primary NPC specimens.12, 14 However, LMP1 expression is highly heterogeneous among NPC tumor cells. The population of LMP1-positive cells is low, and LMP1 is usually detected in sparse cells and small clusters. Latent membrane protein 1 is detected in almost 100% of premalignant lesions and is especially concentrated in the basal layer, which suggests that LMP1 mainly contributes to the early stage of tumor development, and only a small population of cancer cells retains a high level of LMP1 expression in advanced NPC (Table 2).5, 14 Latent membrane protein 1 is a double-edged sword because abundant LMP1 expression inhibits cell growth and induces apoptosis in epithelial cells.15 High LMP1 expression may be involved in the maintenance of stem cell properties and LMP1-expressing epithelial cells may represent a cancer stem cell or progenitor-like cell phenotype.15-17 These reports suggest that the pathogenic role of LMP1 may change during NPC progression (Figure 1).
| Premalignant | Advanced | |
|---|---|---|
| Detection rate (IHC) | 100% | 20%-50% |
| Localization | Basal layer | Sporadically in tumor |
| Expression level | Rather homogeneous | Heterogeneous |
- IHC, immunohistochemistry.
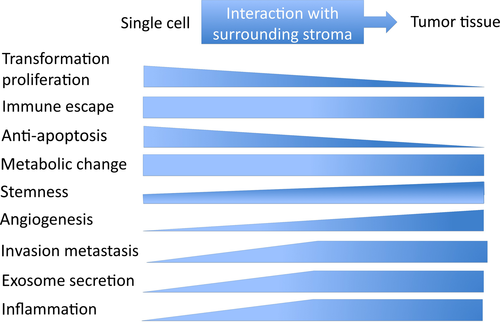
3 INTERPLAY WITH THE MICROENVIRONMENT
The development of cancer tissue depends on the interaction between cancer cells and various stromal components. Here, we focus on the recent progress in understanding the pathogenic role of LMP1 within the NPC microenvironment.
The role of LMP1 in invasion and metastasis has been thoroughly examined since the induction of MMP9 by LMP1 was reported.18 Invasion and metastasis is composed of a multistep interaction between tumor and stromal cells. In addition to the initial transforming property, LMP1 modulates expression of various receptors, enzymes, and cytokines in LMP1-expressing cells, which affects the surrounding microenvironment and promotes metastasis of LMP1-expressing cells.19, 20 The epithelial-mesenchymal transition (EMT) produces cells with properties of stem cells, which suggests that EMT activity may be involved in generating stemness in cancer.21, 22 In particular, LMP1-transformed epithelial cells cause fibroblasts to lose polarity, and upregulation of EMT by LMP1 occurs through upregulation of Twist, Snail, and SatB1 in LMP1-expressing cells.23-25
Most oncogenic viruses are capable of stabilizing or enhancing the transcriptional activity of the angiogenic factor hypoxia-inducible factor-1α (HIF-1α), which integrates several biological pathways for viral carcinogenesis.26, 27 Latent membrane protein 1 also induces HIF-1α.28 Angiogenesis and lymphangiogenesis surrounding LMP1-expressing cells were also promoted through upregulation of angiogenic factors such as vascular endothelial growth factor, basic fibroblast growth factor, and interleukin-8 (IL-8).29-33
4 LATENT MEMBRANE PROTEIN 1 AND THE WARBURG EFFECT
Aerobic glycolysis, glucose consumption, and lactate production even in the presence of oxygen is called the Warburg effect and is a main hallmark of cancer.34 The Warburg effect can also be induced by EBV.35 Latent membrane protein 1 upregulates the HIF-1α pathway regardless of oxygen pressure in the tumor microenvironment. Moreover, glucose is phosphorylated into glucose-6-phosphate in the glycolytic pathway.36 Hexokinase 2 (HK2) catalyzes the first step in this glycolytic pathway, and thus, overexpression of HK2 is a common feature of cancer.
Latent membrane protein 1 reprograms glycolysis by upregulating HK2, which binds to mitochondria in NPC, and c-Myc is required for LMP1-induced upregulation of HK2.35 In addition, LMP1 upregulates the receptor for advanced glycation end products (RAGE) in NPC through the activation of nuclear factor-κB, and microvessel count in NPC tumors is associated with the expression of LMP1 and RAGE.37
Hypoxic conditions help maintain stemness in advanced bulky tumors. Latent membrane protein 1 activates the hypoxic signaling pathway for cells with cancer stem- or progenitor-like properties before these cells become advanced tumor cells under a hypoxic microenvironment, which helps to maintain cancer stem cells in an early stage of NPC development.12, 17
5 LATENT MEMBRANE PROTEIN 1 IS SECRETED THROUGH EXOSOMES
Exosomes are a recently discovered mechanism through which cancer cells and virally infected cells can manipulate the microenvironment. Exosomes are 40-100 nm endosomal-derived vesicles containing transfer proteins, mRNAs, and microRNAs secreted from many cell types to neighboring or distant cells to modulate immune function, angiogenesis, cell proliferation, tumor invasion, and cell-to-cell communication.38, 39
Latent membrane protein 1 localizes at the Golgi and multivesicular compartments, and is packaged into exosomes for release from the cell.40 Exosomes containing LMP1 have been detected in the serum of NPC patients, which induces T-cell anergy and escape from immune surveillance.41, 42 A nanogram amount of purified recombinant LMP1 strongly suppresses activation of T cells and natural killer (NK) cells.42-44 In addition to LMP1 exosomal secretion, LMP1 also increases the concentration and release of basic fibroblast growth factor into exosomes.32 Latent membrane protein 1 induces the expression of the epidermal growth factor receptor in an EBV-negative epithelial cell line, and exosomes produced by these cells also contain high levels of the epidermal growth factor receptor in exosomes.41, 45 Latent membrane protein 1 increases the levels of HIF-1α in exosomes, which are then delivered to surrounding tumor cells for EMT induction and pro-metastatic effects.46 These findings suggest that the effects of EBV and LMP1 on cellular expression also modulate exosome content and properties. It is increasingly evident that viruses, such as EBV, can hijack and utilize the host cell exosome pathway to modulate cell-cell signaling and control the tumor microenvironment.
6 INTERACTION WITH STROMAL CELLS
A dense infiltration of lymphocytes, called lymphoepithelioma, is a well-known pathological characteristic of NPC.47 In addition to exosomal secretion of LMP1, the close association of EBV infection with lymphoepithelioma-like carcinoma implies that the poorly differentiated properties of epithelial cells and an inflammatory environment are involved in viral oncogenesis. To develop and maintain this coexistence of tumor infiltrates lymphocytes (TIL) with EBV-infected cells, clever immune evasion systems from the early phase of EBV infection is necessary. Infiltrating cells consist of different subsets of T-lymphocytes, including CD8+ CTLs, which are capable of killing tumor cells.48 The CD8+ T-cell responses to LMP1 are generally low and rarely detected in healthy virus carriers. In addition to exosome-mediated immune modification, EBV has multiple ways to escape from immune attack.49, 50
Latent membrane protein 1 localizes to lipid rafts in the cell membrane when aggregated.51 An LMP1 mutant that cannot aggregate at lipid rafts is recognized by HLA epitope-sensitized CTLs, but WT and LMP mutants that retain aggregation and localization to lipid rafts are not. These results suggest that aggregation of LMP1 at lipid rafts protects LMP1 from being processed and presented with MHC class I. In addition, LMP-1 limits self-presentation without compromising its ability to modulate trans-presentation of CD8+ T-cell epitopes.52
Programmed cell death protein-1 is a negative checkpoint molecule. Programmed cell death protein-1, expressed by T-lymphocytes, and programmed cell death ligand 1 (PD-L1), expressed by tumor cells, contribute to T-cell inhibition, and thus are correlated with the prognosis of various cancers including NPC.53-56 The LMP1 and γ-interferon pathways co-operate to regulate PD-L1 (Figure 2). Expression of PD-L1 was more abundant in EBV-positive NPC cell lines than in EBV-negative cell lines. Knockdown of LMP1 expression suppressed PD-L1 expression in EBV-positive cell lines. In addition, γ-interferon upregulates PD-L1 expression independently and synergistically with LMP1 in NPC tissue, and PD-L1 expression is associated with worse disease-free survival in patients with NPC.57
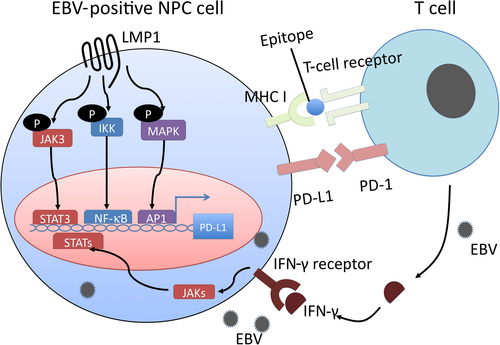
Latent membrane protein 1 promotes myeloid-derived suppressor cell expansion in the tumor microenvironment by promoting extra-mitochondrial glycolysis in malignant cells. In addition to RAGE, LMP1 promotes the expression of multiple glycolytic genes such as GLUT1. This metabolic reprogramming induces the Nod-like receptor family protein 3 inflammasome, activates the arachidonic cascade, and consequently various cytokines such as IL-1β, IL-6, and granulocyte/macrophage colony-stimulating factor. These changes in the tumor environment result in NPC tumor-derived myeloid-derived suppressor cell induction.58
7 PERSISTENT EBV INFECTION IN NPC TUMOR CELLS
In contrast to EBV-associated lymphomagenesis, a major obstacle to elucidate the mechanisms of EBV-mediated NPC initiation is that the genomes of EBV in NPC cell lines are gradually lost after prolonged in vitro culture.59, 60 This suggests that EBV is not essential for in vitro proliferation of NPC tumor cells. The copy number of the EBV episome decreases without drug selection or cell sorting. Analysis of the distributions of EBV-derived plasmids in single live cells throughout the cell cycle revealed that 16% of plasmids failed to duplicate, leading to a net loss of 8% of the plasmids from a population of cells.61 Nevertheless, a high proportion of NPC tumors retain the EBV genome regardless of the endemic or non-endemic area of NPC. The histological feature of EBV-positive NPC that obviously differs from EBV-negative NPC is the undifferentiated state of tumor cells and heavy lymphoid cell infiltration in stroma.47 Expression of LMP1 prevents epithelial cell differentiation.62 Interaction of NPC tumor cells with TIL is thought to be maintained through cytokine production and exosome excretion. The ubiquitous existence of EBV in advanced NPC tumors, recurrent NPC tumors, and passaged NPC xenografts in nude mice suggests that EBV infection in epithelial cells provides an advantage for EBV-infected epithelial cells grown in vivo.63, 64 Similarly, inflammation induced by EBV infection and expression of EBV genes may enhance initiation and progression of NPC in vivo.65 In addition, LMP1-mediated cytokine production might attract migration of inflammatory cells, including activated macrophages and T regulatory cells, to support EBV-infected cell growth and immune avoidance. Moreover, stromal lymphoid cells in NPC tissues secrete IL-6 to stimulate cell growth of EBV-infected NPC cells.66, 67 This mutually dependent relationship between cancer cells and stroma cells suggests that LMP1 expression levels and the LMP1-positive NPC tumor cell population is also regulated by the tumor-stroma interaction.
8 CONCLUDING REMARKS
Although EBV products, including LMP1, enhance the invasive and metastatic capabilities of EBV-infected malignant cells, the overall impact of EBV for the prognosis of NPC patients is somewhat limited.67 Similar to human papillomavirus (HPV)-associated oropharyngeal carcinoma, which has a better prognosis than non-HPV-associated oropharyngeal carcinoma, patients with EBV-associated NPC have a better prognosis compared to patients with non-EBV-associated NPC.47, 68, 69 This might seem contradictory considering the results discussed in this review, but EBV-associated NPC is sensitive to chemoradiotherapy treatment and immune checkpoint inhibitors.
LMP1 is not the only EBV gene expressed in NPC. Emerging evidence has revealed that EBV-encoded non-coding RNAs, such as EBERs, BARTs, and miR-BARTs, also contribute directly to NPC development. For example, EBERs contribute to immune evasion by inducing IL-10 through protein kinase R-independent pathways.70 Thus, the function of these abundantly expressed RNAs suggests an interplay with tumor cells and the microenvironment.11, 12, 71, 72 In addition, for Hodgkin's lymphoma and nasal NK/T-cell lymphoma, which are distinct type II latency tumors originating in B cells and NK/T cells, LMP1 may have different roles in a cell type-specific manner.73-75
Finally, investigation into the role of heavily infiltrated lymphoid stroma will be important for understanding the pathogenic role of EBV in epithelial malignancies, including NPC. Hopefully, this will provide clues to develop new therapeutic targets in addition to emerging immune checkpoint inhibitors.
ACKNOWLEDGMENTS
This work was supported by the Japan Society for the Promotion of Science (KAKENHI Grant Nos. JP26293367, JP26670739, 17K19718, and 17H01590).
CONFLICT OF INTEREST
The authors have no conflict of interest.




