Immunological evaluation of peptide vaccination for cancer patients with the HLA-A26 allele
Funding Information:
Japan Agency for Medical Research and Development; Ministry of Education, Culture, Sports, Science and Technology of Japan; Sendai Kousei Hospital.
Abstract
To develop a peptide vaccine for cancer patients with the HLA-A26 allele, which is a minor population worldwide, we investigated the immunological responses of HLA-A26+/A26+ cancer patients to four different CTL epitope peptides under personalized peptide vaccine regimens. In personalized peptide vaccine regimens, two to four peptides showing positive peptide-specific IgG responses in pre-vaccination plasma were selected from the four peptide candidates applicable for HLA-A26+/A26+ cancer patients and administered s.c. Peptide-specific CTL and IgG responses along with cytokine levels were measured before and after vaccination. Cell surface markers in PBMCs and plasma cytokine levels were also measured. In this study, 21 advanced cancer patients, including seven lung, three breast, two pancreas, and two colon cancer patients, were enrolled. Their HLA-A26 genotypes were HLA-A26:01 (n = 24), HLA-A26:03 (n = 10), and HLA-A26:02 (n = 8). One, 14, and 6 patients received two, three, and four peptides, respectively. Grade 1 or 2 skin reactions at the injection sites were observed in the majority of patients, but no severe adverse events related to the vaccination were observed. Peptide-specific CTL responses were augmented in 39% or 22% of patients after one or two cycles of vaccination, respectively. Notably, peptide-specific IgG were augmented in 63% or 100% of patients after one or two cycles of vaccination, respectively. Personalized peptide vaccines with these four CTL epitope peptides could be feasible for HLA-A26+ advanced cancer patients because of their safety and higher rates of immunological responses.
As a result of recent advances in cancer immunotherapy, immune checkpoint inhibitors have achieved durable clinical responses in at least one-fifth of patients with various types of advanced cancer.1-3 However, the clinical activity of immune checkpoint inhibitors was dependent on the presence of T lymphocytes at tumor sites. Therefore, clinical benefits could not be expected in cancer patients whose tumors had no or fewer tumor-infiltrating lymphocytes. Moreover, the clinical efficacy of chemotherapy with cytotoxic reagents has been well correlated with the number of tumor-infiltrating lymphocytes.4 We previously reported that personalized peptide vaccination (PPV) rapidly induced proliferation of CD45RO+ activated lymphocytes at tumor sites in association with clinical benefits in patients with advanced prostate cancer or bladder cancer.5-7 These results suggest that sequential cancer therapy consisting of PPV followed by either immune checkpoint inhibitors or chemotherapy with cytotoxic reagents could be more efficacious than monotherapy. Indeed, we recently reported that PPV followed by chemotherapy was associated with a favorable clinical outcome in refractory ovarian cancer patients.8
Although many clinical trials of peptide-based cancer vaccines for HLA-A2+ and HLA-A24+ cancer patients have been carried out in the past two decades, cancer patients with the other alleles, including HLA-A1, -A3, -A11, -A26, -A31, or -A33, were excluded from these clinical trials because of the relatively lower allele expression worldwide.9, 10 To develop cancer vaccines applicable for these minor HLA-A alleles, we previously identified large numbers of CTL epitope peptides from alleles other than A2 or A24, and some of them were provided for the clinical trials of peptide vaccines for the HLA-A3 superfamily (HLA-A11, -A31, and -A33) or HLA-A26+ cancer patients under PPV regimens in the past 7 years.11, 12 The HLA-A26 allele is found in approximately 11% of the Japanese population, 4% of the Caucasian population in the USA, and 7.5% of the African population of Cape Town.10
In this study, we retrospectively analyzed the results of PPV for HLA-A26+/A26+ advanced cancer patients from the various regimens of PPV carried out in the past 7 years to investigate the feasibility of using PPV for HLA-A26+/A26+ advanced cancer patients from the viewpoints of safety and immunological responses.
Patients and Methods
Peptides
The four different CTL epitope peptides used in this study were SART3109–118 VYDYNCHVDL, ppMAPkkk432–440 DLLSHAFFA, HNRPL501–510 NVLHFFNAPL, and WHSC2103–111 ASLDSDPWV peptides. All were previously shown to be capable of inducing peptide-specific and tumor-reactive CTL activity in HLA-A26 cancer patients.10 These peptides were prepared under the conditions of Good Manufacturing Practice by the PolyPeptide Laboratories (San Diego, CA, USA) and American Peptide Company (Vista, CA, USA), as reported previously.7, 13-18
Patients
Patients diagnosed with cancer were eligible for this study. They had to show positive IgG responses to at least two of the four peptide candidates applicable for HLA-A26+ cancer patients. Other inclusion criteria were as follows: age between 20 and 80 years; an Eastern Cooperative Oncology Group performance status of 0 or 1 at the time of first visit; life expectancy of at least 12 weeks; and adequate hematologic, hepatic, and renal function. Exclusion criteria included: pulmonary, cardiac, or other systemic diseases; an acute infection; a history of severe allergic reactions; pregnancy or nursing; and other inappropriate conditions for enrolment as judged by clinicians. The protocol was approved by the Kurume University (Kurume, Japan) Ethical Committee and registered with the UMIN Clinical Trials Registry (UMIN nos. 1482, 1839, 1844, 1850, 1854–1856, 1881–1883, 2908, 2984, 2987, 6493, and 10068). All patients were given a full explanation of the protocol and provided their informed consent before enrolment.
Clinical protocol
This was a phase II study to evaluate the safety, immunological responses, and clinical benefits from the viewpoint of overall survival in heavily treated cancer patients under PPV. Peptides for vaccination to individual patients were selected in consideration of the pre-existing host immunity before vaccination, as assessed by the titers of IgG specific to each of the four different vaccine candidates.7, 11, 12, 16-18 A maximum of four peptides (3 mg/each peptide), which were selected based on the results of HLA typing and peptide-specific IgG titers, were s.c. administered with incomplete Freund's adjuvant (Montanide ISA51; Seppic, Paris, France) once a week for 6 consecutive weeks (UMIN nos. 1482, 1839, 1844, 1850, 1854–1856, 1881–1883, 6493, and 10068), or once a week for 4 consecutive weeks followed by four biweekly treatments (UMIN nos. 2908, 2984, and 2987) as the first cycle. After the first cycle of vaccinations, up to four antigen peptides that were reselected according to the titers of peptide-specific IgG were given six or eight times on a biweekly basis. After the first cycle of vaccinations, up to four antigen peptides that were reselected again were given every 4 weeks until the 24th vaccination. During the PPV, patients were allowed to receive combination therapies, such as chemotherapies or radiotherapies. Adverse events were monitored according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0. Complete blood counts and serum biochemistry tests were carried out before and after each cycle of vaccinations. Tumor assessments by computed tomography or MRI scans were carried out before and after PPV, and evaluated according to Response Evaluation Criteria in Solid Tumors version 1.1.
Laboratory markers
Humoral immune responses specific to each of the four peptide candidates were determined by peptide-specific IgG levels using the Luminex system (Luminex, Austin, TX, USA), as previously reported.19-22 If the titers of peptide-specific IgG to at least one of the vaccinated peptides at the end of the first cycle were more than twofold higher than those in the prevaccination plasma, the changes were considered to be significant, as previously reported. The CTL responses specific to the vaccinated peptides were evaluated by γ-interferon ELISPOT assays using PBMCs before and at the end of the first cycle, as previously reported.19-22
Cell surface markers and cytokines
As cell surface markers, myeloid-derived suppressor cells (CD33, CD11b, CD14, CD15, CD3, CD16, CD19, CD56, and HLA-DR), regulatory T cells (CD4, CD25, and FoxP3), BTLA, PD-1, CTLA-4, and LAG-3 were measured by a BD FACSCanto II System with FACS Diva software (BD Biosciences, San Diego, CA, USA). In addition, the levels of interleukin (IL)6, IL8, and γ-interferon-induced protein 10 (IP-10) in plasma before and after one cycle of vaccination were examined by ELISA using kits from eBioscience (San Diego, CA, USA), as reported previously.23
Statistical analyses
All data were analyzed retrospectively. Comparison of each group was carried out using anova. Overall survival was calculated from the first day of peptide vaccination until the date of death or the last date when the patient was known to be alive. Values of P < 0.05 were considered to indicate statistical significance. All statistical analyses were carried out using JMP software, version 12 (SAS Institute, Cary, NC, USA).
Results
Patients' characteristics
Between December 2008 and August 2014, 21 A26+/A26+ advanced cancer patients were enrolled to this study. Their genotypes were A26:01 (n = 24), HLA-A26:03 (n = 10), and HLA-A26:02 (n = 8). Their diagnosis was lung cancer (n = 7), breast cancer (n = 3), pancreatic cancer (n = 2), colon cancer (n = 2), and one case each of prostate cancer, stomach cancer, liver cancer, kidney cancer, bladder cancer, uterine cancer, and ovarian cancer. The patients' characteristics are shown in Table 1. The median age was 65 years (range, 46–75 years). The median lymphocyte number at the time of enrolment was 1600/mm3 (range, 1004–2035/mm3). Performance status at the time of enrolment was grade 0 (n = 18) or grade 1 (n = 3). The numbers of previous chemotherapy regimens were one (n = 8), two (n = 2), three (n = 4), and more than three (n = 4). Fifteen patients received combined chemotherapy or other therapy such as hormone therapy along with PPV. Sixteen patients completed the first cycle of vaccinations, whereas the remaining five patients did not due to rapid disease progression. The median number of vaccinations was 12 (range, 1–32).
| No. | HLA-A genotype | Primary | Pathology | Age, years | Gender | Stage | No. of lymphocytes | PS | Previous chemotherapy | Previous other therapy† | Combined chemotherapy | Combined other therapy† | No. of vaccinations | Overall survival | Clinical response at 3 months after first vaccination | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of regimens | Cytotoxic agent | Targeted agent | No. of regimens | Cytotoxic agent | Targeted agent | |||||||||||||||
| 1 | 26:01 | 26:03 | Lung | Small cell carcinoma | 70 | M | IV | 2035 | 1 | 1 | − | + | − | 1 | + | − | − | 4 | 70 | Death |
| 2 | 26:01 | 26:03 | Lung | Squamous cell carcinoma | 65 | M | IV | 1180 | 0 | 1 | − | + | + | 1 | + | − | − | 4 | 80 | Death |
| 3 | 26:01 | 26:03 | Lung | Adenocarcinoma | 72 | M | Rec. | 1034 | 0 | 0 | − | − | + | 0 | − | − | − | 17 | 435‡ | SD |
| 4 | 26:01 | 26:03 | Lung | Adenocarcinoma | 56 | F | Rec. | 1134 | 0 | 3 | + | − | − | 2 | + | − | − | 24 | 692 | SD |
| 5 | 26:01 | 26:03 | Lung | Adenocarcinoma | 70 | M | III | 1007 | 0 | 1 | + | − | + | 0 | − | − | − | 13 | 312‡ | SD |
| 6 | 26:01 | Lung | Adenocarcinoma | 75 | M | Rec. | 1189 | 0 | 3 | + | − | − | 3 | + | + | − | 17 | 367 | SD | |
| 7 | 26:01 | 26:02 | Lung | Adenocarcinoma | 69 | M | IV | 1005 | 0 | 3 | + | + | − | 2 | + | + | − | 3 | 47‡ | Unknown |
| 8 | 26:01 | Breast | Invasive ductal carcinoma | 55 | F | Rec. | 1004 | 0 | 2 | + | − | − | 2 | + | − | − | 9 | 381 | PD | |
| 9 | 26:01 | Breast | Invasive ductal carcinoma | 68 | F | IV | 1550 | 1 | 6 | + | − | − | 0 | − | − | − | 1 | 20 | Death | |
| 10 | 26:03 | Breast | Mucinous carcinoma | 55 | F | Rec. | 1390 | 0 | 3 | + | + | + | 0 | − | − | + | 18 | 703‡ | SD | |
| 11 | 26:01 | 26:03 | Pancreas | Adenocarcinoma | 74 | M | I | 2011 | 0 | 0 | − | − | − | 0 | − | − | − | 32 | 1058‡ | SD |
| 12 | 26:02 | Pancreas | Adenocarcinoma | 64 | M | IV | 1803 | 0 | 1 | + | − | − | 1 | + | − | − | 6 | 74 | Death | |
| 13 | 26:01 | 26:02 | Colon | Adenocarcinoma | 60 | M | IV | 1365 | 0 | 4 | + | + | + | 0 | − | − | − | 4 | 54 | Death |
| 14 | 26:01 | Colon | Adenocarcinoma | 51 | F | Rec. | 1282 | 0 | 5 | + | + | - | 2 | + | + | − | 9 | 202 | SD | |
| 15 | 26:01 | 26:03 | Prostate | Adenocarcinoma | 62 | M | IV | 1492 | 0 | 1 | − | + | + | 2 | + | − | + | 30 | 949 | SD |
| 16 | 26:01 | Stomach | Adenocarcinoma | 70 | M | IV | 1200 | 0 | 0 | − | − | − | 1 | + | − | − | 12 | 701‡ | SD | |
| 17 | 26:01 | 26:03 | Liver | Hepatocellualar carcinoma | 46 | M | IV | 1050 | 0 | 4 | + | + | + | 1 | − | + | − | 6 | 209 | SD |
| 18 | 26:01 | Kidney | Clear cell type | 53 | M | Rec. | 1673 | 0 | 1 | − | + | − | 1 | − | + | − | 9 | 1820 | SD | |
| 19 | 26:02 | Bladder | Transitional cell carcinoma | 66 | M | Rec. | 1612 | 0 | 1 | + | − | − | 1 | + | − | − | 12 | 346‡ | PD | |
| 20 | 26:01 | 26:02 | Uterus | Leiomyosarcoma | 52 | F | Rec. | 1430 | 0 | 1 | + | − | − | 1 | + | − | − | 12 | 489 | SD |
| 21 | 26:01 | 26:02 | Oval | Adenocarcinoma | 65 | F | Rec. | 1300 | 1 | 2 | + | + | − | 0 | − | − | − | 18 | 642‡ | SD |
- †Other therapy included radiation therapy, hormone therapy and intra-arterial injection therapy. ‡Patients are alive. +, Used; −, not used; F, female; M, male; NA, not assessed; OS, overall survival; PD, progression desease; PS, performance status; Rec., Recurrence; SD, stable disease.
Adverse events
Table 2 shows the adverse events during the PPV. Grade 1 or 2 injection site reactions were observed in 15 of 21 (71.4%) patients. Severe adverse events were seen in four patients (grade 3, two neutropenia and one hypertension; grade 4, one neutropenia). All of them were concluded to be not directly associated with the PPV, but with disease progression or combined therapy according to evaluation by the independent safety evaluation committee.
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | |||||
|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | No. | % | |
| Injected site reaction | 8 | 38.1 | 7 | 33.3 | 0 | 0.0 | 0 | 0.0 |
| General disorder | ||||||||
| Fever | 0 | 0.0 | 1 | 4.8 | 0 | 0.0 | 0 | 0.0 |
| Blood/bone marrow | ||||||||
| Anemia | 0 | 0.0 | 3 | 14.3 | 0 | 0.0 | 0 | 0.0 |
| Leukopenia | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Neutropenia | 0 | 0.0 | 1 | 4.8 | 2 | 9.5 | 1 | 4.8 |
| Lympocytopenia | 1 | 4.8 | 3 | 14.3 | 0 | 0.0 | 0 | 0.0 |
| Thrombocytopenia | 1 | 4.8 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Metabolic and laboratory | ||||||||
| AST increased | 3 | 14.3 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| ALT increased | 1 | 4.8 | 2 | 9.5 | 0 | 0.0 | 0 | 0.0 |
| γ-GTP increased | 4 | 19 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| ALP increased | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Hypoalbuminemia | 7 | 33.3 | 2 | 9.5 | 0 | 0.0 | 0 | 0.0 |
| Creatinine increased | 1 | 4.8 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Electrolyte imbalance | 0 | 0.0 | 1 | 4.8 | 0 | 0.0 | 0 | 0.0 |
| Neurological | ||||||||
| Tumor pain | 2 | 9.5 | 1 | 4.8 | 0 | 0.0 | 0 | 0.0 |
| Vascular disorder | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Hypertension | 0 | 0.0 | 0 | 0.0 | 1 | 4.8 | 0 | 0.0 |
| Gastrointestinal disorder | ||||||||
| Diarrhea | 0 | 0.0 | 1 | 4.8 | 0 | 0.0 | 0 | 0.0 |
| Skin and subcutaneous tissue disorder | ||||||||
| Alopecia | 0 | 0.0 | 1 | 4.8 | 0 | 0.0 | 0 | 0.0 |
| Rash acneiform | 1 | 4.8 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
- ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; γ-GTP, γ-glutamyl transpeptidase.
Immune responses
Both peptide-specific CTL and IgG responses were analyzed in blood samples from 21, 16, and 11 patients before vaccinations, after the first cycle of vaccinations, and after the second cycle of vaccinations, respectively. Peptide-specific IgG reactive to each of the 31 different peptides, including both the vaccinated and non-vaccinated peptides, were measured by the Luminex system. The numbers of peptides used for the first cycle of vaccinations were two, three, and four in 1, 14, and 6 patients, respectively. The IgG responses before vaccination were observed in all the patients. The IgG responses specific to at least one of the vaccinated peptides were increased after the first cycle of vaccination in 11 of 16 (69%) patients tested, and after the second cycle of vaccination in all 11 patients tested (Table 3).
| Patient no. | Peptide | IgG responses to vaccinated peptide† | CTL response‡ | ||||
|---|---|---|---|---|---|---|---|
| Pre | Post 1 cycle | Post 2 cycles | Pre | Post 1 cycle | Post 2 cycles | ||
| 3 | SART3-109 | 60 | 43 | 4625 | 0 | 0 | 0 |
| ppMAPkkk-432 | 103 | 96 | 868 | 0 | 554 | 0 | |
| WHSC2-103 | 19 | 16 | 31 | 238 | 0 | 0 | |
| 4 | SART3-109 | ND | ND | 746 | NA | NA | NA |
| ppMAPkkk-432 | 353 | 319 | 281 | NA | NA | NA | |
| HNRPL-501 | 122 | 124 | 30 500 | NA | NA | NA | |
| WHSC2-103 | 229 | 217 | 729 | NA | NA | NA | |
| 5 | ppMAPkkk-432 | ND | 25 | 16 | 0 | 0 | 0 |
| HNRPL-501 | 172 | 197 | 1530 | 0 | 0 | 0 | |
| WHSC2-103 | 4115 | 2460 | 1869 | 0 | 0 | 0 | |
| 6 | SART3-109 | 21 | 55 | 21 478 | NA | NA | NA |
| HNRPL-501 | 13 | 23 | 7715 | NA | NA | NA | |
| WHSC2-103 | ND | ND | 12 | NA | NA | NA | |
| 8 | SART3-109 | 109 | 110 | NA | 200 | 0 | NA |
| HNRPL-501 | 62 | 51 | NA | 149 | 0 | NA | |
| WHSC2-103 | 24 | 22 | NA | 0 | 0 | NA | |
| 10 | SART3-109 | ND | ND | 388 | NA | NA | 0 |
| ppMAPkkk-432 | ND | ND | 55 | NA | NA | 0 | |
| HNRPL-501 | 17 | 185 | 27 281 | 0 | 722 | 0 | |
| WHSC2-103 | 34 | 42 | 119 | 0 | 0 | 0 | |
| 11 | SART3-109 | 23 | 20 | 9540 | 0 | 0 | 0 |
| ppMAPkkk-432 | ND | 11 | 11 | 0 | 0 | 0 | |
| HNRPL-501 | ND | ND | 10 | 0 | 0 | 0 | |
| WHSC2-103 | 22 | 14405 | 5951 | 0 | 0 | 0 | |
| 12 | SART3-109 | 76 | 76 | NA | 0 | 0 | NA |
| ppMAPkkk-432 | 86 | 94 | NA | 0 | 0 | NA | |
| HNRPL-501 | 43 | 39 | NA | 0 | 0 | NA | |
| WHSC2-103 | 642 | 654 | NA | 0 | 0 | NA | |
| 14 | SART3-109 | 18 | 8432 | NA | 0 | 0 | NA |
| HNRPL-501 | 253 | 21 599 | NA | 0 | 0 | NA | |
| WHSC2-103 | 14 | 43 | NA | 0 | 0 | NA | |
| 15 | SART3-109 | 69 | 210 | 8276 | 0 | 0 | 0 |
| ppMAPkkk-432 | 141 | 144 | 18 124 | 0 | 223 | 816 | |
| WHSC2-103 | 82 | ND | 10 876 | 0 | 0 | 0 | |
| 16 | SART3-109 | 23 | ND | 20 743 | 0 | 0 | 0 |
| WHSC2-103 | 42 | 112 | 7474 | 0 | 0 | 0 | |
| 17 | SART3-109 | 132 | ND | NA | NA | NA | NA |
| ppMAPkkk-432 | 200 | ND | NA | NA | NA | NA | |
| HNRPL-501 | 146 | ND | NA | NA | NA | NA | |
| 18 | SART3-109 | 2930 | 12 149 | NA | 359 | 132 | NA |
| ppMAPkkk-432 | 15 | 16 | NA | 0 | 0 | NA | |
| HNRPL-501 | 454 | 473 | NA | 0 | 0 | NA | |
| 19 | SART3-109 | 29 | 280 | 25 446 | 0 | 0 | 0 |
| ppMAPkkk-432 | 248 | 290 | 36 864 | 0 | 0 | 486 | |
| HNRPL-501 | ND | 11 | 7523 | 0 | 0 | 0 | |
| WHSC2-103 | 3086 | 2906 | 2847 | 0 | 0 | 0 | |
| 20 | SART3-109 | 11 | 16 | 23 089 | 0 | 0 | 0 |
| ppMAPkkk-432 | 15 | 21 | 41 | 0 | 0 | 0 | |
| HNRPL-501 | ND | ND | 24 | 0 | 0 | 0 | |
| 21 | ppMAPkkk-432 | 62 | 87 | 134 | 0 | 0 | 0 |
| HNRPL-501 | 18 | 38 589 | 36 619 | 0 | 0 | 0 | |
| WHSC2-103 | 23 | 66 | 1877 | 0 | 342 | 0 | |
- †If the titers of peptide-specific IgG at the end of the first or second cycles were more than twofold higher than those in the prevaccination plasma, or were newly appeared, these changes were considered to be enhanced. The augmented IgG responses are underlined. ‡CTL responses were determined by the number of spots per 105 PBMCs reactive with the vaccinated peptides in γ-interferon ELISPOT assay before and after the first or second cycles of vaccination. The augmented T cell responses are underlined. NA, not assessed; ND, not detected.
The CTL responses to the vaccinated peptides before vaccination were detectable in 3 of 13 (23%) patients (Table 3). They became detectable after the first cycle of vaccination in 5 of 13 (38%) patients and after the second cycle of vaccination in 2 of 9 (22%) patients.
Collectively, after the first cycle of vaccination, 3 patients showed both increased CTL and IgG responses to the vaccinated peptides, 11 of 16 patients showed either increased CTL or IgG responses, and the remaining 5 patients showed neither CTL nor IgG boosting. All 11 patients showed either increased CTL or IgG responses to the vaccinated peptides after the second cycle of vaccination.
Cell surface marker and inflammatory cytokines
We also measured immune cell subsets in pre- and post-vaccination (after the first cycle) of PBMCs, including myeloid-derived suppressor cells, regulatory T cells, or BTLA-, PD-1-, CTLA-4-, and LAG-3-postive cells, by flow cytometry. In addition, we measured pre- and post-vaccination (after the first cycle) of plasma levels of cytokines, including IL6, IL8, and IP-10. Neither the immune cell subsets nor the cytokine levels in prevaccination samples were significantly different from those of the post-vaccination samples (Table 4).
| Before | After 1 cycle | P-value‡ | |
|---|---|---|---|
| MDSCs, %lymphocytes | 0.84 (0.25–1.70) | 0.96 (0.67–2.13) | 0.48 |
| Regulatory T cells, %lymphocytes | 1.33 (0.55–2.97) | 1.54 (0.90–3.17) | 0.23 |
| BTLA+CD4+ T cells, %lymphocytes | 29.9 (15.8–54.8) | 31.4 (20.1–44.8) | 0.51 |
| BTLA+CD8+ T cells, %lymphocytes | 13.6 (7.9–21.2) | 14.2 (8.0–24.5) | 0.56 |
| PD-1+CD4+ T cells, %lymphocytes | 1.37 (0.45–2.54) | 1.63 (0.40–3.38) | 0.13 |
| PD-1+CD8+ T cells, %lymphocytes | 0.56 (0.25–1.01) | 0.47 (0.17–0.93) | 0.06 |
| CTLA-4+CD4+ T cells, %lymphocytes | 0.05 (0.02–0.16) | 0.06 (0.02–0.13) | 0.65 |
| CTLA-4+CD8+ T cells, %lymphocytes | 15.1 (7.4–27.3) | 16.0 (11.6–25.9) | 0.57 |
| LAG-3+CD4+ T cells, %lymphocytes | 0.18 (0.03–0.53) | 0.23 (0.06–0.47) | 0.28 |
| LAG-3+CD8+ T cells, %lymphocytes | 0.43 (0.06–1.58) | 0.45 (0.10–1.33) | 0.58 |
| IL6, pg/mL | 9.88 (5.30–37.67) | 7.76 (5.15–17.71) | 0.24 |
| IL8, pg/mL | 22.2 (1.8–86.6) | 26.1 (2.2–149.8) | 0.79 |
| IP–10, pg/mL | 150 (63–354) | 172 (110–412) | 0.45 |
- †Eleven of 16 patients who ended one cycle of treatment with personal peptide vaccine were measured. Five patients could not be measured because their samples were insufficient. ‡Paired t-test was used to examine P-values. IL, interleukin; IP-10, γ-interferon-induced protein 10; MDSCs, myeloid-derived suppressor cells.
Clinical response
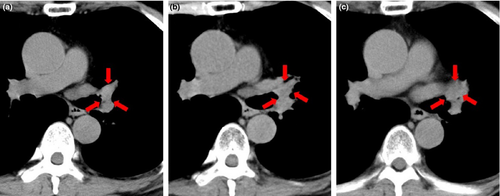
Best clinical responses were evaluated by radiological findings. There were no cases of complete response, no cases of partial response, 13 cases of stable disease (SD), and 7 cases of progressive disease (PD) (Table 1). Computed tomography findings of 1 patient of SD case before vaccination and after 2 cycles of vaccination are shown in Figure 1. This case (case #3 in Table 1) was a 72-year-old man with recurrent lung cancer after radiation therapy treated with PPV alone. At 1 year after the 1st vaccination, the tumor had grown slightly, but showed stable disease (SD). Both the cellular immune responses and IgG responses to vaccinated peptides were well boosted in this case (case #3 in Table 3).
Discussion
To the best of our knowledge, this is the first clinical trial of peptide-based cancer vaccines for HLA-A26+ cancer patients. The HLA-A26 allele is found in approximately 11% of the Japanese population and includes various genotypes.10 The frequencies of the different HLA-A26 genotypes in the Japanese population have been reported as follows: HLA-A2601: 7.35%; HLA-A2603: 2.23%; HLA-A2603: 1.81%; HLA-A2605: 0.07%; and HLA-A2606: 0.01%.24 Their HLA-A26 genotypes of the 21 patients in this study were HLA-A26:01 (n = 24), HLA-A26:03 (n = 10), and HLA-A26:02 (n = 8). Thus the trend among the present patient population was similar to that for Japan as a whole, although it is difficult to compare the two sets of results because of the extremely small size of our subject group (21 patients).
From the viewpoint of adverse events, a grade 1 or 2 injection site reaction was observed in 70% of the patients. Severe adverse events were seen in 4 patients, and all of them were concluded to be not directly associated with the PPV, but with the disease progression or combined therapy. These results were consistent with the previous results on PPV for the HLA-A24+ or -A2+ cancer patients reported previously.11, 12
This study showed the successful boosting of HLA-A26 restricted peptide-specific immune responses in blood samples after the 6th vaccination of PPV. Boosting for peptide-specific IgG responses, which were clearly detectable in all 21 patients, was especially apparent in 63% or 100% of patients after the 1st cycle or 2nd cycle of vaccination, respectively. We have reported that boosting of IgG responses is well correlated with overall survival.21, 25
In contrast to IgG responses, peptide-specific CTL responses of each of the vaccine candidates were rarely detectable. There were several possible explanation for this discrepancy between IgG and CTL boosting. One of them could be deletion or depression of the expression of HLA-A26 molecules on the surface of cancer cells from these HLA-A26 homogenous advanced cancer patients. PBMCs from HLA-A26 heterogeneous advanced cancer patients were provided for in vitro determination of these 4 peptides that could induce peptide-specific CTL activity for HLA-A26+ cancer patients as reported previously.10 Their clinical stages were not as advanced as to those of the 21 patients who received PPV in this study. The other explanation could be that the numbers of pooled peptides were too small to correspond to antigenic heterogeneity of tumor cells and also diverse immune responses among cancer patients. Indeed, 9 of 30 (30%) HLA-A26+/A26+ cancer patients could not be enrolled in this study because they did not have more than 2 positive IgG responses among the 4 pooled peptides. Therefore, the development of additional HLA-A26 restricted peptides is needed. In addition, if patients have both the HLA-A2, -A24, or A3 superfamily and HLA-A26, it might be appropriate to use both the peptides matched with the HLA-A2, -A24, or A3 superfamily and those matched with the HLA-A26 allele, as we have been conducting the vaccination using both the peptides with the successful boosting of peptide-specific CTL responses as reported previously.11, 12, 21, 23
This was a small study with a limited number of patients for investigation of the PPV-induced immunological responses in HLA-A26+/A26+ cancer patients. Therefore, clinical benefits were not set as the secondary objective. However, it could be important to provide available information on the clinical outcome of these patients under PPV. We have previously reported the possibility of extending the overall survival in clinical trials of PPV in each organ cancer patients, and especially for patients who exhibit humoral responses and T cell responses. As a result, there were no complete response, no partial response, 13 stable disease (SD), 7 progressive disease, and 1 unknown. Five patients (one each with stage IV small-cell lung cancer, adenocarcinoma lung cancer, invasive ductal breast cancer, pancreatic cancer, and colon cancer patients) could not receive the first cycle of vaccination because of rapid disease progression and died within 80 days of the first vaccination. The other 16 patients received at least six vaccinations (median, 15 vaccinations; range, 3–32 vaccinations), and their median survival time was 949 days (range, 47–1820 days). Best clinical responses were SD (n = 12), progressive disease (n = 2), and unknown (n = 1).
In this study, there was no significant difference in either cell surface markers or inflammatory cytokines between before and after PPV. This could be mainly due to small numbers of patients with various types of cancers. These results, however, were partly similar to the previous cell surface marker study in which PPV induced increases and decreased the frequency of PD1+CD4+ T cells and that of PD1+CD8+ T cells in association with favorable overall survival.25
In summary, this study showed that PPV with these four different CTL epitope peptides could be feasible for HLA-A26+ advanced cancer patients because of the safety of the regimens and high rates of immunological responses.
Acknowledgments
This study was supported in part by the Japan Agency for Medical Research and development, AMED, a research program of the Regional Innovation Cluster Program of the Ministry of Education, Culture, Sports, Science and Technology of Japan, and a grant from the Sendai Kousei Hospital.
Disclosure Statement
Akira Yamada is a Board member of the Green Peptide Co., Ltd. Kyogo Itoh received bureau honorarium and is a consultant/advisory board member. Kyogo Itoh received research funds from Taiho Pharmaceutical Co., Ltd. No conflicts of interests were declared by the other authors.




