Heterogeneity of tumor endothelial cells
Abstract
Tumor blood vessels play important roles in tumor progression and metastasis. Thus, targeting tumor blood vessels is an important strategy for cancer therapy. Tumor endothelial cells (TECs) are the main targets of anti-angiogenic therapy. Although tumor blood vessels generally sprout from pre-existing vessels and have been thought to be genetically normal, they display a markedly abnormal phenotype, including morphological changes. The degree of angiogenesis is determined by the balance between the positive and negative regulating molecules that are released by tumor and host cells in the microenvironment. Reportedly, tumor blood vessels are heterogeneous with TECs differing from normal endothelial cells (in contrast to the conventional view). We recently compared characteristics of different TECs isolated from highly and low metastatic tumors. We found TECs from highly metastatic tumors had more proangiogenic phenotypes than those from low metastatic tumors. Elucidating the variety of TEC phenotypes and identifying TEC molecular signatures should lead to more complete understanding of the mechanisms of tumor progression, discovery of new therapeutic targets, and development of biomarkers. This review considers current studies on TEC heterogeneity and discusses the therapeutic implications of these findings.
Tumor blood vessels provide nutrition and oxygen, eliminate waste from tumor tissue, and act as gatekeepers for tumor cells to metastasize to distant organs.1, 2 Since Folkman proposed that tumor growth was dependent on angiogenesis,3 tumor blood vessels have been recognized as an important target for cancer therapy, and many anti-angiogenic drugs have been discovered and tested with promising results.1 The degree of angiogenesis is determined by the balance between the positive and negative regulating molecules that are released by tumor and host cells in the microenvironment.4 Tumor blood vessels differ morphologically and phenotypically from their normal counterparts.5, 6
We previously reported that tumor endothelial cells (TECs) differ from normal endothelial cells (NECs) in characteristics such as cell proliferation, migration, gene profile, and responses to growth factors and several chemotherapeutic drugs.7-14 As tumor blood vessels are reportedly heterogeneous15 and TECs play an important role in metastasis, we wished to investigate possible differences between TECs of tumors of differing malignancy.
We recently investigated phenotypic differences between TECs isolated from highly and low metastatic tumors, to analyze the correlation between TEC characteristics and malignancy status of the tumor.28
Tumor angiogenesis
Angiogenesis is necessary for tumor progression and metastasis. The onset of angiogenesis or the “angiogenic switch” occurs at any stage of tumor progression, and depends on the type of tumor and on its microenvironment.
Tumor vessels are unorganized, whereas normal vasculature shows a hierarchal branching pattern of arteries, veins and capillaries.6 Pericytes are present among TECs, but have uneven associations with these cells.16 These abnormalities result in leakiness of tumor blood vessels. Tumor blood vessels are often thin, fragile, and defective in barrier function. They have focal regions that lack endothelial cells or basement membrane.17, 18 Tumor vessels exhibit chaotic blood flow and are often leaky and hemorrhagic owing in part to irregular endothelial cell interconnections.5 High tumor interstitial fluid pressure causes blood vessel collapse and impedes blood flow, leading to hypoxia in tumor tissue, despite high vascularization.19 Abnormalities of tumor blood vessels may come from unbalanced expression of angiogenic factors and inhibitors. Altered expression of pro-angiogenic and anti-angiogenic factors cause excess formation of neovasculature.20 Tumor cells produce several angiogenic factors that promote new vessel formation, including vascular endothelial growth factor (VEGF), basic fibroblast growth factor, angiopoietins, hepatocyte growth factor, chemokines, and placental-derived growth factor (PDGF).20 Although VEGF is the major angiogenic factor, other growth factors such as the angiopoietin family and the ephrin family play important roles in tumor angiogenesis.21 In addition, mural cells or fibroblasts secrete growth factors, such as PDGF or transforming growth factor β (TGF-β) families, which are considered essential for new blood vessel formation.
Properties of TECs
Recent studies have reported molecular differences between TECs and NECs.22, 23 We have isolated TECs and reported that TECs have several abnormalities,9, 24 including differences in the responsiveness to epidermal growth factor (EGF),8 adrenomedullin,14 and VEGF12 compared with NECs. These growth factors are important in proangiogenic phenotype in TEC. Especially, VEGF stimulates cell migration and enhances survival of TEC in an autocrine manner. Tumor endothelial cells are also resistant to serum starvation, unlike NECs, which is caused, at least in part by the VEGF autocrine loop.12 Tumor endothelial cells show some cytogenetic abnormalities, such as aneuploidy or abnormal centrosomes, in mouse tumors.9 These abnormalities are not caused by tumor cell contamination, because we found that TECs can be purified without contaminating tumor cells by using diphtheria toxin (DT), which binds to human tumor cells expressing heparin binding-EGF like growth factor, a DT receptor, but not to mouse cells.9 These cytogenetic abnormality was found in human renal carcinoma.7 Because aneuploidy in TECs is suggestive of genetic instability, the question arises as to whether TECs are drug resistant. Some TECs are resistant to certain drugs.25, 26 For example, renal carcinoma ECs are resistant to vincristine,25 while hepatocellular carcinoma ECs are resistant to 5-fluorouracil and adriamycin.26, 27 However, the mechanism of drug resistance in TECs has not been elucidated. We recently demonstrated that TECs are resistant to paclitaxel, and that tumor-derived VEGF causes drug resistance in endothelial cells.28
Heterogeneity in endothelial cells
There have been several reports about endothelial cell heterogeneity at the level of cell morphology, function and gene expression. For example, rolling velocity and arrest frequency for leukocyte were different at endothelial cell junctions versus more central areas of endothelial cells.29 Interorgan differences in endothelial cells have been also reported.30 Furthermore, stem-like endothelial cells were observed in pre-existing blood vessels and they have proangiogenic phenotype.31 In rat brain blood vessels, the expressions of P-gp and endothelial barrier antigen were heterogeneous at the single cell level, suggesting that the blood–brain barrier is not uniform (Table 1).32
| Findings | Reference |
|---|---|
| Differences in leukocyte adhesion at endothelial cell junctions versus central areas of endothelial cells. | Mundhekar et al. 29 |
| Interorgan differences in endothelial cells | Langenkamp and Molema 34 |
| Stem-like endothelial cells in preexisting blood vessels | Naito et al. 31 |
| Heterogenious expression of P-gp and endothelial barrier antigen in rat brain blood vessels | Saubaméa et al. 32 |
| Heterogeneity of the tumor vasculature in terms of the structure, pericyte coverage | Nagy et al. 35 |
| Differences between tumor endothelial cell versus normal endothelial cell | St Croix et al. 22 |
| Bussolati et al. 25 | |
| Hida et al. 9 | |
| Dudley et al. 33 | |
| Differences between high metastic tumor derived-EC versus low metastic tumor derived-EC | Ohga et al. 27 |
Heterogeneity in TEC
Proangiogenic phenotype
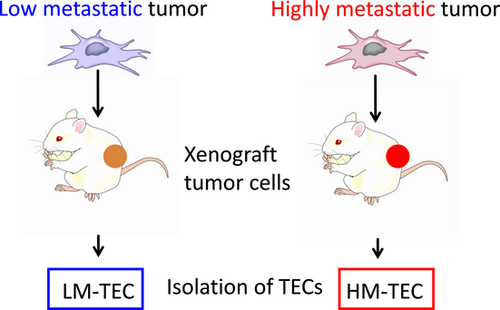
It has been accepted that TECs and NECs are different. One example is endothelial heterogeneity (Table 1).9, 22, 25, 33 Heterogeneous vascular morphology or pericyte coverlage have been described in various tumor types, at different stages of tumor progression, and even within a single tumor stage (Table 1).30, 34, 35 However, heterogeneity of TEC among tumor type has not been unraveled until recently. We addressed the question of whether tumors with different malignancy status have heterogeneous characteristics besides morphological differences in their blood vessels.27 For this purpose we recruited low metastatic melanoma, A375 (LM tumor) and high metastatic melanoma A375-SM cells (HM tumor) to investigate TEC heterogeneity with regard to metastatic ability (Fig. 1). The HM-TECs were more proliferative, motile, sensitive to VEGF, and invasive to ECM than LM-TECs. In addition, HM-TECs showed upregulation of the angiogenesis-related genes VEGFR-1, VEGFR-2 and VEGF. Furthermore, we previously suggested that autocrine VEGF was necessary for cell survival and tube formation in HM-TECs.11, 12 The PI3K/Akt signaling pathway plays a crucial role in angiogenesis.15, 36 Under basal conditions, Akt phosphorylation levels in HM-TECs were higher than those in LM-TECs, suggesting that PI3K/Akt signaling activation by the VEGF autocrine loop causes the HM-TECs phenotype to be more pro-angiogenic than that of LM-TECs in the tumor microenvironment.
During tumor neovascularization, EC can breach their basement membrane, degrade ECM, and migrate to the tumor. These events are necessary for tumor metastasis. The gelatinase/collagenase IV metalloproteases (MMP-2 and MMP-9) are involved in this process and promote angiogenesis. Cell migration, invasion, and matrix remodeling are essential for the morphogenesis of TEC into capillaries.15
The PI3K/Akt signaling pathway functions simultaneously with MMP-2, MMP-9, and VEGF production in EC.15 We showed that HM-TEC upregulates expression of MMP-2 and MMP-9 and displays increased Akt phosphorylation under basal conditions compared with LM-TEC. MMP-2/MMP-9 upregulation, which occurs through the activation of PI3K/Akt signaling pathway, may be responsible for the invasive potential of HM-TEC.
Stem-like phenotype in HM-TEC
Recent studies revealed that prostate EC displayed mesenchymal-like differentiation.33 In another study, bone marrow-derived stem cells (Sca-1+, c-kit+) or tissue resident progenitors were shown to contribute to tumor vasculature and give rise to functional EC.37 HM-TECs have relatively marked stem cell characteristics; they transdifferentiate into bone-like cells at a higher rate than LM-TECs or NECs. We hypothesized that these phenotypic differences between HM-TECs and LM-TECs may be because TECs could be recruited from different sources, that is, EPC or resident progenitors of EC, in addition to sprouting EC. We found expression levels of the Sca-1 and CD90 genes in HM-TEC were higher than in LM-TEC. These results suggest that progenitor cell incorporation during HM tumor vascularization is more frequent than that during LM tumor vascularization.
Drug resistance and cytogenetic abnormality in HM-TEC
We have recently showed TECs were resistant to anti-cancer drug, paclitaxel with MDR1/P-gp upregulation.28 HM-TECs are more resistant to paclitaxel and show higher mRNA expression levels of MDR1 than LM-TECs.
We also reported that TEC were karyotypically aneuploid, whereas NECs cultured under the same conditions were diploid.7, 9, 10 Karyotypes of LM-TECs and HM-TECs were analyzed by GTG-banding. HM-TECs had more complex abnormal karyotypes than LM-TECs.27 Complex abnormal karyotype and high aneuploidy rates in cancer cells are related to frequent occurrence of multidrug resistance genes.38 Although the consequences or mechanism of HM-TEC abnormality is unclear, further research on the mechanism of genetic instability in HM-TECs could provide a practical strategy to establish effective anti-angiogenic therapy. The differences between HM-TEC and LM-TEC are summarized in Table 2.
| HM-TEC characteristics (compared to LM-TEC) | |
|---|---|
| Angiogenesis related genes | VEGF-A↑, VEGF-R1↑, VEGF-R2↑, HIF-1α↑, CXCL12↑ |
| Proanigogenic phenotype | Proliferation↑, Motility↑ |
| Invasion related genes | MMP-2↑, MMP-9↑, TIMP-1↓ |
| Drug resistance | Multi drug resistance gene (MDR-1) ↑ Resistance to anti cancer drug ↑ (5FU, Paclitaxel) |
| Stemness | Sca-1↑, CD90↑, CD133↑, Sphere forming activity↑, Bone differentiation activity↑ |
| Chromosomal abnormality | Aneuploidy rate↑, Abnormality in karyotype↑ |
Mechanism of TEC heterogeneity
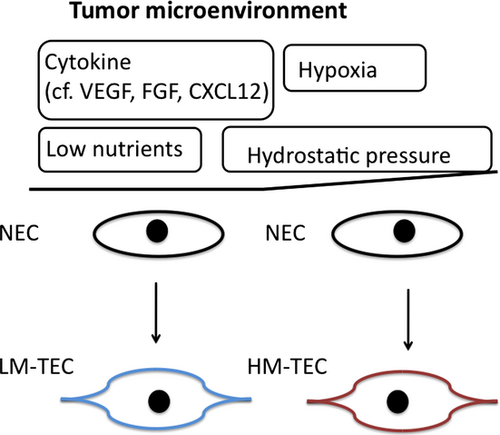
Crosstalk between tumor cells and stromal cells is reportedly essential for tumor progression, acceleration of metastasis, and increased tumor malignancy. Factors secreted by HM tumors may alter NEC characteristics to a pro-angiogenic phenotype. Co-culture with HM tumor cells led to increased expression of pro-angiogenic genes and phenotypic change in NECs. HM-TECs probably acquire their pro-angiogenic characteristics through soluble factors secreted by HM tumors, which are highly angiogenic. Also, hypoxia may be another mechanism by which HM-TECs acquire a pro-angiogenic phenotype. Tumor blood vessels were found adjacent to areas of tumor necrosis; an area of tumor necrosis was induced by rapidly growing tumor, which depleted nutrients and oxygen, ultimately resulting in tumor hypoxia. Tumor vasculature is often leaky because of its immaturity, causing high tissue pressure inside the tumor and shrinkage of tumor vessels, resulting in hypoxia.39, 40 HM tumor blood vessels were more immature and showed less pericyte coverage than LM tumor blood vessels when immunostained with α-SMA antibody.27 This may be a reason why HM tumors are more hypoxic than LM tumors. Furthermore, in HM tumors, some tumor blood vessels and surrounding areas are exposed to hypoxia. Hypoxia in HM tumors may lead to excessive VEGF production, vascular permeability, and gene instability in HM-TECs.41 The resulting decrease in blood flow around tumor vessels in HM tumors may also decrease nutrient and oxygen delivery, imposing physiological stress on the tumor. Like cytokines such as VEGF and CXCL12, hypoxia induces mobilization of progenitor cells from the bone marrow to the leading edge of necrotic tumors to promote revascularization.42 Thus, hypoxia and high expression levels of cytokines may be responsible for the higher progenitor cell incorporation in HM tumor vasculature (Fig. 2).
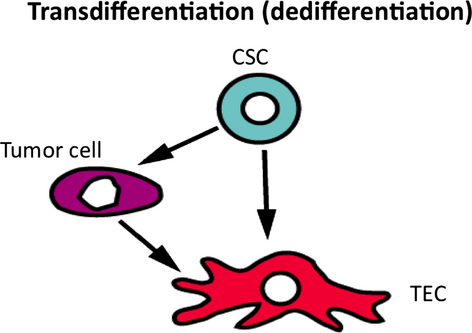
In addition, glioblastoma cells reportedly sometimes differentiate into tumor endothelial cells.43, 44 However, recent study has shown that glioblastoma cells transdifferntiated into pericyte, not endothelial cells.45 Another report suggested that tumor cells transdifferentiated into TEC in lymphoma.46 This may also be a mechanism of TEC heterogeneity. However, it was not supported by our cross-species model (human tumor and mouse endothelial cells) (Fig. 3). Further studies are required to elucidate the mechanism of TEC heterogeneity.
Therapeutic implications
Although anti-angiogenic therapy is an important treatment option for cancer, its use is still limited because of inadequate understanding about the benefit of these agents, and the occurrence of side-effects or drug resistance.47 The mechanism involved in resistance to anti-angiogenic therapy is still not fully understood.
Our results suggest that the heterogeneity of different types of TECs differs by tumor malignancy. Tumor endothelial cells may be related to their parental tumors by tumor-mediated factors, that is, growth factors, chemokines, and microRNA. Furthermore, TECs may differ by tumor microenvironment, stage of tumor growth, progression, and metastasis. It may be possible to develop TEC markers into biomarkers that reflect heterogeneous tumor blood vasculature, allowing better selection of anti-angiogenic therapies.
Conclusion
This mini-review summarized the significance of heterogeneity in tumor endothelial cells. Although the mechanism of the effects of TEC heterogeneity on monomorphous anti-angiogenic therapy is still unclear, our results raise several important issues in cancer therapeutics. The most critical points are the observations that even stromal cells can be abnormal in the tumor microenvironment, and that TECs are heterogeneous. A novel anti-angiogenic therapy that targets TEC heterogeneity could improve overall anti-tumor efficacy. Further studies on TEC heterogeneity will facilitate the selection of suitable anti-angiogenic therapies.
Acknowledgments
We thank the members of the Department of Vascular Biology of the University of Hokkaido and Dr. Shindoh for helpful discussions.
Funding
This work was supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Science, and Culture of Japan (to K. Hida) and by grants from the Akiyama Foundation (to K. Hida).
Disclosure statement
The authors have no conflict of interest.




