Drought and soil nutrients effects on symbiotic nitrogen fixation in seedlings from eight Neotropical legume species
Associate Editor: Jennifer Powers
Handling Editor: Norbert Kunert
Abstract
enSymbiotic nitrogen fixation is a dominant source of nitrogen to many terrestrial ecosystems and thus may influence their responses to global change. High legume species diversity and abundance are thought to lead to high rates of symbiotic nitrogen fixation in Neotropical forests. However, how changes in water and nutrient availability will affect symbiotic nitrogen fixation has only recently been explored, even as droughts begin to increase in severity and frequency in the Neotropics. To explore these effects, we grew eight species of Neotropical woody legume seedlings in a shadehouse for four months while manipulating soil water, phosphorus, and molybdenum availability. Overall, drought reduced nodule biomass, nitrogenase activity (acetylene reduction g-1 nodule), and acetylene reduction per seedling by 33%, 27%, and 41%, respectively, but reduced seedling biomass by only 18%. Species varied in the manifestation of drought effects. For example, drought reduced the probability that nodules formed in some species, but in others reduced the nitrogenase activity or acetylene reduction per seedling. In contrast, the effects of phosphorus and molybdenum availability were more species-specific. However, fertilization did affect (both positively and negatively) one or more symbiotic nitrogen fixation response variables in two of the eight species. Our results indicate drought reduces symbiotic nitrogen fixation in Neotropical legume seedlings, but the mechanism of response may be species-specific. Therefore, predicting the response of symbiotic nitrogen fixation in the Neotropics to a changing climate, with expected increases in drought frequency and severity, may require grappling with the diversity of responses among nitrogen-fixing legumes.
Resumen
frA fixação simbiótica de nitrogênio é uma das principais fontes de nitrogênio para muitos ecossistemas terrestres e, portanto, pode influenciar nas respostas desses ecossistemas às mudanças climáticas globais. Acredita-se que a alta diversidade e abundância de espécies de leguminosas levem a altas taxas de fixação simbiótica de nitrogênio nas florestas neotropicais. No entanto, só recentemente tem sido investigado como as mudanças na disponibilidade de água e nutrientes afetam a fixação simbiótica de nitrogênio, mesmo com as secas começando a aumentar em severidade e frequência na região neotropical. Para explorar esses efeitos, cultivamos oito espécies de leguminosas lenhosas neotropicais em uma casa de vegetação por quatro meses, manipulando experimentalmente a disponibilidade de água, fósforo e molibdênio no solo. No geral, a seca reduziu a biomassa do nódulo, a atividade da nitrogenase (redução do acetileno em g-1 por nódulo) e a redução do acetileno por muda em 33%, 27% e 41%, respectivamente, porém a biomassa da muda reduziu em apenas 18%. As espécies variaram na resposta aos efeitos da seca. Por exemplo, a seca reduziu a probabilidade de formação de nódulos em algumas espécies, mas em outras reduziu a atividade da nitrogenase ou redução do acetileno por muda. Já os efeitos da disponibilidade de fósforo e molibdênio foram mais específicos à espécie. No entanto, a fertilização afetou (positiva e negativamente) uma ou mais variáveis de resposta à fixação simbiótica de nitrogênio em duas das oito espécies. Nossos resultados indicam que a seca reduz a fixação simbiótica de nitrogênio em mudas de leguminosas neotropicais, mas o mecanismo de resposta pode ser específico da espécie. Portanto, prever a resposta da fixação simbiótica de nitrogênio na região neotropical sob um clima em mudança, com aumentos esperados na frequência e severidade das secas, depende de um melhor entendimento da diversidade de respostas entre as espécies leguminosas fixadoras de nitrogênio.
1 INTRODUCTION
Biological nitrogen (N) fixation is the dominant N input to many tropical forests and is important for nutrient cycling and other ecosystem properties (Cleveland et al., 1999, Vitousek et al., 2002; Vitousek et al., 2013a, Batterman, Hedin, et al., 2013). In the Neotropics, symbiotic N fixation (SNF) is commonly the dominant pathway of biological N fixation because of the high abundance of trees in the Fabaceae family (hereafter legumes); many of which have roots colonized by N-fixing bacteria (Gei et al., 2018; Ter Steege et al., 2006). The role of SNF in Neotropical nutrient cycling may influence how these forests respond to disturbance and/or global changes (Davidson et al., 2007, Houlton et al., 2008, Hedin et al., 2009, Menge & Levin, 2017, Nagy et al., 2017, Winbourne et al., 2018, Taylor et al., 2019). Soil nutrient demand may increase under elevated atmospheric CO2 (Fleischer et al., 2019; Thornton et al., 2007), altering nutrient cycling dynamics. Thus, understanding how the abiotic factors likely to be altered with global change (e.g., drought or nutrient cycling) influence SNF may provide insight to how Neotropical forests will function in the future.
The abiotic controls on SNF have long been debated (Barron et al., 2011; Batterman et al., 2013; Hedin et al., 2009; Houlton et al., 2008; Menge & Levin, 2017; Menge et al., 2008; Vitousek & Howarth, 1991; Vitousek et al., 2013b). Theory and empirical work suggest SNF in the Neotropics is primarily facultative (Barron et al., 2011; Menge et al., 2017) and thus upregulated or downregulated based on environmental conditions (Batterman, Wurzburger, et al., 2013; Taylor & Menge, 2018; Trierweiler et al., 2018). Facultative fixation allows N-fixing legumes to upregulate SNF when the carbon cost of fixation is low relative to other N acquisition strategies and downregulate when conditions are not ideal or N demand is not as high (Barron et al., 2011; Batterman, Wurzburger, et al., 2013; Hedin et al., 2009; Menge & Levin, 2017).
While the existence of facultative fixation has been demonstrated (Barron et al., 2011; Batterman, Wurzburger, et al., 2013; Hedin et al., 2009; Menge et al., 2015; Taylor & Menge, 2018), the mechanisms that trigger SNF downregulation are less well understood. Further, the diversity of Neotropical forests makes it difficult to predict how SNF at ecosystem scales may respond to changing environmental conditions, as there may be myriad legume species in a particular area, each of which may upregulate or downregulate SNF independently. Species-specific SNF responses to abiotic conditions might be expected, as there is evidence that N-fixing legumes in the Neotropics differ in the rate at which they fix N under similar abiotic conditions (Batterman, Hedin, et al., 2013, Wurzburger & Hedin, 2016).
There are several pathways by which abiotic factors might influence SNF. Low availability of soil nutrients and/or water needed for SNF might directly decrease allocation to SNF without affecting plant biomass, such as changes in the amount of N fixed by bacteria and/or N provided to the plant (Simms & Taylor, 2002). Further, high soil N availability might stimulate SNF downregulation (Batterman, Wurzburger, et al., 2013; Hedin et al., 2009). Alternatively, low soil nutrients or water availability might indirectly limit SNF by decreasing overall plant biomass, leaving SNF per unit plant biomass unchanged. These potential mechanisms are not mutually exclusive, and there is a continuum between these end members.
Regardless of the mechanism, SNF may be limited by different abiotic factors that vary in space, time, or in their relative importance to particular N-fixing legume species. For example, P can limit SNF in tropical ecosystems where soils are highly weathered (Augusto et al., 2013; Vitousek et al., 2010). The mechanism(s) by which low P limits SNF remain poorly resolved; however, low P conditions can directly affect nodule development and function, and indirectly limit SNF by limiting overall plant growth (Augusto et al., 2013; Batterman, Wurzburger, et al., 2013; Binkley et al., 2003; Nasto et al., 2017; O’Hara, 2001; Robson, 1983). Similarly, the availability of molybdenum (Mo), a co-factor in the nitrogenase enzyme, can limit free-living N fixation in lowland tropical rainforest (Barron et al., 2009, Wurzburger et al., 2012, Reed et al., 2013), and Mo can limit SNF in agricultural legumes, and wild legumes grown under elevated CO2 conditions (O’Hara, 2001; Trierweiler et al., 2018; Wurzburger & Hedin, 2016; Zahran, 1999). Water availability can also limit SNF through decreasing nodule function and plant growth (Binkley et al., 1994; Dovrat et al., 2018; Dovrat & Sheffer, 2019; Marino et al., 2007; Serraj, 2003; Streeter, 2003; Weisz et al., 1985; Zahran, 1999). However, SNF upregulation under drought (Wurzburger & Miniat, 2014) and high legume abundance in tropical dry forests (Gei et al., 2018; Pellegrini et al., 2016) has also been observed.
Despite these complexities, understanding SNF responses to environmental conditions is particularly important in the current era of rapid global change. SNF may influence how Neotropical forests respond to elevated atmospheric CO2 by mitigating N limitation that may develop (Norby et al., 2010; Terrer et al., 2018; Thornton et al., 2007). SNF could also play an important role in young secondary tropical forests, which now constitute the majority of tropical forests (Chazdon, 2008; FAO, 2015) and likely to have net primary productivity limited by N (Davidson et al., 2007; Davidson et al., 2018, LeBauer & Treseder, 2008, Yang et al., 2010, Wright et al., 2011, Batterman, Hedin, et al., 2013, Nagy et al., 2017, Taylor et al., 2019).
Further, droughts are predicted to increase in Neotropical forests as anthropogenic climate change accelerates (Dai, 2013). Drought can limit plant growth through lowered stomatal conductance and metabolic processes (Flexas & Medrano, 2002), and can limit SNF directly by decreasing nodule function (Serraj et al., 1999). Legumes preferentially support SNF on root sections with ample water relative to root sections of the same plant grown under dry conditions (Marino et al., 2007), suggesting SNF downregulation under drought (Dovrat et al., 2018; Dovrat & Sheffer, 2019; Marino et al., 2007; Serraj, 2003; Streeter, 2003; Weisz et al., 1985; Wurzburger & Miniat, 2014; Zahran, 1999) even if plant growth is less affected under drought conditions than non-legume species (Pellegrini et al., 2016; Thrall et al., 2005; Wurzburger & Miniat, 2014). Despite the importance of drought, and the diversity of legumes, to our knowledge there has never been a study that explores the interacting effects of water and soil nutrient availability on SNF for Neotropical N-fixing trees.
In this context, we grew seedlings from eight Neotropical N-fixing woody legume species in a shadehouse and manipulated water, P, and Mo availability in a full factorial combination. We hypothesized that low soil water, P, and/or Mo additions might limit SNF in these seedlings. We were particularly interested in species-specific effects on SNF (e.g., if SNF in one species is downregulated under drought, but SNF in another species responds only to low P). We were also interested in the mechanisms by which SNF might be affected by these treatments and assessed whether seedlings did not form nodules at all, had fewer nodules, or had altered nitrogenase activity in response to particular treatments.
2 METHODS
2.1 Shadehouse experiment
We selected eight species of Neotropical woody N-fixing legumes and obtained seedlings from two nurseries in Bahia, Brazil (Table S1), all of which (Swartzia macrostachya, Inga edulis, Inga marginata, Inga microcalyx, Abarema turbinata, Andira ormosioides*, Andira anthelmia*) formed nodules. It is worth noting that seedlings for six of the eight species came from the same nursery, while the two others (denoted with a “*” in the text) came from another nursery and potentially experienced different inoculants before transplanting into our shadehouse. Species were selected because seeds were readily available, and they are common species used for reforestation in the study area. While these eight species do not represent the full extent of legume diversity, they do represent four genera and two sub-families within the Fabaceae family. The relatively little trait information available for these species is presented in Table S1.
All seedlings were started from seed and grown in small cones (150 ml) containing the same brand of pine compost for approximately three months prior to the experiment. At the start of the experiment, the two species not grown at our main greenhouse were brought onsite to a common shadehouse with 50% light reducing shadecloth, and all species were transplanted into 1.5-liter plastic potting bags with a mix of silica sand and native soil (90:10) to expose seedlings to a suite of native symbionts. Five species had nodules present on the day of replanting (Table S1), and the nodules were left in place during replanting, as nodule excision would have caused excessive damage to root systems.
Six hundred and forty seedlings (80 per species) were grown in full factorial treatment groups that varied water, P, and Mo (n = 10 per treatment per species with eight treatments). Seedlings received nutrient treatments (standard Hoagland solutions diluted in water with manipulated levels of P and Mo) once a week for four months and received additional water twice a week (Table S2). Our nutrient treatments contained no N. Chemical analyses of the water used showed no detectable N or P. The drought treatment received half as much water (1,500 mm year-1) as the non-drought treatment (3,000 mm year-1, slightly more than mean rainfall in wet forests in the area; Table S2). We halved the mean precipitation from wet forests in the area for our drought treatment, which is slightly more rainfall than seen in severe El Niño years (~1,200 mm year-1). This rainfall amount has increased tree mortality rates in other parts of the Atlantic Forest (Rolim et al., 2005). The mean annual temperature and precipitation in Ilhéus, Bahia (the nearest meteorological station to the experiment), is 24.5°C and 2,000 mm; however, these meteorological parameters vary throughout the state of Bahia where these species are native. Over the course of the experiment each plant in the high P addition treatment received the equivalent of 14.5 g P m-2 year-1, whereas each plant in the low P addition treatment received the equivalent of 0.4 g P m-2 year-1. The starting 150 ml of pine compost contained approximately 1.5 g m -2 of N and P fertilizer and an undetermined Mo amount. Thus, the high and low P addition treatments received a total of 16 g P m-2 year-1 mg versus 1.9 g P m-2 year-1 of readily available P per plant over the course of the seedlings’ growth. Neem oil was sprayed one month into the experiment to control herbivory.
We harvested the seedlings four months after the transplant to the sand:soil mixture and the initiation of treatments. Harvesting took place over the course of 10 days, with one individual from each species from each treatment harvested daily. A random number generator was used to select the order seedlings were harvested each day to randomize when species or treatments were measured throughout the day. Upon harvest, nodules were collected for measurements of SNF, and the plants were separated into leaves, shoots, and roots and dried at 60ºC for at least two days before weighing.
2.2 Response variables
We report four response variables: total biomass (g), nodule biomass (g), nitrogenase activity (μmol C2H4 hr-1 g-1 of nodule), and acetylene reduced (AR) per seedling (μmol C2H4 hr-1 per seedling). High intraspecific variability in initial biomass precluded calculating accurate growth rates so we focus on final biomass as our primary growth metric (initial biomass provided in Table S1).
We chose three SNF variables (nodule biomass (g), nitrogenase activity (μmol C2H4 hr-1 g-1 of nodule), acetylene reduced (AR) per seedling (μmol C2H4 hr-1 per seedling)) to assess the response of SNF to our experimental treatments. Nodule biomass is an integrated measure of SNF potential, whereas nitrogenase activity and AR per seedling are both proxies for SNF rate at the time of harvested. We report all three of these variables, as these variables are all plausible aspects of SNF that may change in response to our treatments. We explore the pros and cons of each measurement in the Discussion.
2.3 Estimating symbiotic N fixation
At the time of harvest, the root system of each seedling was gently washed and 10 nodules on new root material (e.g., nodules that were growing in the sand:soil mix rather than the starting pine compost) were randomly selected. Selecting nodules in the sand:soil mixture ensured that the nodules measured were not present at transplant and were no older than four months. Excised nodules were immediately placed in a 60-mL gas tight jar fitted with septa. These nodules were exposed to an atmosphere of 10% acetylene (made from calcium carbide each day) and incubated for one hr. Additional nodules, if present, were separated, counted, dried, and weighed to estimate nodule biomass and AR per seedling. In addition to the nodules incubated with acetylene, controls for each species in each treatment were performed on randomly selected seedlings to detect background ethylene levels by placing 10 nodules from each plant in a jar with no added acetylene and incubating for one hr. When there were fewer than 20 nodules present, the nodules were evenly divided into control or acetylene treatments. Five blanks were also performed each day, in which acetylene was added to jars with no nodules and incubated for an hr to determine background ethylene levels.
2.4 Statistical analysis
To determine the interactive effect of the water and soil nutrient treatments on seedling biomass, we used a three-way ANOVA. Tukey's honest significant differences were used as post hoc tests when appropriate. All data were log transformed to meet normality and homoscedasticity assumptions.
We used maximum likelihood estimates (MLE) to determine whether the treatments affected SNF, and MLE analyses were done with all the species pooled together and for each species separately. These analyses included two-part models evaluated using MLE framework for SNF-related response variables (Lai et al., 2018, Taylor & Menge, 2018, Taylor et al., 2019). We chose this approach because the SNF data were zero-inflated with non-zero values log-normally distributed. This method is conceptually similar to an ANOVA framework, determining which treatments have similar or different means while adhering to the log-normal, zero-inflated, structure of these data.
We expected treatments to have several possible effects on SNF. With two-part models, we simultaneously parameterized the probability a treatment produces zeros (nodules absent, or no acetylene reduced) and parameterize the mean and standard deviation of the non-zero values based on the model constraints (Table S4). We selected the model that best parameterizes the data by comparing the AICc scores (Anderson, 2008) of each model and selecting the model with lowest AICc, with the best model considerably better than another if the ΔAICc > 2. We report the ΔAICc values of best model compared with the null model (M1 an intercept-only model that fits the same mean and probability to all the treatments; Table S4) within the results. The ΔAICc of all the models are in Table S5.
To test the effects of drought, P and Mo on SNF-related response variables, we used a series of 16 maximum likelihood models (Table S4). In addition to testing individual treatment effects, we test for a drought-P interaction effect, and a three-way interaction effect between drought, P, and Mo. We do not report other potential interactions (drought-Mo or P-Mo interactions), as preliminary results indicated no significant effects on the response variables. While we discuss results of all the species together (e.g., SNF decreased with drought in seven of eight species), we do not to directly compare SNF response variables between species (e.g., species A had a larger reduction in SNF under drought than species B). There were several reasons for this decision. First, we did not perform 15N2 calibrations with our ARA measurements. While it is common to assume that the ratio of N fixed to AR is constant, there is considerable evidence that the ratio can vary widely among Neotropical species (Batterman et al., 2018). Further, there are potential differences in the bacteria populations that seedlings from different nurseries were exposed to, which could confound our interpretation of SNF rates between species. Five of the eight species had nodules at the time of transfer from the pine compost substrate into the sand:soil mixture. The number of nodules in the 150 ml starting soil was far lower than the number on the roots that grew during the four-month experiment. Therefore, we compare how seedlings of the same species respond to the experimental treatments, as all individuals from a given species were exposed to the same conditions and do not compare between species. All statistical analyses were performed in R 3.4.3. statistical software using the bbmle package (version 1.0.23.1) for maximum likelihood tests.
3 RESULTS
3.1 Drought effect
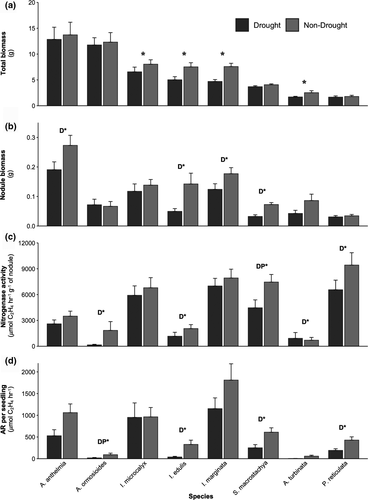
Plant biomass negatively responded to drought (F1,582 = 1.943, p = 0.0003) for all species pooled together (Figure 1a). Drought significantly decreased plant biomass in four species, as a main effect (I. marginata, F1,71 = 9.956, p = 0.002; and A. turbinata F1,71 = 4.189, p = 0.044), a two-way interaction effect (drought*Mo; I.edulis, F1,63 = 4.852, p = 0.03), or a three-way interaction effect (drought*P*Mo; I. microcalyx, F1,69 = 7.195, p = 0.009). When interactions were significant, total biomass was on average lower in the drought treatment with varying responses to P and/or Mo within a drought or non-drought treatment. For example, Mo additions in I. edulis increased total biomass within a water treatment, but the opposite pattern was observed in I. microcalyx. While these four species were sufficient to drive an 18% experiment-wide reduction of biomass from 7.21 ± 0.48 g to 5.91 ± 0.44 g under drought, there was not a consistent effect of drought on plant biomass for the four-remaining species (Figure 2a). While it might be expected that the largest species were the most water stressed, this was not the case. The two species with the highest final biomass did not have reduced biomass under drought (Figure 2a).
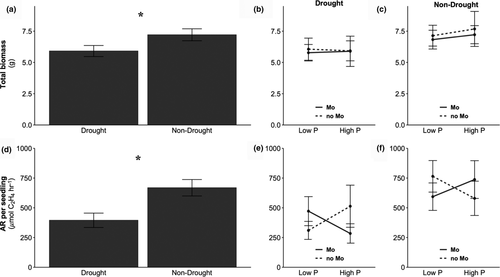
Nodule biomass (g), nitrogenase activity (μmol C2H4 hr-1 g-1 of nodule), and AR per seedling (μmol C2H4 hr-1 per seedling) were more substantially reduced by drought conditions (ΔAICc = 200.79, ΔAICc = 11.54, ΔAICc = 20.86, respectively) with all species pooled together. Nodule biomass was reduced by 33% from 0.124 ± 0.009 g to 0.082 ± 0.006 g, nitrogenase activity by 27% from 4,980 ± 371 to 3,613 ± 311 μmol C2H4 hr-1 g-1 of nodule, and AR per seedling (μmol C2H4 hr-1 per seedling) by 41% from 669 ± 69.1 to 395 ± 60.4 μmol C2H4 hr-1 per seedling in drought seedlings compared with non-drought seedlings (Figure 1b). Nodule biomass per gram of total biomass was 15% less under the drought treatment.
We saw a relatively consistent species-level response of SNF response variables to drought. In seven of the species in this experiment, at least one SNF variable showed a drought response, and in nearly all cases drought reduced SNF (Table 1). All but one case (the nitrogenase activity of A. turbinata) exhibited a negative drought response. The SNF response variable affected by drought differed among species; however, this was not reflective of the nursery of origin or the nodulation status at the time of transplantation (Table 1).
| Species | Total biomass (g) | Nodule biomass (g) | Nitrogenase activity (μmol C2H4 hr-1 g-1 of nodule) | AR per seedling (μmol C2H4 hr-1 seedling-1) | Nodule status |
|---|---|---|---|---|---|
| Abarema turbinata | drought↓ (p = 0.0003) |
Mo↓ (ρ) |
drought↑ (ρ) |
Mo↑ (μ) |
A |
| Ingaedulis | drought↓*Mo↓ (p = 0.031) | drought↓ (μρ) |
drought↓ (ρ) |
drought↓ (μρ) |
P |
| Inga marginata | drought↓ (p = 0.002) | drought↓ (μρ) |
P↑ (ρ) |
P ↓ (ρ) |
P |
| Inga microcalyx |
drought↓*P↓*Mo↑ (p = 0.009) |
Mo ↑ (μρ) |
Null | Null | P |
| Plathymenia reticulata | n.s. |
Null |
drought↓ (μ) |
drought↓ (μ) |
A |
| Swartzia macrostachya | n.s. | drought↓ (μρ) |
drought↓*P↑ (ρ) |
drought↓ (μρ) |
A |
| Andira anthelmia | n.s. |
drought↓ (μ) |
Null | Null | P |
|
Andira ormosioides |
n.s. | Null |
drought↓ (μ) |
drought↓*P↑ (μ) |
P |
Note
- Nodule status indicates whether nodules were absent (A) or present (P) when the seedlings were replanted at the start of the experiment. Significant treatment effects and P-values for total biomass are provided, with n.s. indicating no significant treatment effect. For complete three-way ANOVA table see Table S3. For all other presented variables (nodule biomass, nitrogenase activity, and AR per seedling), the treatment (drought, P, Mo or drought*P interaction) that best explained the variation in that variable is indicated (Table S5). μ indicates the model fitting different means with the probability of having zero nodules held constant, ρ indicates the models fitting different probabilities of zero nodules with means held constant, and μρ indicates models that fit different means and zero-probabilities to the drought treatments. Gray background in rows indicates species that were started in the same nursery in which the experiment was run. Arrows indicate the direction of individual treatment effects, with “↓” indicating lower values in the drought, low P, or no Mo treatment compared with their non-drought, high P, or Mo treatment counterpart (respectively), and “↑” indicating comparatively higher values associated with drought, low P or no Mo treatments.
In four species (S. macrostachya, ΔAICc = 14.73; A. anthelmia* (with * indicating that the species came from a different nursery), ΔAICc = 3.65; I. edulis, ΔAICc = 6.55; and I. marginata, ΔAICc = 4.92), the drought treatment best explained the nodule biomass (g) variability (Figure 2b, Table S5), with 56%, 30%, 65%, and 30%, respectively, reduction in nodule biomass under drought. Drought also influenced the probability of nodules forming in three of these species (S. macrostachya, I. edulis, and I. marginata; Table 1).
Nitrogenase activity (μmol C2H4 hr-1 g-1 of nodule) in four of the species also decreased with drought (Figure 2c, Table S5), but one species (A. turbinata, ΔAICc = 0.18) had 31% increase in nodule efficiency under drought. The four species (S. macrostachya, ΔAICc = 11.06; I. edulis, ΔAICc = 2.44; P. reticulata, ΔAICc = 0.82; and A. ormosioides*, ΔAICc = 2.40) with decreased nitrogenase activity under drought showed 40%, 43%, 30%, and 91% (respectively) reduction compared with non-drought treatment seedlings. The negative drought effect in three of these species (S. macrostachya, I. edulis, and A. turbinata) was driven by a decreased probability in nitrogenase activity, while in the other two species (P. reticulata and A. ormosioides*, Table 1) the negative drought effect was driven by a decrease in the mean nitrogenase activity.
Acetylene reduced (AR) per seedling (μmol C2H4 hr-1 per seedling) decreased by 59%, 88%, 80%, and 55% for four (S. macrostachya, ΔAICc = 13.40; I. edulis, ΔAICc = 9.09; A. ormosioides*, ΔAICc = 4.50; and P. reticulata, ΔAICc = 5.16; respectively) of the species under drought conditions (Figure 2d, Table S5). The drought treatment decreased both the mean and probability of AR per seedling for two of these species (S. macrostachya, and I. edulis). Drought only decreased the mean AR per seedling for the other species (A. ormosioides* and P. reticulata).
3.2 Nutrient effects
P and Mo treatments did not have a significant experiment-wide effect on the response variables (Figure 1a and b) when all species were pooled. However, individual species analysis showed nuanced effects of soil nutrient treatments that were masked in the pooled species analyses (Table 1, Table S5). For example, nodule biomass (g) variability in I. microcalyx was best explained by Mo treatments (ΔAICc = 4.21), with a 62% increase under the no Mo addition treatment. P treatments best explained the variability in nitrogenase activity and AR per seedling in one species (I. marginata; ΔAICc = 2.32, ΔAICc = 2.32, respectively). Under low P treatments, there was an 8% increase in nitrogenase activity and 14% decrease in AR per seedling. Within a species, variation in different SNF response variables was sometimes best explained by different treatments. For example, the nodule biomass of I. marginata decreased under drought, where the low P treatment influenced nitrogenase activity (positively) and AR per seedling (negatively). The variability in nodule biomass and AR per seedling of A. turbinata was best explained by the Mo treatment (ΔAICc = 6.85, ΔAICc = 0.36, respectively), with 33% decrease and 48% increase, respectively, under the no Mo addition treatment.
4 DISCUSSION
Drought reduced at least one proxy for SNF in seven of the study species. Lower SNF under drought could be a result of plant growth limited by water availability. This, in turn, might lead to lower nodule biomass and/or SNF rates because the plants were smaller. Alternatively, drought could limit SNF through a disproportionate decrease in carbon allocation to SNF or directly limit N-fixing bacteria activity. In this case, plant growth would be the same, but fixation would be less in the drought treatment. Our data are more suggestive of the latter, as we see a larger reduction in the three SNF variables (27%–41%) than the reduction in total biomass (18%) in the drought treatment. We found nodule biomass g-1 plant biomass was 15% lower under the drought treatment, suggesting drought may have limited SNF by reducing the proportional biomass investment in SNF.
Although eight species constitute a tiny fraction of known legume diversity, a more uniform response to a particular treatment (e.g., drought in this experiment) might suggest a generalizable result. Species-specific responses to a given treatment (e.g., soil nutrients in this experiment) would suggest caution in extrapolating the results of the necessarily low-diversity experiments and observational studies of SNF, especially in the Neotropics where legume diversity is high. We believe the results from this study suggest a strong SNF decrease in response to drought across the majority of the eight species we examined, at least at the seedling stage. Drought influenced more of the measured response variables, on average, in the species that had larger species ranges (Table 1, Table S1), indicating this decrease in SNF may be observed throughout the Neotropics. This suggests potential implications for Neotropical forest nutrient cycling with increased drought severity and frequency, whereas the response to soil nutrient limitation may be more nuanced and species-specific.
Studies comparing legumes with non-legumes have shown high growth rates, SNF, or abundance for N-fixing legume trees under drought or dry conditions (Adams et al., 2016; Gei et al., 2018; Wurzburger & Miniat, 2014). Thus, the decrease in SNF under drought may be because the species from this study are typically found in wetter conditions and/or that their seedlings are particularly drought sensitive. However, our reduction in water conditions was within the range of what these species may experience in a dry year. We recognize that drought responses may differ in adult legumes, as has been demonstrated in legume shrubs (Dovrat et al., 2018). Nevertheless, drought may reduce the ability of N-fixing legumes to maintain high SNF levels during drier conditions, and the mechanism by which SNF reduction occurs (nodule biomass versus nitrogenase activity versus AR per seedling) may be species-specific.
Interestingly, there was a reduction in SNF, but not total biomass, under drought conditions in several species. There are several possible explanations for this result. Legumes are known for their generally high N demand (Mckey, 1994); however, seedlings can be plastic in their C:N ratio by increasing nutrient use efficiency under low N conditions. Seedlings with drought limited SNF could maintain equal plant biomass with their non-drought treatment counterparts by increasing their C:N ratio. Alternatively, it is possible that some seedlings were not water limited by the “drought” treatment and/or these seedlings were not limited by N (see below for further discussion) and luxury SNF only occurred under non-drought treatments.
Based on the relative pros and cons of each SNF variable, we compiled a set of SNF variables to examine multiple levels at which SNF regulation can occur and be influenced by the treatments. Nodule biomass may not always scale directly with SNF, as not all nodules may be actively fixing N or fixing N with the same efficiency. Both nitrogenase activity and AR per seedling were quantified using an ARA as a proxy for how much SNF is occurring at the time of measurement (Hardy et al., 1973; Vessey, 1994). Scaling acetylene reduction rates to SNF rates is not always straightforward for a number of reasons including nodule excision effects (Minchin et al., 1986; Witty & Minchin, 1988; Witty et al., 1984). Further, the ratio between acetylene reduction and N fixation varies between plant species (Batterman et al., 2018). We did not calibrate with 15N2 gas incubations to determine the acetylene to N reduction ratio for these study species. Therefore, we conservatively estimate differences in treatment effects on ARA-derived variables by only reporting AR as a proxy for nitrogenase activity and AR per seedling and make no attempt to scale to N fixed or compare between a species.
High soil N can downregulate SNF in Neotropical legume tree seedlings (Batterman, Wurzburger, et al., 2013; Taylor & Menge, 2018; Trierweiler et al., 2018; Wurzburger & Hedin, 2016). Though our experimental treatments did not include any N fertilization, seedlings were exposed to approximately 4 mg of N from the starting mixture. If we conservatively assume the foliar biomass of these seedlings is 1%N, then the median N amount in the foliar samples is approximately 9 mg. Despite the presence of some added N in the initial soil, we do not think the initial soil provided the seedlings enough N to stifle SNF. However, seed reserves can provide a substantial amount of N and this may be particularly important in large seeded species (Table S1). In the absence of a N fertilization treatment and/or species-specific seed N content, we cannot determine whether the seedlings experienced N poverty in this experiment.
Phosphorus availability can also affect SNF in Neotropical legume seedlings (Batterman, Wurzburger, et al., 2013; Nasto et al., 2017; Trierweiler et al., 2018). However, a study found P did not influence SNF but seasonal drought did in Mediterranean N-fixing legume species (Dovrat et al., 2018). We found no consistent P effect on SNF, and SNF responses to P availability were more nuanced species-specific responses. In I. marginata, the P treatment best explained the variation in two SNF variables (nitrogenase activity and AR per seedling), and a P-drought interaction best explained the variability in SNF variables for two other species (A. ormosidoides and S. macrostachya). In both of these species, the difference between the response variables in the P treatments was greater within the drought treatment compared to within the non-drought treatment. These seedlings were started in a potting mixture before being transplanted into the low nutrient sand:soil mixture. This potting mixture only made up approximately 10% of the pot size during the experiment. Nevertheless, it is possible the starting soil contained sufficient P and Mo to alleviate limitation to SNF by these elements for some species. One study found no P addition limited SNF for one Neotropical species growing in native soil (Batterman, Wurzburger, et al., 2013), but saw no P limitation with application rates of 20 g P m-2 year-1. Comparatively, our treatment levels were 1.9 g P m-2 year-1 and 16 g P m-2 year-1 (when including approximately 4 mg of P from potting mixture) and thus may not have been low enough to induce P limitation to SNF for all of our study species. However, a lack of strong P effect on SNF across all species may be a result of a stronger influence of drought and/or species-specific differences in P demand both for growth and SNF. Pervasive P limitation in one Neotropical forest was correlated with differences in tree species community composition and not with differences in biomass, suggesting some species are better adapted for low soil P conditions (Turner et al., 2018). Therefore, it is possible across our eight-study species some are better adapted to low P conditions than others and may not exhibit as strong of a P limitation effect.
The no Mo treatment may not have had experiment-wide effects on biomass or SNF, perhaps because so little Mo is needed to ensure adequate supply (Ishizuka, 1982; Kaiser et al., 2005). Unlike free-living N fixation, which can be limited by Mo (Barron et al., 2009), the plant partner of SNF may be able to obtain or provide the Mo needed for N fixation more easily than free-living N-fixing bacteria. However, Mo can limit SNF under high concentrations of atmospheric CO2 (Trierweiler et al., 2018), and therefore, Mo may become increasingly limiting to Neotropical SNF in the future. Although there was no experiment-wide effect of Mo, there were some specific-specific exceptions in three species (A. turbinata, P. reticulata, and A. ormosioides*). However, we are cautious when interpreting these results, as the next best models in some cases varied between drought, P, and null models. A Mo model may have best explained the variation of these variables in these particular species but perhaps did not explain the variation well. There were other instances where the best model had several other models with ΔAICc values < 2 and this was not unique to when a Mo treatment model was the best model (Table S5). We present the models with the lowest ΔAICc values as the models that best explain the data variation, but models within ΔAICc values < 2 should also be considered as plausible (Table S5). While the nutrient effects in this study were small relative to those of drought, five the species had at least one SNF variable best explained by P, drought-P, or Mo model.
Finally, this study demonstrates the ways in which legumes may respond to abiotic drivers. There are two main potential points of regulation of SNF. First, environmental conditions may affect whether and how many nodules form. Second, once nodules do form, environmental conditions may affect how much fixation occurs. Many legumes are capable of regulating their bacterial symbionts by manipulating oxygen and/or carbon to nodules (Kiers & Denison, 2008; Kiers et al., 2003; Simms et al., 2006; West et al., 2002). Legumes appear better at suppressing poor performing N-fixing bacteria than at choosing good performing N-fixing bacteria at the onset (Simms et al., 2006). Our results suggest that environmental conditions can affect different components of SNF. A logical follow-up to these results would be genetic analyses of the bacterial populations in different species and treatments, as we allowed for these seedlings to be exposed to a suite of native symbionts. Therefore, some of the SNF variation within a plant species may be attributable to different bacterial strains.
5 CONCLUSIONS
SNF in Neotropical legume seedlings decreased under drought, but there was variability in SNF responses to abiotic drivers across eight N-fixing species. This highlights the difficultly of predicting a single SNF response to environmental change. Species differences in foliar chemistry (Osborne et al., 2017; Townsend et al., 2007), response to N fertilization (Alvarez-Clare et al., 2013; Wright et al., 2018), and response to soil P availability (Turner et al., 2018) necessitate that researchers account for the enormous tree species diversity as we continue to refine our understanding of tropical biogeochemistry. These results add to the list of important biogeochemical properties and processes that may depend on which species are present to experience particular abiotic drivers. We do observe a largely consistent response to drought, which may be of particular importance in the Neotropics where drought is predicted to increase in severity and frequency (Dai, 2013). Nevertheless, the different ways species responded to treatments suggest a more nuanced approach is needed for integrating N-fixing legumes and SNF into global change models.
ACKNOWLEDGEMENTS
We thank Instituto de Floresta Viva and their greenhouse staff in Bahia, Brazil, for use of their facilities and assisting in the experiment. We also thank Viveiro Symbiosis for their help acquiring seedlings from their nursery. We are grateful to K. Fenn and S. Balint for their assistance in the field and laboratory. We appreciate feedback and guidance on our statistical analyses by B. Taylor. We thank the Kellner and Porder Lab Groups for helpful comments in early versions of this manuscript. The authors are grateful for funding from the Institute at Brown for Environment and Society and an anonymous donor to Brown University to support research into "Biome Restoration in Brazil's Atlantic Forest.”
AUTHOR CONTRIBUTIONS
L.A.M. and S.P. conceived of and designed the study. L.A.M. and D.P. performed the research. L.A.M. analyzed the data. L.A.M. and S.P. wrote the manuscript, and all authors contributed to revisions.
Open Research
DATA AVAILABILITY STATEMENT
Data available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.wh70rxwm7 (McCulloch et al., 2020).




