A meta-analysis of tropical land-use change effects on the soil microbiome: Emerging patterns and knowledge gaps
Associate Editor: Jennifer Powers
Handling Editor: Jennifer Powers
Abstract
enModifications to vegetation and soil due to changes in land use have the potential to alter the soil microbiome, with consequences for carbon and nutrient cycling. Despite the important function of soil microorganisms, little is known about their response to land-use change, especially in tropical regions where current rates of land conversion are greatest. The aim of this meta-analysis was to examine how land-use change influences soil microbial properties in tropical ecosystems and to identify current trends and knowledge gaps in the literature. We identified 83 published paired studies that reported data on microbial biomass, abundance, composition, and enzyme activity under representative land-use changes in the tropics. We calculated response ratios for studies that compared the following: reference forests to (a) agriculture, (b) pastures, (c) plantations, and (d) secondary forests. Soil microbial biomass decreased with forest conversion to agriculture and plantations. Microbial biomass response to land-use change depended on rainfall classes, although this was the only microbial variable which had sufficient data to test for a rainfall effect. Microbial abundance and enzyme activity showed variable results depending on the type of forest conversion. Microbial diversity and richness did not show any pattern with forest conversion or recovery to secondary forests. Published studies were not representative of the range of biophysical conditions observed in the tropics. Sites in moist regions in the American tropics were overrepresented. To better predict how land-use change affects the soil microbiome and its contribution to nutrient cycling, research should reflect observed environmental variation in the tropics.
RESUMEN
esLas modificaciones en la vegetación y el suelo como resultado de cambios en el uso de la tierra tienen el potencial de alterar el microbioma del suelo, afectando el carbono y los nutrientes. A pesar de la importante función de los microorganismos en el suelo, se sabe poco sobre su respuesta al cambio de uso de terreno, especialmente en las regiones tropicales donde las tasas actuales de conversión de la tierra son mayores que en otras regiones. El objetivo de este metaanálisis fue examinar cómo el cambio de uso de la tierra influye en las propiedades microbianas en los ecosistemas tropicales, e identificar las tendencias actuales y las lagunas de conocimiento en la literatura. Identificamos 83 estudios que contenían datos sobre biomasa microbiana, abundancia, composición y actividad enzimática bajo cambios de uso de la tierra en los trópicos. Calculamos las proporciones de respuesta para los estudios que compararon la diferencia entre bosques de referencia con (a) agricultura, (b) pastos, (c) plantaciones y (d) bosques secundarios. La biomasa microbiana del suelo disminuyó con la conversión de bosques a agricultura y plantaciones. La respuesta de la biomasa microbiana al cambio de uso de la tierra dependió de las categorias de lluvia (seco, húmedo, o lluvioso), aunque ésta fue la única variable que tenía datos suficientes para probar este efecto. La abundancia microbiana y la actividad enzimática mostraron resultados variables según el tipo de conversión forestal. La diversidad y riqueza microbiana no mostró ningún patrón con la conversión forestal o la recuperación a bosques secundarios. Los estudios publicados no fueron representativos del rango de condiciones biofísicas observadas en los trópicos. Los sitios en las regiones húmedas de los trópicos americanos estaban sobrerrepresentados. Para predecir mejor cómo el cambio de uso de la tierra afecta el microbioma del suelo y su contribución al ciclo de nutrientes, la investigación debe reflejar la variación ambiental observada en los trópicos.
1 INTRODUCTION
Tropical regions are currently experiencing some of the fastest rates of land-use change globally (Aide et al., 2012). From 2010 to 2015, tropical forests were lost at a rate of 5.5 M ha/year, greater than the loss of boreal forests (0.08 M ha/year), and in contrast with an increase in temperate and subtropical forests (Keenan et al., 2015; Veldkamp et al., 2020). Habitat loss is the primary driver of plant and animal diversity declines worldwide (Millennium Ecosystem Assessment, 2005), yet the effects of changes in land use and land cover (hereafter, land-use change) on the abundance and diversity of soil microorganisms and their function has not been well documented. Soil microorganisms are key players in multiple ecosystem processes, such as plant fitness (Friesen et al., 2011), litter decomposition, nutrient availability, and soil organic matter (SOM) transformations (Turner et al., 2013; Wieder et al., 2013). Losses of soil biodiversity and food web complexity can affect carbon (C) and nutrient cycling (de Graaff et al., 2015), yet whether land-use change results in microbial species richness declines or shifts in community assemblages and activity is still unknown.
Land-use change, such as deforestation or forest clearing for agriculture and livestock grazing, can influence the soil microbiome through shifts in plant communities and management practices, which can alter the amount, timing, and spatial distribution of substrates for microbial growth (Cai et al., 2018; Krashevska et al., 2018). For example, perennial and annual cropping systems differ in rhizosphere inputs, which influence fungal communities and total microbial biomass (Liang et al., 2012; Zhang et al., 2018, 2019). Grazing and fertilization can affect soil resources and alter the abundance of microbial groups involved in nitrogen (N) cycling (Meyer et al., 2013). Management practices, such as the use of mechanization for clearing and tillage, can transform soil physical and chemical properties and the spatial relationship among organisms, which can alter heterotrophic food webs and biogeochemical cycling (Mathew et al., 2012).
Modifications in pathways and rates of biogeochemical processes coupled to changes in overall microbial biomass or the abundance of key microbial groups (Bai et al., 2019; Docherty & Gutknecht, 2012; Potthast et al., 2012) may result from the preferential use of different substrates by distinct microorganisms (Paterson et al., 2008; Strickland et al., 2009; Zhong et al., 2020). For example, the forest soil microbiome may be better adapted to degrading lignin-rich litter than the microbiome of grassland soils (Cleveland et al., 2003). Bacteria are expected to assimilate more decomposed substrates, whereas fungi may preferentially degrade more recent plant C inputs (Frey, 2019; Poll et al., 2006).
Enzyme activities can be used as a proxy for microbial function and are sensitive to changes in plant cover and management (Sinsabaugh et al., 2002; Zhao et al., 2018). Enzymes involved in plant litter decomposition catalyze the breakdown of compounds, such as cellulose, hemicellulose, and lignin, and control the release of plant- and microbe-available nutrients from organic forms (Horwath & Paul, 2015). These activities can be sensitive to changes in plant cover and management (Sinsabaugh et al., 2002). For example, some studies have found that agricultural practices can reduce levels of enzyme activity compared with forest soils due to soil disturbance by tillage (Acosta-Martínez et al., 2007). Other studies have found that rates of enzyme activity can increase with pasture establishment after deforestation (Tischer et al., 2015). However, it is important to consider how plant diversity effects on decomposition and nutrient cycling create biogeochemical heterogeneity, as well as the concept of microbial functional redundancy, to understand how the soil microbiome is affected by forest clearing and conversion to agriculture or livestock grazing, especially in species-rich tropical ecosystems (Chaer et al., 2009; Smith et al., 2015; Townsend et al., 2008).
Here, we conduct a meta-analysis on the response of soil microbial biomass, composition, and functional activity under different land-cover types representative of major land-use change transitions in tropical regions. We focused on soil bacteria and fungi. We identify current knowledge gaps in the literature, including geographic biases in available data. Our understanding of the diversity of tropical soil microorganisms lags behind temperate regions. Identifying how tropical land-use change alters interactions among soil microbial community composition and function can provide insight into feedbacks between tropical ecosystems and global change factors, such as climate change (Graham et al., 2016; Powell et al., 2015). We hypothesized that forest conversion to agriculture, pasture, and tree plantation would decrease soil microbial biomass, abundance, and function, with consequences for biogeochemical cycling. We expected that secondary forests regrowing from human land use would recover microbial communities and function. Because microbial communities are dependent on soil resource availability, we expected that changes in microbial communities would mirror expected changes in soil pH, carbon, nitrogen, and phosphorus with land-use change.
2 METHODS
We conducted a literature review using Web of Knowledge and Google Scholar databases to find studies within the tropical latitudes (between 23°26′16′′N and 23°26′16′′S) using the terms “tropical,” “land use,” “land cover,” “soil microbes,” “microbial biomass,” “fungi,” and “mycorrhizal fungi” (published up until February 2019, the last date we updated our search). This search resulted in 110 primary sources. Eighty-three of those reported data for paired comparisons between two or more land-use types. We used PlotDigitizers software to estimate values when data were only reported in figures (Huwaldt & Steinhorst, 2015).
We selected studies that were conducted using a paired plot approach to compare reference forests to other land use or vegetation cover types: (a) forests to agriculture (b) forests to pastures, (c) forests to plantations, and (d) forests to secondary forests (Table S1). The studies needed to have a reference forest to be able to compare changes. Studies that only included regrowing forest and secondary forest without reference forests were not selected for this meta-analysis. We averaged data reported from plot replicates within a site (location). However, most studies reported mean values for different land covers.
Most of the studies grouped the data by soil depth, date collected, or by season. Due to the limited number of studies reporting each possible combination of categories, we created consolidated variables. We limited our analysis to data for the topsoil layer (0–20 cm depth) to include the largest number of studies. If reported depths fit into the category of 20 cm depth (i.e., 0–5, 5–10, 0–10, 10–20, 0–15 cm), we averaged the data for the different layers up to 20 cm. If a study reported two or more sampling collection dates or seasons (e.g., wet and dry), we calculated average values. We acknowledge that sampling collection date and season are important factors that can influence microbial communities, yet few studies reported seasonal measurements of enough microbial variables of interest for our meta-analysis.
Land use and vegetation covers were grouped under five categories: “forest,” “agriculture,” “pasture,” “plantation forest,” and “secondary forest.” We primarily used site identification provided by the authors. Forests were those presented as reference points by study authors and included unmanaged forests largely undisturbed by human activities. Agriculture included crops and systems with some level of resource inputs. Pastures included managed and unmanaged grasslands and forage systems that were actively being used to support cattle or other livestock or were recently abandoned but still under grass cover. Plantations were defined as managed systems of woody trees or palms. Secondary forests were identified as successional forests establishing after clearing or after cessation of other land-use activities and could include sites recovering from logging, pasture, agriculture, and other non-forest uses.
We included a large number of microbial and environmental variables in our meta-analysis (Table 1). Studies reported many different microbial measurements depending on their research objective. For microbial variables, we grouped the data based on the type of measurements. For example, we analyzed studies using phospholipid fatty acid analysis (PLFA) and DNA sequencing to measure microbial abundance separately, recognizing that different approaches have different assumptions that can lead to different responses.
| Environmental variables | Microbial Biomass | Colonies | OTU Abundance | PLFA | Enzymes | Bacterial Diversity | Fungal variables | Fungal Diversity |
|---|---|---|---|---|---|---|---|---|
| Site information | Carbon | Fungi | Archea | Total | a-Galactosidase | Similarity | Spore Density | Spores Shannon Diversity |
| Land change | Nitrogen | Bacteria | Bacteria | Bacterial | B-Glucosaminidase | Simpson | AMF Spore Abundance | Spores Simpson |
| Mean annual temperature (MAT) | Phosphorus | Fungi | Gram Positive | B-Glucosidase | Shannon Diversity | AMF Spore Abundance | AMF Spore Richness | |
| Mean annual precipitation (MAP) | Actinomycetes | Gram Negative | a-Glucosidase | Whitaker Global | AMF Total Spore Species | AMF Diversity Jackknife | ||
| Temperature class | Proteobacteria | Fungal | Xylanase | Pielou Evenness | Extractable Glomalin | AMF Shannon Diversity | ||
| Rainfall class | Actinobacteria | B-Xylosidase | Fisher Diversity | Total Glomalin | AMF Shannon Evenness | |||
| Soil properties | Acidobacteria | Acetylglucosaminidase | OTU Jackknife | AMF Root Colonization | AMF Pielou Evenness | |||
| Organic carbon | Firmicutes | Phosphatase | OTU Richness | ECM Root Colonization | AMF Simpson | |||
| Total carbon | ECM | Phophomonoesterase | OTU Richness | |||||
| Total nitrogen | Urease | OTU True B Diversity | ||||||
| Available phosphorus | Acid Phosphatase | |||||||
| Total phosphorus | Alkaline Phosphatase | |||||||
| pH |
Cellobiohydrolase Chitinase Phenol Oxidase Peroxidase Laccase Cellulase Arylsulfatase |
2.1 Data analysis
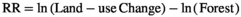
We performed a one-way ANOVA Type III for an unbalanced design to analyze differences between response ratios across rainfall classes for each land-use change comparison only for microbial biomass carbon. We did not perform the aforementioned analysis with other variables because the number of samples was too low to fit all the land-use change and rainfall classes. To test if microbial biomass mirrored the magnitude of changes in soil properties, we used simple linear regression models between the response ratio of microbial biomass carbon (C) and nitrogen (N) and the response ratio of soil properties, specifically pH, organic C, total N, and total phosphorus (P) across all land-use changes. Statistical significance for these analyses is reported at p < 0.05 unless otherwise indicated.
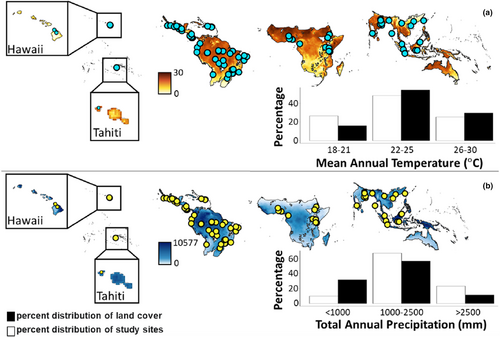
To identify potential geographic biases in available data on tropical soil microbiome response to land-use change (sensu Powers et al., 2011), we assessed how well the literature represented variation in mean annual precipitation (MAP), mean annual temperature (MAT), ecoregions, and soil type observed across the tropics. We calculated the percentage of land in the tropics represented by each climatic category, ecoregion, or soil type using ArcGIS (ArcGIS 10; ESRI, 2010). We performed a chi-squared analysis to assess how well the studies represented MAP and MAT (Powers et al., 2011). When missing from the original publication, we used the WorldClim version 2 Global Climate Data set (Hijmans et al., 2005) for compiling climate data, and the Food and Agriculture Organization (FAO) Harmonized World Soil Database v 1.2 (Fischer et al., 2008) to identify soil types. We used The Nature Conservancy's terrestrial ecoregions map, which is based on the World Wildlife Fund ecoregions classification (Olson et al., 2001). Ecoregions represent potential vegetation types based on climatic variables and were classified as moist broadleaf forest, dry broadleaf forest, coniferous forest, and grassland, savanna, and shrubland. Mean annual temperature was subdivided into three categories (18–21°C, 22–25°C, and 26–30°C) which accounted for the Koppen climate classification system criterion (Köppen, 1900; Kottek & Hantel, 2005). Mean annual precipitation was divided into rainfall classes associated with different tropical forest life zones: dry (<1000 mm), moist (1000–2500 mm), and wet (>2500 mm) representing 17%, 59%, and 23% of the sites, respectively. When analyzing for biases, we pooled studies from all land-use transitions as the number of studies was too small to compare each category individually. There were too few studies to perform the chi-squared on soil type and ecoregion type, given the large number of categories for each variable, but expected representation of each category was compared qualitatively to observed representation in the literature.
3 RESULTS
We recorded 10,314 observations of 87 variables from 83 studies (Supplementary database). Thirty-six studies included multiple pairs of sites of the same land-use change comparison. Field sites were located in 25 countries, with the greatest proportion in Brazil (33%), India (7%), and Costa Rica (6%) and the lowest proportion in Argentina, Benin, Cameroon, Colombia, Dominican Republic, Ethiopia, Hawaii, Nigeria, Sumatra, and Tahiti, Taiwan (1%).
3.1 Microbial biomass response to land-use change
On average, forest conversion resulted in losses of soil microbial biomass carbon and nitrogen (Figure 1, Table S2). For microbial biomass C, these results were primarily observed in conversion of forest to agriculture and plantations. Conversion to pasture did not affect microbial biomass C. Only conversion of forest to plantation showed a significant decline in microbial biomass N. Microbial biomass P was not affected by forest conversion. Secondary forests did not show any difference in microbial biomass C, N, or P compared with forest values.
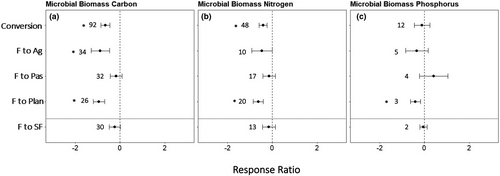
Microbial biomass carbon was the most represented variable among the three rainfall classes: dry (23% of studies), moist (52%), and wet (25%) life zones. Wet secondary forests had a greater microbial biomass carbon response ratio than moist secondary forests (F = 3.35, p = 0.04, Figure 2, Table S3). There was no effect of rainfall class on microbial biomass carbon during conversion of forest to plantations. In forest conversion to pastures (F = 6.17, p < 0.01) and in agricultural lands (F = 18.27, p < 0.01), microbial biomass carbon response ratio was greater in dry systems compared with moist systems.
3.2 Microbial abundance response to land-use change
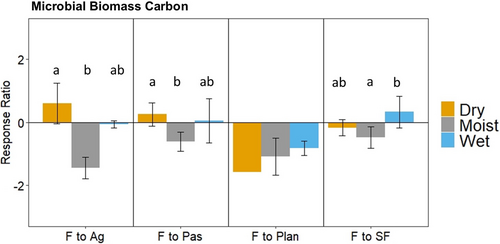
On average, forest conversion resulted in a decrease or no change in microbial abundance measured by DNA sequencing and phospholipid fatty acid analysis (PLFA; Figure 3, Table S2). Four studies performed sequencing analysis (one study conducted pyrosequencing and the rest conducted Illumina sequencing). Forest replacement by plantations decreased total bacteria PLFAs and Gram- bacteria PLFAs. Conversion of forests to agriculture resulted in a decrease in the abundance of proteobacteria DNA sequences, total bacteria PLFAs, and Gram- bacteria PLFAs. In contrast, the conversion of forests to pastures increased the abundance of actinobacteria DNA sequences and total fungal PLFA. Secondary forests had greater abundance of Firmicutes and lower actinobacteria DNA sequences than forests.
3.3 Enzyme activity response to land-use change
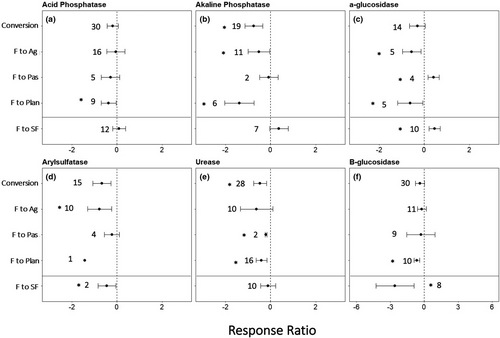
Extracellular enzyme activity generally decreased with forest conversion (Figure 4, Table S2). Plantations showed lower activities for all enzymes reported. Conversion to pastures increased α-glucosidase and urease activity. Deforestation for agriculture decreased activities of alkaline phosphatase, α-glucosidase, and arylsulfatase. Secondary forests had greater levels of α-glucosidase activity and lower levels of ß-glucosidase and arylsulfatase than reference forests. Activities of acid phosphatase, alkaline phosphatase, and urease did not differ between forests and secondary forests.
3.4 Bacterial diversity response to land-use change
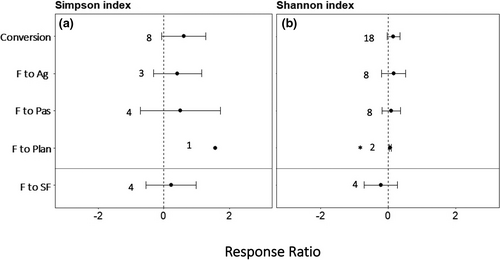
Forest conversion resulted in no change in soil bacterial diversity as measured by Shannon and Simpson indices (Figure 5, Table S2). However, OTU Chao1 richness decreased with forest conversion to agricultural lands (Table S2).
3.5 Fungal response to land-use change
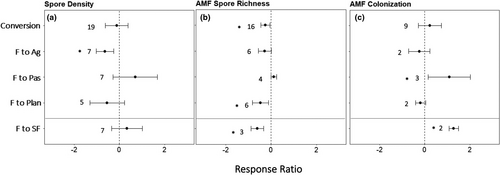
Fungal measurements showed variable responses to land-use change (Figure 6, Table S2). Conversion from forest to plantation decreased arbuscular mycorrhizal fungi (AMF) richness. Fungal spore density from all species in the soil and AMF richness decreased with conversion to agriculture. AMF colonization of roots increased in pastures. Secondary forests had greater arbuscular root colonization and less AMF richness than reference forests.
3.6 Microbial biomass responses to changes in soil properties
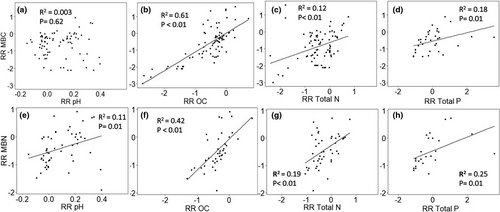
We tested for relationships between average response ratios for microbial biomass and the response ratios for soil properties across all four land-use change transitions. The response ratios for microbial biomass N (Figure 7e) and microbial biomass P (Figure S2) were positively correlated with the response ratio for soil pH. Microbial biomass C response ratios did not show any relationship with soil pH (Figure 7a). Microbial biomass C and microbial biomass N response ratios increased with organic C, total soil N, and total P response ratios.
3.7 How representative are microbial studies of tropical environments?
The available literature reporting microbial biomass and compositional variables for paired land uses does not reflect the diversity of biophysical variables represented in tropical regions. The range of MAP observed in the study sites (from 500 mm to 4,400 mm) was not representative of rainfall variation in the tropics (chi-square p < 0.001; Figure 8a). Sites in the wet (>2500 mm) and moist (1000–2500 mm) life zones were overrepresented at the expense of drier sites. The range of MAT was also not representative of the temperature variation in the tropics (chi-square p = 0.035; Figure 8b). There were not enough studies to perform a chi-square analysis on the representativeness of the study sample set by ecoregion or soil order, although visual interpretation of the data suggests some strong biases in the literature. Grasslands, savannas, shrublands, coniferous forest, dry broadleaf forest were underrepresented while moist broadleaf forests were overrepresented compared with their observed distribution across tropical landscapes (Figure S3a). Moist broadleaf forests composed 71% of field observations. The diversity of tropical soils also is underrepresented in the literature. The FAO global soil map identifies 27 soil units in the tropics, yet only 16 of these were represented in the literature reviewed (Figure S3b). Twenty-one percent of the study sites were on Ferrasols and Acrisols. Cambisols were the third most studied soil order (12% of field observations).
4 DISCUSSION
Our findings show that land-use cover changes in tropical regions affect soil microbial communities and functions. Notably, forest conversion to intensely managed systems decreases microbial biomass, abundance, and enzyme activities. Forest conversion to pastures showed increases, decreases, or no changes to microbial communities and functions, suggesting some microbial variables may depend on the response of soil properties after deforestation. After abandonment of pasture, agricultural or plantation forest management, secondary forest succession can recover microbial communities and function. Moist ecosystems were overrepresented in the literature, whereas dry ecosystems were greatly underrepresented. Therefore, our understanding of how microbial communities and function are affected by land-use change is biased toward moist and wet regions.
4.1 Greater changes in microbial biomass and composition with replacement of forest by more intensively managed land
Measured declines in soil microbial biomass carbon, nitrogen, and phosphorus with conversion of forests to agriculture and plantations are consistent with expectations for changes in soil properties in more intensely managed systems. The overall decrease of microbial biomass C and N can be associated with the decrease of soil C and N stocks common in agricultural systems (Bossio et al., 2005). Losses of soil C and N are attributed to the removal of plant residues and nutrients through biomass harvest and to accelerated decomposition of organic matter through soil disturbance during tillage and other soil preparation techniques (Montecchia et al., 2011). Management practices that improve organic matter inputs to soils can remedy observed decreases in microbial biomass in agricultural soils. On sandy soils in the tropical dry forest of eastern Amazonia, the addition of manure fertilizer coupled with irrigation increased microbial biomass C relative to the adjacent forest during the dry season (Medeiros et al., 2015). Banger et al., (2008) found that manure additions increased microbial biomass C on tropical agricultural fields beyond that observed with inorganic fertilizers. Similarly, conversion of conventional agriculture to organic farming in Brazil increased soil microbial biomass C and soil C (Santos et al., 2012). Observed decreases in soil microbial biomass after establishment of plantations have been attributed to reductions in soil nutrients due to woody biomass harvest and residue management (Liu et al., 2012). Allen et al., (2015) found that reduction in microbial biomass with establishment of plantations in Indonesia was related to soil nutrient availability in highly weathered Acrisols.
Surprisingly, conversion of forests to pastures did not alter soil microbial biomass C, N, or P pools. However, this result could be related to carbon dynamics in soils. Forage grasses common in many tropical ecosystems are characterized by large root C inputs (Fischer et al., 1994) and many studies report no change or even gains in soil C pools with pasture establishment (Cleveland et al., 2003; Marín-Spiotta et al., 2008; Neill et al., 1997; Trumbore et al., 1995). Consistent with our findings for more intensively managed sites, soil C stocks and microbial biomass have been found to decline with increasing pasture age and grazing intensity in the eastern Amazon (Melo et al., 2012) and in Hawai'i (Elmore & Asner, 2006).
4.2 Changes in microbial composition but no change in microbial diversity with forest conversion
Forest conversion altered the abundance of different microbial groups depending on the land-use conversion. Conversion to agriculture and tree plantation decreased the abundance of bacterial PLFA biomass, consistent with observed declines in total soil microbial biomass pools. Fungal PLFA biomass, spore abundance, and root colonization were greater in pasture soils compared with forests, which could be due to increased fine-root derived C inputs under pasture (Picone, 2000). Distinct microbial communities between tropical forests and pastures have been attributed to differences in root biomass, soil pH, moisture, and nutrient availability (Borneman & Triplett, 1997; Picone, 2000).
Replacement of forest with alternative land uses did not alter microbial community diversity as measured by traditional richness indices, although AMF spore richness decreased with conversion to pastures and in secondary forests. A recent meta-analysis of tropical rain forest soils found an increase in bacterial alpha diversity and changes in composition with forest conversion to pastures and plantation (Petersen et al., 2019). However, most of these results were not strictly attributed to conversion from forest to other land uses, but to variability in soil properties such as pH and available C for microorganisms.
4.3 Reductions in enzyme activity with conversion to intensively managed systems
Extracellular enzymes catalyze the decomposition of C-rich macromolecules into smaller, soluble compounds that plants and microorganisms can use for energy and nutrient acquisition. As such, they are often measured as indicators of microbial function, activity, and soil health. Overall, our meta-analysis found that extracellular enzyme activity decreased or showed no change with deforestation. Agricultural soils and plantation soils tended to have reduced enzyme activities, whereas pastures and secondary forests showed reduction, increases, or no net change (Acosta-Martínez et al., 2007; Sotomayor-Ramírez et al., 2009). Decreases in microbial functional capacity resulting from land-cover change and long-term agricultural practices can affect microbial influences on nutrient and carbon dynamics.
Variability in enzyme activity response with land-use change can be due to a variety of methodological and ecological factors. Enzyme assay conditions are not standardized across laboratories, which may affect findings (Burns, 1982; German et al., 2011, 2012). At the same time, extracellular enzymatic activity is regulated by environmental factors such as temperature, moisture, soil pH, and substrate availability (Burns & Dick, 2002; Tabatabai, 1994; Tate III, 2002). Soil texture, structure, and mineralogy also affect enzymatic activity (Burns, 1982; Quiquampoix et al., 2002; Tate III, 2002). Variability in environmental properties within and across land uses in a study can mask potential effects of changes in microbial biomass or composition with land management. For example, differences in soil types among replicate sites masked potential effects of land use in Western Kenya (Bossio et al., 2005). Large variability among soil sample replicates and strong seasonal differences in enzyme activity along a forest successional chronosequence on former pastures in Puerto Rico may have also obscured differences between land use and cover types (Smith et al., 2015).
4.4 Recovery of forest microbiome during secondary succession
The meta-analysis revealed few differences between secondary forests and reference forest sites. In summary, secondary forests and reference forests had similar amounts of microbial biomass C, N, and P, total bacterial and fungal PLFA biomarkers, abundance of Acidobacteria and Proteobacteria, activity rates of acid phosphatase, alkaline phosphatase, and urease, and AMF spore density. This suggests that forest succession can reverse some of the observed declines in microbial biomass properties during deforestation. However, it could also suggest that the variability among studies is too high and values can be either positive or negative resulting in a non-significant response ratio.
Reforestation provides the opportunity to study successional trajectories of soil microbial communities. Mycorrhizal fungi can persist through at least 30–40 years of pasture use (Fischer et al., 1994) and potentially facilitate plant colonization during reforestation. In Costa Rica, studies have found greater microbial biomass carbon, fungal abundance, and diversity in secondary forests compared with pastures used for cattle (Hafich et al., 2012; McGee et al., 2019). This result was attributed to increased levels of soil N, organic C, and phosphate with forest succession. As secondary forests aged in a successional chronosequence in Puerto Rico, the amount of fungal PLFA biomarkers decreased, whereas Gram-positive bacteria and anaerobic Gram-negative bacteria became more abundant, resembling the community of primary forest soils (Smith et al., 2015).
Many tropical studies show that succession and restoration can return properties of the soil microbial community to pre-disturbance levels with the recovery of aboveground biomass (Araújo et al., 2013; Knief et al., 2005), yet recovery times can vary. Variability in the length of time for microbial recovery with reforestation is likely affected by environmental properties, such as soil type and climatic controls on primary production and plant inputs, as well as the severity of disturbance associated with land-use change (Carpenter et al., 2001). Recovery of soil microbial biomass and activity comparable to undisturbed old forest in the Dominican Republic occurred within 5–7 years after agricultural abandonment (Templer et al.,2005). In highland wet forests in Western Amazon, bacterial community composition shifted to that of the primary forests within 5–30 years of forest regeneration (Jesus et al., 2009). In north-eastern Brazil, soil microbial biomass and enzyme activity in areas that had been logged recovered in less than 10 years, but stoichiometry of microbial biomass carbon-to-nitrogen ratios differed, reflecting potential legacy effects of N losses from land use (Araújo et al., 2013). Overall, the soil microbiome during tropical reforestation shows relatively rapid recovery to levels of biomass and community composition of undisturbed forests, although time to recovery can vary greatly with past land-use intensity and soil type.
4.5 Microbial response to forest conversion is positively related to changes in soil properties
Soil physical and chemical properties play a strong role in regulating microbial communities by influencing gradients of environmental conditions, such as pH, oxygen, temperature, and moisture, that affect microbial physiology (Morris & Blackwood, 2007; Russo et al., 2012). The response of certain microbial variables, including microbial biomass C, N, and P, was positively correlated to response ratios of soil pH, and soil organic carbon, total nitrogen, and total phosphorus concentrations. Experimental manipulations in a lowland tropical forest showed significant shifts in microbial community structure in response to plant litter and throughfall inputs, attributed to changes in the amount and bioavailability of C and N substrates reflecting top-down control in soils (Nemergut et al., 2010). In contrast, along a soil fertility gradient and nutrient addition experiment in Hawaii, the effect of invasive tree species on soil microbes was mediated by soil N and P levels, highlighting the important bottom-up control of soil resources on microbial composition (Kao-Kniffin & Balser, 2008). High levels of N availability result in greater microbial biomass and activity in soils (Potthast et al., 2012) and low P availability constrains processes such as decomposition and microbial use of available C in highly weathered tropical soils (Cleveland et al., 2003).
A recent synthesis concluded that tropical deforestation can have long-term effects on soil properties, such as pH, base saturation, and bulk density, in addition to changes in organic matter (Veldkamp et al., 2020). Considering a soil's physical, chemical, and biological component is important for helping us understand land-use change effects on the soil microbiome. Measurements at the appropriate spatial and temporal scale that consider heterogeneity in factors influencing soil microbial communities and their function are necessary to reveal directional responses to temporal seasonality and environmental changes. Other challenges in quantifying and predicting the response of soil microbes to land-use change are paucity in environmental data, including soil properties, or even lack of soil descriptions and classification in many microbial studies.
4.6 Gaps in our knowledge of microbial response to tropical land-use change
Our current understanding of the response of the tropical soil microbiome to some of the most common land-use conversions come from a limited number of sites that are not representative of the diversity of environmental conditions found in the tropics. Similar geographic biases have been reported for the literature on soil C response to land-use change in the global tropics (Marín-Spiotta & Sharma, 2013; Powers et al., 2011). Identifying these gaps in our knowledge is crucial, given the strong influence of environmental variables on soil microbial processes.
Our finding that drier regions (<1000 mm/y MAP) were underrepresented in the literature is important because soil microbial community composition and function are known to be especially sensitive to changes in soil moisture (Barbhuiya et al., 2004; Cleveland et al., 2003; Costa et al., 2013; Liu et al., 2012; Saynes et al., 2005; Singh et al., 2010). Moisture fluctuations play an important role in microbial communities and on their contribution to biogeochemical cycling of C, N, and other important plant nutrients (Bouskill et al., 2016; Liptzin & Silver, 2015; Yuste et al., 2017). Particularly, transitions from dry to wet seasons increase respiration and denitrification rates resulting in greater CO2 and N2O fluxes from soil to the atmosphere (Calvo-Rodriguez et al., 2020; Waring & Powers, 2016). Yet, uncertainties in our ability to predict microbial response to land-use change are magnified by projected increased variability in rainfall and frequency or severity of droughts in the tropics (Mora et al., 2013; Uriarte et al., 2016). Changes in the length and temporal distribution of wet and dry seasons may affect microbial function and community structure through changes in soil water content, affecting a soil's capacity to buffer microbes from extreme temperatures (Schipper et al., 2007; Tucker & Reed, 2016; Wood et al., 2013).
Bias in the types of soils studied also require caution when extrapolating results across the tropics, because soil types can influence substrate availability, enzyme activity, and soil microbial biomass (Cleveland et al., 2003). Expanding soil microbial research on sites that represent large land areas of the tropics yet are poorly studied will enhance our understanding of biogeochemical consequences of potential changes in microbial communities. Currently, most of the available data on soil microbial response to land-use change comes from the American tropics, especially Brazil, whereas other areas, such as the African tropics, are under studied. This geographic bias, identified also for research on soil C response to land-use change (Powers et al., 2011), has many causes and implications (e.g., Wilson et al., 2016) but is especially problematic for informing regional projections and land management policies.
5 CONCLUSIONS
Tropical deforestation to intensively managed systems alter soil microbial community structure and function with consequences for biogeochemical cycling and nutrient pools. Our meta-analysis revealed the following trends in the literature: (1) Conversion of forests to agriculture and tree plantations typically yielded reductions in microbial biomass and enzyme activity and shifts in community composition, (2) Differences between pastures and forests were more variable, with some studies reporting gains, losses, or no net change in microbial biomass with pasture establishment, reflecting reported heterogeneity in the response of soil C pools in the literature, and (3) Secondary forests recovered microbial biomass and diversity but microbial composition and structure remained different from that of reference forests.
The diversity of ecoregions, rainfall classes, and soil orders found across the global tropics was not well represented in the literature on soil microbial response to land-use change. Microbial land-use change studies have been conducted in only 60% of the FAO soil orders expected in the tropics. Given the importance of soil physical and chemical properties on microbial processes, these limitations affect our understanding of the mechanisms controlling C cycling and nutrient availability in tropical soils. Moist and wet sites are overrepresented compared with drier sites, which hinders predictions about the response of tropical soil microbes to future climate change, especially given projected variability in rainfall for many regions of the tropics. Despite these geographic uncertainties, emergent trends indicate differential response of soil microbial communities and related ecosystem functions to land-use conversions.
ACKNOWLEDGMENTS
This research was supported by a Society of Woman Geographers Pruitt National Minority Fellowship to EJDV, a Morris K. Udall Scholarship to MS, a College of Agricultural and Life Sciences Wisconsin Distinguished Graduate Fellowship to APS, and U.S. National Science Foundation Awards DEB-1050742, DEB-0644265, and BCS-1349952 to EMS. We thank two anonymous reviewers and the editor for helpful feedback.
DISCLOSURE STATEMENTS
The corresponding author confirms on behalf of all authors that there have been no involvements that might raise the question of bias in the work reported or in the conclusions, implications, or opinions stated.
Open Research
DATA AVAILABILITY STATEMENT
Data available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.r7sqv9sb9 (Diaz-Vallejo et al., 2021).




