Seed dispersal effectiveness by oilbirds (Steatornis caripensis) in the Southern Andes of Colombia
Associate Editor: Jennifer Powers
Handling Editor: Erin Kuprewicz
Abstract
enOilbirds are specialized frugivores that disperse seeds; however, no study has evaluated their seed dispersal effectiveness (SDE). We estimated SDE for nine plant species and tested if high-altitude plants are more effectively dispersed than lowland species and if there are high SDE scores in non-breeding periods of oilbirds. The quantity factor was estimated from seed rain data in the main cave of Cueva de Los Guacharos National Park (Colombia). The quality component considered germination chance, the probability that seeds reach suitable habitats (using data from GPS devices on three birds) and the probability of establishment in different habitats (literature review). Our model showed that the habitat with more seedrain was the cave (41%), followed by dense forest and mosaics of crops and forests (both ca. 20%). Seed germination was evidenced in 7 of the species, and the other two indicated viability (using tetrazolium tests). The most dispersed species corresponded to palms (Prestoea acuminata and Geonoma undata), but, in terms of biomass, P. acuminata and Dacryodes olivifera (Burseraceae) were the top species. D. olivifera, G. undata, and P. acuminata showed the highest SDE values. No relationship was found between the probability of arrival to suitable habitats and the altitudinal level of the species, but the oilbirds were more effective dispersing seeds in non-breeding periods. Despite a large amount of seeds dropped at the caves, oilbirds disperse seed to suitable habitats, move seeds long distances, and disperse large seeds. As some of these ecosystem services are unique, oilbird's conservation seems imperative.
Resumen
esLos guácharos son considerados como frugívoros especializados, con potencial de dispersar semillas; pero ningún estudio ha evaluado su efectividad como dispersores (EDS). El objetivo de este trabajo fue evaluar su EDS para nueve especies de plantas, y probar si las especies de montaña son mejor dispersadas que las de tierras bajas y si los guacharos son más eficientes en épocas de no anidación. Se cuantificó la cantidad de la dispersión a partir de datos de lluvia de semillas en la cueva principal del PNN Cueva de Los Guacharos (Huila, Colombia); y la calidad de la dispersión teniendo en cuenta la tasa de germinación de las semillas dispersadas, la probabilidad de que las semillas lleguen a hábitats adecuados (con dispositivos GPS) y la probabilidad de establecimiento en diferentes tipos de cobertura (de literatura). Según nuestro modelo, estimamos que cayeron más semillas en la cueva (41%), seguido de bosques densos y mosaicos de cultivos y bosques (ca. 20%). La sombra de semillas fue mejor explicada por los patrones de uso de hábitat, que por la disponibilidad de coberturas. La germinación de semillas se evidenció en 7 de las especies y las otras 2 indicaron viabilidad (con pruebas de tetrazolio). En términos de número de semillas, las especies más dispersadas correspondieron a palmas (Prestoea acuminata y Geonoma undata), pero al utilizar biomasa P. acuminata y Dacryodes olivifera (Burseraceae) fueron las más importantes. D. olivifera, G. undata y P. acuminata mostraron los mayores valores de EDS. No se encontró una relación entre la probabilidad de llegada a hábitats adecuados y el nivel altitudinal de las especies, pero los guácharos fueron más eficientes dispersando semillas en épocas de no-anidación. A pesar de que depositan muchas semillas en las cuevas, estas aves llevan semillas a hábitats adecuados, mueven semillas a muy largas distancias (incluyendo ambientes pristinos e intervenidos) e ingieren semillas grandes. Dado que algunos de estos servicios son únicos, la conservación de guácharos parece clave para mantener procesos ecosistémicos.
1 INTRODUCTION
Dispersal refers to the movement of individuals from the place of origin to other sites where they may establish and reproduce. In plants, this process mainly occurs through seed dispersal, and three hypotheses have been advanced to explain its relevance: escape, colonization, and directed dispersal (Howe & Smallwood, 1982). The escape hypothesis states that below and near parental trees there is a higher chance of mortality due to attack by pathogens and predators, as well as intra-specific competition (Connell, 1971; Gillies & St. Clair, 2010; Janzen, 1970). The colonization hypothesis assumes that each species has particular micro-habitats for establishment, and dispersal increases the chance of arriving in those sites; directed dispersal implies that frugivores disproportionately disperse seeds to suitable sites for establishment (Howe & Smallwood, 1982). Since plants are sessile organisms, seed dispersal is a key process performed by animals (zoochory), or by other means (winds: anemochory, water: hydrochory, gravity, or mechanical dehiscence) (Nathan et al., 2008; van der Pijl, 1972; Ridley, 1930). When animals swallow seeds (endozoochory), the mutualistic process usually involves frugivores getting nutritional rewards and seeds being dispersed (Ganzhorn et al., 2009). Since most Neotropical tree species depend on animals for seed dispersal (Howe & Smallwood, 1982), the study of mutualistic relationships is relevant for the diversity and dynamics of natural and disturbed tropical systems (García et al., 2010; Gilbert, 1980; McConkey et al., 2012; Sekercioglu, 2017).
Schupp (1993) showed that plant fitness may be affected by seed dispersers and created the framework of seed dispersal effectiveness (SDE). The concept includes two components, the quantity and the quality of seed dispersal, and the multiplication of these factors may lead to the estimation of how a plant may be effectively dispersed by a frugivore (Schupp et al., 2010). Quantity of seed dispersal is simply the number of seeds that are dispersed. Quality of seed dispersal has two sub-components, seed treatment (i.e., survival probability in the digestive tract) and deposition quality (including the chance of getting to suitable habitats, and the effects of seed mixing and feces). Proper seed treatment in the frugivore's gut may be indicated by high germination rates (Schupp, 1993), which depends on seed viability, dormancy, gut temperature, mechanical, and chemical abrasion of the seed coat (Baskin & Baskin, 2004; Stevenson et al., 2002; Traveset et al., 2008; Traveset & Verdú, 2002). Furthermore, the most challenging component to measure SDE is the quality of seed dispersal, because it is usually difficult to know how and where seeds are dropped and to evaluate plant survival in every potential site (Schupp, 1993). For example, seed and seedling mortality in natural conditions tend to be high (for seeds usually >90%, e.g., Notman & Gorchov, 2001), generating low numbers surviving. Thus, it is difficult to generalize plant establishment patterns for all potential regeneration sites, with low sample sizes.
The seed rain refers to the spatial patterns of seed droppings (Nathan & Muller-Landau, 2000) and may be studied from local scales (e.g., parental trees) to large geographic scales (Nathan et al., 2008). Two approaches have been used to estimate seed shadows: phenomenological (fitting mathematical functions to observed data) and mechanistic (including plant traits and frugivore behavior). The mechanistic models have been the most commonly used for animal dispersed seeds (Nathan & Muller-Landau, 2000). These models usually include as independent factors seed retention time in the gut and patterns of animal movement (e.g., Stevenson et al., 2014), allowing researchers to infer seed dispersal distances and potential habitat types where the seeds may be dropped.
Although oilbirds (Steatornis caripensis, Humboldt 1817) are medium-sized birds, reaching 400–450 gr (Holland et al., 2009), they may swallow seeds 3-cm wide; such seeds are generally dispersed by the largest living frugivores in the Neotropics (Peres & Van Roosmalen, 2002). The oilbird is the only strictly nocturnal frugivorous bird in the continent which disperses and deposits intact seeds (Amico & Aizen, 2005; McAtee, 1922). Their diet includes dozens of plant species, and it has been associated with lipid-rich fruits (Bosque & de Parra, 1992; Rojas-Lizarazo, 2016; Stevenson et al., 2017; Tannenbaum & Wrege, 1978), mainly from the Arecaceae and Lauraceae plant families. These birds usually inhabit caves (Tello et al., 2008; Thomas, 1999), where they drop many seeds in unsuitable conditions for establishment. However, individuals do not always spend the day in caves and they may travel between 44 and 300 km away (Cárdenas et al., ; Holland et al., 2009; Karubian et al., 2012), suggesting that seeds may get dispersed to suitable places for establishment (Karubian et al., 2012). However, no studies have been done on the effect of oilbirds on plants using the SDE framework.
The main aim of this study was to assess seed dispersal effectiveness for selected plant species dispersed by oilbirds in Cueva de Los Guácharos National Park. Specifically, we wanted to (a) estimate the number of seeds dispersed to different habitats and how this may be related to the patterns of habitat use, (b) quantify seed germination rates for seeds dispersed by oilbirds, and (c) estimate seed dispersal effectiveness for 9 of the most consumed plant species. Given that individuals of this colony center their activities in a cave located ca. 2000 masl, we proposed the hypothesis that seed dispersal effectiveness will be higher for high-altitude plants, assuming extensive use of high forests. We tested this idea by analyzing for a negative relationship between the chance of establishment and the mid-range elevation of the plant species. In addition, we tested the hypothesis that SDE will depend on breeding periods. Since oilbirds spend more time away from the cave in non-breeding periods (Cárdenas, et al., 2020; Holland et al., 2009), we predicted that SDE will be higher in these periods than in breeding periods, when the cave is frequently used.
2 METHODS
2.1 Study site
We centered our study in Cueva de Los Guácharos National Park (1°36.14'N; 76°8.13'W), located in Huila and Caquetá departments, Colombia. The park has Andean (2,400–2,700 m asl) and Sub-Andean forests (1,100–2,400 m asl), including both primary and old secondary stands (Prada & Stevenson, 2016). The park has at least three caves commonly used by oil birds, and we focused on the main cave (Cardona, 2016; Stevenson et al., 2017). There are at least 74 families of woody plants in the park (Prada & Stevenson, 2016), and oilbirds have access to plants in an area of at least 4,517 km2 surrounding the cave (Cárdenas, et al., 2020).
2.2 Seed dispersal effectiveness
We included factors related to seed dispersal quantity and quality (Schupp et al., 2010). Dispersal quantity was based on the number of seeds found in six fruit traps that were placed near the walls of the main cave in Cueva de Los Gúacharos NP and were monitored for five days each month between January and December 2015 (Cardona, 2016; Stevenson et al., 2017). Seeds were identified at the lowest taxonomic level possible, according to its morphology and a reference collection from the Park. The seed quantity estimates were generated for each species from the number of seeds dispersed by oilbirds in the main cave (# .m-2.yr-1). Biomass was determined from the total number of seeds multiplied by the mean dry weight, which was calculated from 5 seeds per species. This approach did not include fleshy parts and was used because recruitment generally is higher for larger seeds (Harms et al., 2000; Stevenson & Guzmán-Caro, 2013); however, we did not include here an approach to estimate SDE from biomass (which provided similar results, Cárdenas, et al., 2020). SDE approaches are intended to evaluate the fitness of individual trees; however, in our approach we do not know how many trees the seeds were dispersed from. For this reason, we corrected the estimates by the relative density of plants in the region (from 71 vegetation plots and 5,227 trees; Cárdenas, et al., 2020). For instance, for a species with 10 individuals per ha, the seed dispersal estimate from the cave was divided by 10 and for a species with 2 trees per ha, divided by 2 and so on.
The quality of dispersal was assessed using seed germination rates and the probability that dispersed seeds arrive in suitable habitats. Germination tests for nine species (Table 1) were achieved in soil (or petri dishes in cases of small seeds less than 4 mm long), where seeds recently collected in the traps were placed after washing in glasshouses (both in the field and in the city). Four to ten replicates (each one with ten seeds) were used for each species, and seed germination was observed for 6–10 mo. For Oenocarpus bataua and Trattinnickia lawrancei, no germination occurred during the study period (perhaps due to low temperatures for these lowland species), and tetrazolium test (solution of 0.5%) was used to estimate viability, as a proxy of germination chance (Delouche et al., 1962). The seeds were immersed in the solution for 24 hr and then crushed to assess embryo coloration.
| Species | Mean elevation (m asl) | Lower limit (msnm) | Upper limit (msnm) | Density (ind/ha) | Seed quantity (#/m2 year) | Seed weight (g) | Total biomass (g/m2 year) |
|---|---|---|---|---|---|---|---|
| Hedyosmum cuatrecazanum a | 2,264 | 1,145 | 3,382 | 3.8 | 422 ± 34 | 0.01 | 0.4 ± 0.0 |
| Geonoma undata b | 2,175 | 1,200 | 3,150 | 0.2 | 1,785 ± 201 | 0.09 | 17 ± 2 |
| Licaria applanata c | 2,150 | 1,600 | 2,700 | 3.1 | 127 ± 47 | 1.44 | 19 ± 7 |
| Prestoea acuminata b | 2,075 | 1,500 | 2,650 | 3.5 | 3,359 ± 238 | 0.31 | 110 ± 8 |
| Nectandra purpurea c | 1,950 | 1,300 | 2,600 | 4.1 | 264 ± 36 | 2.48 | 69 ± 9 |
| Ocotea rugosa c | 2,050 | 1,600 | 2,500 | 0.3 | 401 ± 195 | 0.37 | 15.6 ± 8 |
| Dacryodes olivifera d | 1,095 | 490 | 1,700 | 0.1 | 186 ± 56 | 5.15 | 101 ± 30 |
| Trattinnickia lawrancei d | 673 | 140 | 1,205 | 0.1 | 63 ± 13 | 0.36 | 2.4 ± 1 |
| Oenocarpus bataua b | 475 | 50 | 900 | 0.1 | 264 ± 35 | 3.91 | 109 ± 14 |
- a Chloranthaceae.
- b Arecaceae.
- c Lauraceae.
- d Burseraceae.
To estimate how many seeds reach suitable habitats, we used a simple mechanistic model using seed retention times in oilbirds (from Bosque & Parra, 1992 and by fitting a polynomial function; Figure S1) and movement patterns (from GPS devises attached to three individuals). GPS telemetry devices (e-obs GPS-Tags WGS84-Height, Global Positioning System of e-obs digital telemetry ©) were attached to 5 individuals captured using mist nets in the in the main cave's entrance of Cueva de Los Guacharos NP. Data were recovered for 3 out of 5 individuals: one individual (tag G29) during the non-breeding season on December 2015, and the other two individuals (tags G32 and G33) during breeding season on March 2016 (Cárdenas, et al., 2020; Cardona, 2016). Two individuals captured during the non-breeding season did not provide information, probably because they did not visit the cave when we downloaded data at close proximity. The devices corresponded to approximately 6% of an individual's body weight and were located on birds of unknown sex greater than 200 g, close to the individual's gravity center, in order to not affect their mobility. The devices provided information on location (WGS-84), flight speed, elevation, and flight direction for each bird at 30-min intervals, from 18:00 to 6:00 hr COT during 56, 31 (tags G32 and G33) and 17 nights (tag G29).
As GPS data were set to indicate the location every 30 min, we estimated the number of seeds that could be dropped at half an hour intervals from the feeding tree up to 15 hr later. Also, for each plant species, we generated the elevation range of distribution from museum collections (Missouri Botanical Garden, Herbario Nacional Colombiano, Herbario Amazónico and GBIF), and our field data comprising 71 vegetation plots of 0.1 ha (Cárdenas, et al., 2020). These plots were placed in 25 sites of high visit frequency by the oilbirds with GPS devices (with at least five independent visits, each visit defined as the use of the site in different days) and 46 additional plots within the same region (located to represent the floristic composition of different forest types in the region—e.g., Prada & Stevenson, 2016—and built prior to this study). When potential feeding trees of the nine focal species were detected in the high visit frequency plots, we used them as the starting points where we set the dispersal kernel (Figure S1). From every independent visit, we assumed that feeding time started at the moment of the first GPS fix at the site and we estimated the location of deposition for one seed that could be dispersed to different places (i.e., where the individuals were detected according to point samples of the GPS at 30-min intervals). This procedure was replicated 100 times for each tree to assess the probability of the seeds getting to different sites, just by integrating the probability of seed delivery at 30-min intervals from the function of gut retention times (blue line in Figure S1). For example, 30 min after a visit we expected 0.8% of the seeds to be dropped, after 1 hr 1.9% of the seeds, and so on, with a maximum of 6.1% seeds expected at 7 hr after the visit, and no more seeds after 15 hr. The number of trees varied between species (P. acuminate: 23, L. applanata: 16, D. olivifera: 11, T. lawrancei: 10, Ocotea rugosa: 7, G. undata: 5, N. purpurea: 4, H. cuatrecazanum: 1, O. bataua: 1). Furthermore, we used a modification of CORIN land cover map (Cárdenas, et al., 2020), to estimate how many of the seeds of each species were landing in different habitats (including the cave as an independent landscape unit).
Finally, to establish whether estimated places of seed dispersal were suitable for the seeds, we assumed that only seeds landing within the elevation range of the species could survive. We also assumed no recruitment in caves, because of low light intensity, as well as high predation rates and fungal attack; and no recruitment in pastures and croplands where establishment probability is low or nil for mature forest plants (Holl & Lulow, 1997; Nepstad et al., 1991, 1996; Zimmerman et al., 2000).
2.3 Analyses
We assembled a database with 4,934 geographic coordinates of places where oilbirds were detected by GPS devices, and we established the habitat type for each point based on the CORIN land cover map. Daytime locations were assigned to the caves in Cueva de Los Guácharos, in cases when the GPS lost satellite signal and the individuals were directly traveling to the main cave just before sunrise. In fact, 2,740 (56%) points were assigned to caves, and only 111 records could not be assigned to a particular habitat.
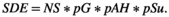 (1)
(1)The approach was based on the number of seeds (NS) found in seed traps in the cave during one year, which was corrected by plant density. In addition, the probability of germination (pG) was included, as well as the probability that seeds get to suitable habitats (pAH), according to the elevation range of each species. Finally, we included the probability of survival (pSu) of seeds, seedlings, and saplings from a literature review. This probability was set different for each plant species (using seed mass, Table 1), given that small seeds tend to show lower establishment rates than large seeds. Thus, we weighted pSu using the patterns of survival found by Baraloto et al., (2005). In the literature review we did not find information from all the study species. Therefore, as predation rates are high in initial plant stages and vary according to the habitat (Notman & Gorchov, 2001; Stevenson, 2007), we assumed that these species would show patterns similar to dispersed seeds and seedlings of other plants that have been studied in other tropical habitats. In the search, we used the terms “seed predation in tropical forests,” “seed survival in tropical forests,” and “seedling survival in tropical forests” in Scopus, Web of Science, and Google Scholar. Overall, we gathered 796 records of survival away from parental plants (35 sites and 310 plant species), after excluding information from pioneer trees (which are not consumed by oilbirds: Stevenson et al., 2017) and data from Asian forest where masting is common (Curran & Leighton, 2000), affecting seed predation rates. In addition, we found that survival in young secondary forests is six times lower than in natural habitats (Table S1), which was also taken into account for the assessment of SDE. We considered similar establishment rates between forests and disturbed areas that still maintain substantial tree cover, because high irradiance in places with minor disturbances may enhance survival. Moreover, we fitted logarithmic models to the data (for seeds, seedlings, and saplings) (Figure S3) and established survival probabilities as the mean indicated by the model at the end of each stage interval (i.e., seeds: 0.10, seedlings: 0.16, saplings DBH 1–5 cm: 0.85 and saplings DBH 5–10 cm: 0.88). Thus, the overall survival rates (pSu) were the multiplication of the probability in each stage. Finally, we used the code for estimating and plotting effectiveness landscapes with R (Jordano, 2014).
To test whether high-altitude species will have greater SDE than lowland species, we used a simple regression between SDE and mid-range elevation. We also used regression models to determine whether seed dispersal to different habitats depends on habitat occurrence in the region or on patterns of habitat use by oilbirds. Although the number of sampled oilbirds was limited, we analyzed SDE values for species dispersed in breeding (January–June) and non-breeding periods (July–December) (Stevenson et al., 2017) using a Mann–Whitney test.
3 RESULTS
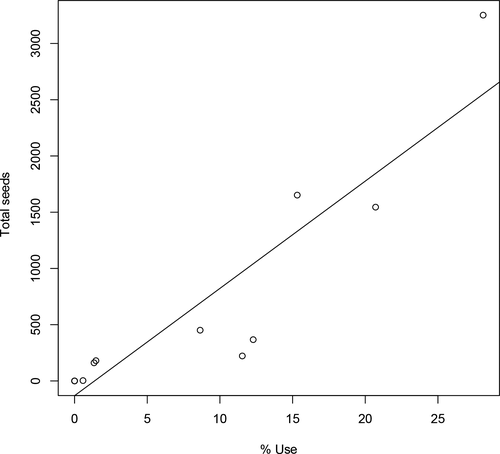
Oilbirds were frequently recorded out of the cave (21% in dense forests, 15% in mosaics of disturbed and natural habitats, 12% in coffee farms). The site with the highest probability of seed dropping was the main cave (41%), followed by mosaics of crops and natural trees, and dense forests (21 and 20%, respectively) (Table 2). The area of each habitat was a poor predictor of seed rain probability (R2 = .06; F = 0.78, df = 11, p = .39), but overall seedrain was associated with the frequency of habitat use (Figure 1, R2 = .82, F = 55.5, df = 11, p < .001). This pattern was observed independently for all plant species (Figure S2). We did not find a negative relationship between the proportion of seeds estimated to reach suitable habitats and elevation (F = 0.08, n = 9, p = .78).
| Habitat type | Oeno. bata. | Trat. lawr. | Dacr. oliv. | Ocot. rugo. | Nect. purp. | Pres. acum. | Lica. appl. | Geon. unda. | Hedy. cuat. | Total seeds (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Cave | 17 ± 1 | 42 ± 1 | 43 ± 2 | 35 ± 1 | 26 ± 1 | 39 ± 2 | 29 ± 1 | 37 ± 1 | 56 ± 3 | 3,252 (41.5) |
| Crops and trees | 1 ± 1 | 0 | 1 ± 1 | 6 ± 1 | 5 ± 1 | 93 ± 2 | 51 ± 1 | 1 ± 1 | 8 ± 0 | 1,652 (21.1) |
| Dense Forest | 2 ± 1 | 27 ± 2 | 29 ± 2 | 3 ± 1 | 5 ± 1 | 25 ± 3 | 24 ± 1 | 3 ± 1 | 36 ± 2 | 1,545 (19.7) |
| Secondary forests | 7 ± 1 | 9 ± 1 | 11 ± 1 | 1 ± 1 | 1 ± 1 | 7 ± 1 | 3 ± 1 | 3 ± 1 | 4 ± 1 | 451 (5.8) |
| Coffee crops | 2 ± 1 | 0 | 2 ± 1 | 7 ± 1 | 7 ± 2 | 3 ± 1 | 6 ± 1 | 0 | 9 ± 1 | 367 (4.7) |
| Pastures and crops | 0 | 0 | 1 ± 1 | 2 ± 1 | 5 ± 1 | 7 ± 2 | 4 ± 2 | 3 ± 1 | 0 | 222 (2.8) |
| Forest Fragments | 0 | 0 | 0 | 1 ± 1 | 0 | 5 ± 1 | 0 | 6 ± 1 | 4 ± 1 | 180 (2.3) |
| Pastures and trees | 1 ± 1 | 1 ± 1 | 2 ± 1 | 4 ± 1 | 0 | 4 ± 1 | 2 ± 1 | 0 | 3 ± 1 | 161 (2.1) |
| Rivers and lakes | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 (0) |
Note
- The SE was calculated from 10 independent model runs. The total number of seeds reaching each habitat is shown in the last column. Shrublands, exotic plantations, urban areas, and degraded habitats without cover did not receive seeds from any of these species (Oeno. bata. = Oenocarpus bataua, Trat. lawr. = Trattinnickia lawrancei, Dacr. oliv. = Dacryodes olivifera, Ocot. rugo. = Ocotea rugosa, Nect. purp. = Nectandra purpurea, Pres. acum. = Prestoea acuminata, Lica. appl. = Licaria applanata, Geon. unda. = Geonoma undata, Hedy. cuat. = Hedyosmum cuatrecazanum).
Prestoea acuminata was the species showing highest seed rain in the cave (1,070 seeds m−2 year−1), followed by another palm (Geonoma undata: 483 seeds m−2 year−1). The study species with lowest values were Trattinickia lawrancei, Hedyosmum cuatrecazanum, and Oenocarpus bataua (11, 37 and 35, respectively). In terms of biomass, G. undata showed the highest value (548 gr m2 year), followed by Dacryodes olivifera (505 gr m2 yearr); the lowest values were found for H. cuatrecazanum and T. lawrancei (2.2 and 11.9, respectively), both species with relatively small seeds. The most abundant species in the region were Licaria applanata, P. acuminata, Nectandra purpurea, and H. cuatrecazanum (>3 ind./ha), while the other species were uncommon in the region. Overall dispersal quantity was highest for Dacryodes olivifera and O. bataua, and lowest for H. cuatrecazanum (Table 3).
| Species | N | % in suitable habitats | Germina. Prob. | Establish.Prob. | SD quantity—number—(#/m2.yr) | SD quantity—biomass—(gr/m2.yr) | WCSMa | SDE |
|---|---|---|---|---|---|---|---|---|
| Hedy. cuat. | 1,193 | 53 | 0.82 | 0.16 | 9.7 | 0.3 | 0.84 | 1.1 |
| Geon. unda. | 537 | 26 | 0.73 | 0.07 | 2,417 | 212 | 0.90 | 111.2 |
| Lica. appl. | 1,192 | 66 | 0.52 | 0.24 | 41 | 48 | 0.95 | 4.8 |
| Pres. acum. | 1,836 | 70 | 0.98 | 0.25 | 306 | 78 | 0.85 | 63.7 |
| Nect. purp. | 497 | 37 | 0.39 | 0.08 | 33 | 42 | 1.08 | 1.1 |
| Ocot. rugo- | 590 | 37 | 0.31 | 0.09 | 394 | 130 | 0.88 | 9.7 |
| Dacr. oliv. | 894 | 51 | 0.94 | 0.14 | 902 | 2,524 | 1.38 | 163.8 |
| Trat. lawr. | 795 | 47 | 0.75b | 0.14 | 109 | 60 | 0.88 | 10.1 |
| Oeno. bata- | 298 | 42 | 0.46b | 0.07 | 354 | 1,506 | 1.23 | 14.0 |
Note
- SDE estimates included seed numbers, the percentage of seeds estimated to get to suitable habitats for establishment, probability of germination, probability of survival (from seeds to saplings in different habitats), and seed quantity (based on the number of seeds dispersed in the cave and tree density). Hedy. cuat. = Hedyosmum cuatrecazanum, Geon. unda. = Geonoma undata, Lica. appl. = Licaria applanata, Pres. acum. = Prestoea acuminata, Nect. purp. = Nectandra purpurea, Ocot. rugo. = Ocotea rugosa, Dacr. oliv. = Dacryodes olivifera, Trat. lawr. = Trattinnickia lawrancei, Oeno. bata. = Oenocarpus bataua.
- a Weighted coefficient seed mass.
- b Tetrazolium viability test.
All species dispersed by oilbirds show potential germination (range: 31%–98%) or viability (Table 3). The highest germination scores were found in P. acuminata (98%), D. olivifera (94%), and H. cuatrecazanum (82%). The lowest values were for O. rugosa (31%) and N. purpurea (39%), which were also attacked by fungi. T. lawrancei and O. bataua did not germinate but were viable based on the tetrazolium tests.
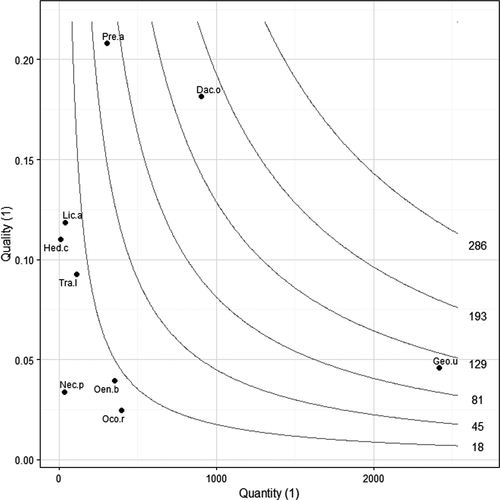
The most effectively dispersed plants were D. olivifera and G. undata; in contrast, H. cuatrecazanum and N. purpurea had the lowest values (Table 3). D. olivifera showed the highest scores of dispersal effectiveness. This may be explained because of high germination and high rates of survival (given their large seeds) and intermediate estimates of seed dispersal quantity and probability of seed deposition in suitable habitats (Figure 2, Table 3). O. bataua showed similar traits, but a lower probability of arriving in suitable habitats (Table 3). G. undata had high SDE scores, because of the large number of seeds dispersed numbers. P. acuminata had intermediate values of SDE because of the high seed rain, germination, and probability of reaching suitable sites, but it had a relatively abundant population and relatively small seeds. N. purpurea had low values of SDE (Table 3), because it had low germination and low probability to reach suitable habitats, despite having high scores of seed dispersal quantity. Some of these traits were observed for O. rugosa, which has high SDE scores because of the low population density (Table 3). L. applanata had high germination scores and probability to reach suitable sites (Table 3) but is relatively common in the region (Table 1). H. cuatrecazanum had low SDE because of low seed dispersal quantity, the small seeds (Table 3), and the abundance of trees in the region (Table 1). T. lawrancei had similar values as H. cuatrecazanum (Figure 2), except in terms of seed dispersal quality, given that it is an uncommon tree in the region (Table 1).
As expected, SDE was lower in breeding than in non-breeding periods (W = 0, n = 13, p = .003). Overall the percentage of seed estimated to reach suitable habitats in the non-breeding period was almost twice that found in breeding periods (Table 4).
| Species | N | % breed | % non-breed |
|---|---|---|---|
| Hedyosmum cuatrecazanum | 1,193 | 43 | 74 |
| Geonoma undata | 537 | 26 | N.A |
| Licaria applanata | 1,192 | 56 | 66 |
| Prestoea acuminata | 1,836 | 45 | 87 |
| Nectandra purpurea | 497 | 37 | N.A |
| Ocotea rugosa | 590 | 24 | 100 |
| Dacryodes olivifera | 894 | 51 | N.A |
| Trattinnickia lawrancei | 795 | 47 | N.A |
| Oenocarpus bataua | 298 | 42 | N.A |
| Average | 870 | 41.2 | 81.8 |
Note
- As many species show seasonal patterns of production, there were cases in which feeding trees were not visited and the percentage of seeds dispersed to suitable habitats was not available (N.A.).
4 DISCUSSION
Our results showed that seeds dispersed by oilbirds are in good condition to germinate (or are viable) and the long travel distance results in some of the swallowed seeds reaching suitable habitats. Not surprisingly, seed shadows were more associated with the patterns of habitat use than habitat area; in particular, use was associated with habitat selection for undisturbed forests where they get nutritious fruits (Cárdenas, et al., 2020; Stevenson et al., 2017). The fact that oilbirds spend more time in caves during breeding periods implies that their effectiveness as seed dispersers is lower during that season than out of the reproductive period. Our approaches to estimate SDE indicated that oilbirds might positively affect plant fitness for the species they consume, even though 41% of the seeds could be dropped in caves; however, this estimate could be lower if we could have a similar sample size in breeding and non-breeding periods. Some highland species (i.e., G. undata) and lowland species (i.e., D. olivifera) had high scores of SDE, and no relationship was found between SDE and the elevation range of the plants, perhaps because of the long gut retention time, allowing seeds of low and high-altitude plants to be delivered in a variety of habitats.
Seed dispersal effectiveness is an approach that attempts to estimate the effect of a frugivore on plant fitness due to the interaction between seed dispersal quantity and quality (Schupp, 1993). In our case, SDE varied among plant species, which is common in studies including multiple species (Figueroa-Esquivel et al., 2009; Graham et al., 1995; Rother et al., 2016) due to different combinations of SDE components (Schupp et al., 2010). The oilbird colony seems to be very effective in terms of seed quantity, moving a large number of seeds of the focal species and more than 43 other plant species (Stevenson et al., 2017). In terms of quality, the role of oilbirds may not be the highest, because of the high probability of dropping seeds in caves, where plant establishment is unlikely.
Species like D. olivifera showed high SDE, which can be associated with the large seeds, which may provide a high chance of recruitment (Baraloto et al., 2005) and the fact that the species has a broad altitudinal range may help dropped seeds reach suitable habitats. Large-seeded plants usually have a small set of seed dispersers (Bueno et al., 2013; Karubian et al., 2012; Stevenson et al., 2015; Terborgh et al., 2008; Wright, 2003), due to size limitation of the disperser's anatomy, such as gape width (Rother et al., 2016), but when dispersers are available plant establishment may be high.
Our SDE values may include some noise because of logistic limitations in our approach that perhaps could be assessed in future studies. Perhaps the main limitation could be that we do not know how many trees are being represented by the seed rain in the traps. This may be overcome by performing paternity analyses; however, a more direct way to estimate seed dispersal quantity could be to monitor individual trees and estimate the number of seeds being dispersed by each one (cf. using infrared trap cameras). In addition, seed and seedling survival experiments in the main habitats used by oilbirds will provide better information on seed dispersal quality. Finally, a larger sample size of oilbirds with GPS devices would be desirable, as well as refined assessments of movement patterns (i.e., data at <30 min intervals) and more information on seed retention times.
Despite the low number of birds studied and the fact that some species could not be compared because they only fruited in one season (Table 4), as predicted, we found differences in SDE between reproductive periods. We estimated that almost half of the seeds are deposited in the cave during breeding periods, while less than 20% were dropped in the cave in the non-breeding period.
Even for species with low SDE scores (e.g., H. cuatrecazanum and N. purpurea), seeds apparently reach suitable places for recruitment. This may be explained by the long travel paths, where oilbirds visit different habitats, especially when they are not sleeping in caves (Holland et al., 2009). As seed transit in the gut of oilbirds is relatively long (on average 6 hr, Bosque & Parra, 1992), and they move on average 54.7 km each night (Cárdenas, et al., 2020), this interaction generates the chance that seeds may be dispersed to a variety of habitats. Since habitat use and seed shadows are positively associated, the fact that dense forest is the main habitat type the birds visit out of the caves may allow many seeds to reach potential sites for establishment. Seed retention times in the gut of birds are usually shorter than in oilbirds (Pigeon: Hemiphaga novaezeelandiae = 37–181 min, Wotton et al., 2008; Toucan: Ramphastos sulfratus = 4–98 min, Kays et al., 2011; Turacos: Musophaga johnstoni = 0.6–1.8 hr, Sun et al., 1997). However, some other frugivores may show similar patterns (hornbills: Ceratogymna spp.: 1–12.7 hr, Holbrook & Smith, 2000).
There is accumulating evidence that frugivores may affect the patterns of plant composition by seed dispersal processes (García et al., 2010; Stevenson, 2011), and frequently preferred sites by frugivores are the places where many seeds may reach, becoming important nurseries to be considered in plant conservation plans (García et al., 2010; Sanford et al., 2008). Habitat connections are usually necessary to allow frugivore visits (Eycott et al., 2012; Fahrig, 2007; Gillies & St. Clair, 2010), and the restoration of connectivity is difficult to implement (Simberloff & Cox, 1987), implying that forest remnants and degraded habitats will not get as many seeds as continuous habitats. Interestingly, oilbirds may be one of the few frugivores able to carry large-seeded species to distant forest fragments in the Neotropics, so their seed dispersal services probably cannot be achieved by other extant frugivores.
Large-seeded species with fleshy fruits are negatively impacted in forests that have been degraded by humans (Chapman & Onderdonk, 1998; McConkey et al., 2012), because the largest frugivores (their main dispersers) are heavily affected (Bueno et al., 2013; Chapman & Onderdonk, 1998; Terborgh et al., 2008; Wright, 2003). However, the relatively smaller oilbirds are accomplishing similar roles as the largest extant frugivores. In addition, the preponderance of Lauraceae seeds in their diet may imply long-term survival of hard-wooded species, and thus the potential of accumulating more biomass in Neotropical forests (King et al., 2006; Scabin et al., 2012). The fact that human activities highly impact the Andean forest where the local extinction of many large frugivorous birds (Kattan et al., 1994) and mammals (Stevenson & Aldana, 2008) has occurred makes frugivorous birds key seed dispersers in these habitats (García et al., 2010). In this scenario, the behavior of oilbirds represents a chance of recruitment for many plant species, which may be too costly to maintain by other means, such as active restoration (García et al., 2010; Howe & Miriti, 2004; Sekercioglu, 2017).
ACKNOWLEDGMENTS
We are grateful to the people that helped us building vegetation plots, in particular to Manuel Lequerica, Cecilia Prada, Laura Molina, and María Paula Kairuz. Santiago Palacios helped us arranging the set-up of vegetation plots, and Laura Cardona was the person providing the information from traps in the cave. Francisco Henao undertook the first analyses to determine the geographic range of the nine species. Luis Miguel Renjifo and Juan Carlos Benavides provided useful comments as evaluators of a MSc thesis at “Facultad de Estudios Ambientales y Rurales de la Pontificia Universidad Javeriana,” where maps and GIS tools were provided. We thank Dr. Eugene Schupp and an anonymous reviewer for the useful comments. Plant identifications were made at “Laboratorio de Ecología de Bosques Tropicales y Primatología” in Universidad de Los Andes, and Rafael Lozano and students of the Ecology course helped with germination experiments. Funds were provided by the Amazon Conservation Team (Colombia), and the research initiatives from Universidad de Los Andes.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available in EUDAT at http://doi.org/10.23728/b2share.68e3c405a974483d9d1cb6243c93861d.




