Population-level plant pollination mode is influenced by Quaternary climate and pollinators
Associate Editor: Ferry Slik
Handling Editor: Nico Bluthgen
Abstract
Patterns in ecology are the products of current factors interacting with history. Nevertheless, few studies have attempted to disentangle the contribution of historical and current factors, such as climate change and pollinator identity and behavior, on plant reproduction. Here, we attempted to separate the relative importance of current and historical processes on geographical patterns of the mating system of the tree species Curatella americana (Dilleniaceae). Specifically, we asked the following: (a) How do Quaternary and current climate affect plant mating system? (b) How does current pollinator abundance and diversity relate to plant mating system? (c) How does mating system relate to fruit/seed quantity and quality in C. americana? We recorded pollinators (richness, frequency, and body size) and performed pollination tests in ten populations of C. americana spread over 3,000 km in the Brazilian savannah. The frequency of self-pollination in the absence of pollinators was strongly influenced by historical climatic instability and not by present-day pollinators. In contrast, seed set from hand-cross and natural pollination were affected by pollinators (especially large bees) and temperature, indicating the importance of current factors on out-cross pollination. Two populations at the Southern edge of the species’ distribution showed high level of hand-cross-pollination and high flower visitation by large bees, but also a high level of autogamy resulting from recent colonization. Our results indicate that historical instability in climate has favored autogamy, most likely as a reproductive insurance strategy facilitating colonization and population maintenance over time, while pollinators are currently modulating the level of cross-pollination.
1 INTRODUCTION
Animal pollination is estimated to occur in approximately 87.5% of the angiosperms and is particularly prevalent in the warm and humid tropics (Ollerton et al., 2011; Rech et al., 2016). In general, there is less pollen limitation when the pollination systems are more generalized, exhibiting a higher probability of pollen being transferred to conspecific stigmas (Knight et al., 2005; Lopes et al. submittted). Generalized pollination systems are therefore more resistant to pollinator species loss, and, hence, they are hypothesized to predominate in environments where the pollinator fauna is highly variable (Waser et al., 1996) or not immediately fitted to the ancestral pollination mode, such as on islands (Armbruster & Baldwin, 1998; Rivera-Marchand & Ackerman, 2006; Sonne et al., 2019). More diverse sets of pollinators can also be functionally more stable over time and space due to the buffering effect of different species responding in different ways to environmental changes, that is, the “biodiversity insurance hypothesis” (Bartomeus et al., 2013; Loreau, 2001). However, we know very little about the influence of current and past climate factors on the functioning of pollination systems.
Plants can also show diverse and complex reproductive strategies related to how to find reproductive partners, resulting in mating systems that range from autogamy (independence of pollen vectors) to exclusively outcrossed, with everything in-between (Goodwillie et al., 2005). Although self-incompatibility usually results in higher-quality progeny and genetic diversity (Dart & Eckert, 2013; Wright et al., 2013), autogamous self-pollination (hereafter called autogamy, see Cardoso et al., 2018) may allow species to colonize new areas or survive within ones where conditions are non-optimal for pollinators (Grossenbacher et al., 2015; Lloyd & Webb, 1992). The idea of autogamy assuring reproduction was originally proposed by Darwin (1877) and formalized by Baker (1955, 1967), and has been named “Baker's rule” or the “reproductive insurance hypothesis.” A similar rationale was later expanded to small populations living at the edges of species distributions, where the lower plant density is likely to reduce cross-pollination (Levin, 2012; Randle et al., 2009). Mating systems may therefore influence the geographical range of plants, with autogamous species having larger ranges due to low mate requirement and high reproductive success at the edges of their range or in colonizing populations (Grossenbacher et al., 2015). Traditionally, mating systems were considered species-level properties and few comparisons considered differences among populations or individuals (Levin, 2012). However, we now know that mating systems may vary among populations according to local environmental conditions (Rech et al., 2018; Whitehead et al., 2018). As with pollination systems, assessing the influence of current and historical factors on mating systems within populations is an untested approach that will improve our understanding of the evolution of plant reproductive strategies.
Historical climate dynamics are likely candidates to affect mating systems since we already know of their effect on species distribution and diversity patterns (Cardenas et al., 2011; Kissling et al., 2012; Sandel et al., 2011), population demography and genetic structure (Cabanne et al., 2007; Grazziotin et al., 2006), and previous studies have suggested an influence of historical climate stability on the structure of mutualistic plant–pollinator assemblages (Dalsgaard et al., 2011, 2013). To understand how historical climate has varied, pollen records have often been used to reconstruct Quaternary paleo-environments, evidencing possible stable areas for genetic diversity increasing after Pleistocene climatic oscillation (Anhuf et al., 2006; Buzatti et al., 2018; de Oliveira Bezerra et al., 2019). In South America, there is considerable debate whether currently forested areas such as the Amazon basin may previously have been savannah, and about the consequences for species diversification in the area (Colinvaux & De Oliveira, 2001; Pennington & Ratter, 2006; Richardson, 2001). In this study we consider the possible impacts of these dynamics on the mating system of a widely distributed tree species associated with open, savannah areas.
We chose Curatella americana L. (Dilleniaceae) as our species model as it is one of the main pollen types used to reconstruct the history of South American savannah environments (Absy et al., 1997; Behling, 1995). Moreover, the association of this species with savannahs and its mixed mating system (Rech et al., 2018) makes C. americana a suitable model to address ecological questions about spatial variability and historical climate stability on plant mating systems. Previous studies have shown that areas of South American savannah have varied in size throughout the Neogene (Ledru et al., 2006; Pennington & Ratter, 2006), and that the disjunct areas of savannah present nowadays in Pará, Roraima, and other areas of Brazil were probably connected and separated many times over the Quaternary (Adrian Quijada-Mascareñas et al., 2007; Werneck, 2011). At the present time, C. americana is likely to be found in most areas of the savannah, also known as the Cerrado, in Brazil (Ratter et al., 2003). It is reported even in small areas of savannah surrounded by forest at the Amazon region (Magnusson et al., 2008; Ratter et al., 2003), thought have been isolated at least from the mid Holocene onward (Mayle & Power, 2008; Werneck, 2011).
Despite the potential for an important relationship among plant–pollinator interactions, mating system, and past and current climate, this relationship has never previously been empirically tested and addressed. To gain insight into current and historical drivers of population-level plant mating systems, in this study we investigated the spatial structure and the determinants of the pollination and mating systems of C. americana across a latitudinal gradient of Brazilian savannah areas, considering both historical and current climates. Specifically, we ask: (a) How do Quaternary and current climates affect the level of cross- and autogamous pollination)? (b) How does current pollinator abundance and functional diversity relate to plant mating system? (c) How does mating system relate to fruit and seed quantity and quality in Curatella americana?
2 METHODS
2.1 Study sites and species
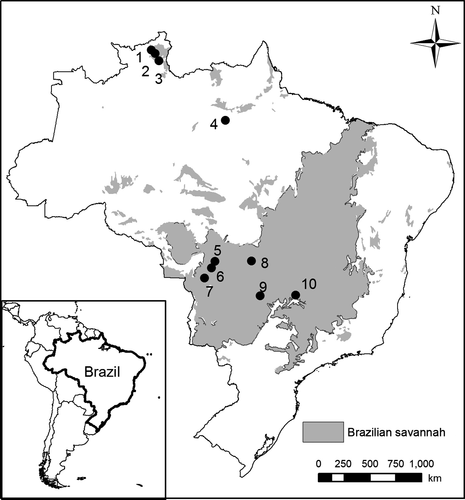
We studied ten populations of Curatella americana in three disjunct areas of savannah (Table S1, Figure 1). Vegetation physiognomies are very similar among sites, but in general plant species diversity decreases northward (Bridgewater et al., 2004; Ratter et al., 2003). We observed animal pollinators and performed experiments on C. americana at all the studied sites. The species flowers from June to September in Central Brazil, mid-August to early October in Pará state, and October and November at Roraima state. Flowers are white, pentamerous and grouped into dense inflorescences, and each flower stays receptive for three to five hours for one single day (see Rech et al., 2018 for more details).
2.2 Mating system
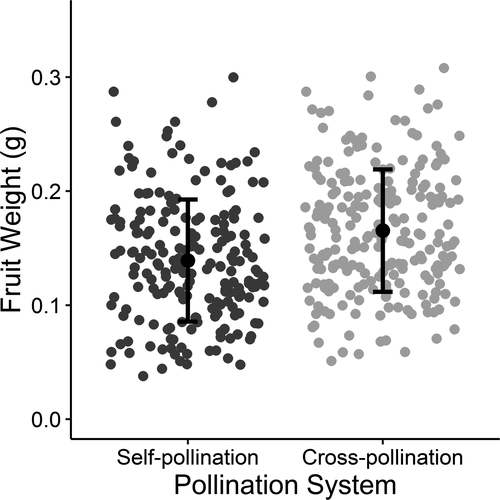
In order to study the reproductive system of C. americana in situ we applied the following pollination tests: hand-cross-pollination, hand-self-pollination, autogamous self-pollination and natural pollination. All pollination tests were performed with flowers previously bagged using cloth insect exclusion bags, except for natural pollination, which involved counting and tagging flowers exposed to flower visitors. In order to mitigate possible differences related to resource allocation we always performed the pollination tests on the same branch (considered as a functional unit). The number of tested flowers was always higher than 20 flowers per individual and a mean of 15 different individuals per test per population. In two of the studied areas (Nova Xavantina and Caldas Novas) we chose 12 individuals and compared the fruit weight from self (n = 107) and cross (n = 102) pollinated flowers, which may represent seed quality (Coomes & Grubb, 2003).
2.3 Flower visitation and pollination
For all populations we recorded daily flower visitors (species richness and abundance) from anthesis until the end of visitation. In order to quantify visitation, we counted all visits to an observable (and counted) set of flowers for ten minutes each half an hour for at least 20 hr (120 x ten minute sessions) in each population. All the visitors touching anthers and/or stigmas were considered and scored as potential pollinators. After observing behavior, flight distance and pollinator size, we grouped the pollinators into two categories: (a) Large-sized bees, and (b) Others, which includes bees the same size or smaller than Apis mellifera, beetles, flies and wasps. We separated pollinators according to size because flight range correlates with body size (Araújo et al., 2004; Gathmann & Tscharntke, 2002; Greenleaf et al., 2007). Based on this premise, we expected a higher level of cross-pollination by large-sized bees.
2.4 Statistical analysis
To test for differences in fruit set related to the mating system and the regions, we used a generalized linear mixed model assuming a binomial distribution. The fixed factors were region, pollination experiment treatment, and the interaction between them. The random factors were the individuals nested within sites and these nested within regions. Our response variable was the production of a fruit from each flower. We performed the models with all fixed factor combinations and only a fixed intercept (Null Model), always keeping the random factor. For the fruit weight comparison we used pollination treatment (self- and cross-pollination) as predictors and generated models using individuals as random factors. All the alternative models were built removing factors or interactions between factors from the full model. A null model using only the intercept was also considered. In order to compare the generated models we used the Akaike information criterion—AIC (Burnham & Anderson, 2004). All tests and models were performed in the R environment (R Core Team, 2018).
For each studied site, we modeled the climate changes since Last Glacial Maximum (LGM) by estimating the mean annual temperature (MAT_LGM) and annual precipitation (MAP_LGM) at each location for 21ky, according to the Community Climate System Model (CCSM) (Gent et al., 2011). We also extracted the current values of temperature (MAT_Current) and precipitation (MAP_Current) from the Global Climate Data (Worldclim 1.4 - http://www.worldclim.org/). For each site, we calculated the anomalies and velocities of change in temperature (MAT_Velocity_21) and precipitation (MAP_Velocity_21), as the long-term average over the last 21ky. Both climate anomaly and velocity are measures of climate stability (or climate change), but they are calculated in two different ways. Whereas climate anomaly simply is the difference in climatic conditions between two time periods (today and 21,000 years ago), climate velocity integrates macroclimatic shifts (i.e., anomalies) with local spatial topoclimate gradients. Velocity is calculated by dividing the rate of climate change through time (i.e., anomaly) by the local rate of climate change across space (Sandel et al., 2011). All calculations are based on a 2.5 min geographical resolution.
We then estimated the effect of climate and pollinator activity on pollination mode. Due to the modest sample size of populations (n = 10) and some predictor variables being strongly correlated (i.e., r ≥ 0.6; Table S2), we took the following modeling approach. First, we modeled the effect of climate on pollination mode using current and past climate predictors, identifying minimum adequate models (MAMs) using the approach outlined in Diniz-Filho et al. (2008). As the temperature and precipitation anomalies used as a measure of past climate stability were strongly correlated, we modeled the effect of temperature and precipitation anomaly separately. The effect of past climate stability was also tested using modeled temperature and precipitation velocity instead of anomaly, giving qualitatively the same results (not shown). Second, we tested whether the four pollinator variables (pollinator richness, visitation frequency, and proportion of large bee visitation calculated both with and without the exotic honey bee) were significantly related to pollination mode. To do this we used single correlation tests using traditional non-spatial correlation analysis and correcting the degrees of freedom using Dutilleul's (1993) method (Table 1), followed by models testing whether each of these pollinator activity variables may have other or additional effects from climate. We examined this by again following the approach of Diniz-Filho et al. (2008) to identify MAMs, but this time only considering climate variables included in the above-identified MAMs and each of the four pollinator variables.
| Autogamous pollination | Natural pollination | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Σ wi | Averaged | MAM | Σ wi | Averaged | MAM | Σ wi | Averaged | MAM | Σ wi | Averaged | MAM | |
| MAT | 0.06 | +0.14 | – | 0.09 | +0.11 | – | 0.04 | −0.04 | – | 0.04 | −0.04 | – |
| MAP | 0.08 | +0.05 | – | 0.11 | −0.23 | – | 0.05 | +0.04 | – | 0.05 | +0.03 | – |
| MAT seas | 0.11 | −0.30 | – | 0.15 | −0.36 | – | 0.99 | +0.91 | +0.91** | 0.99 | +0.91 | +0.91** |
| MAP seas | 0.21 | +0.53 | – | 0.61 | +0.62 | – | 0.05 | +0.09 | – | 0.05 | +0.09 | – |
| MAT anomaly | 0.79 | +0.73 | +0.74* | 0.06 | −0.12 | – | – | |||||
| MAP anomaly | 0.22 | −0.45 | – | 0.08 | +0.16 | |||||||
| AICc | −3.821 | −11.098 | −11.098 | |||||||||
| Moran's I | ≤0.39NS | ≤0.01NS | ≤0.01NS | |||||||||
| CN | 1 | 1 | 1 | |||||||||
| R2 | 0.55 | 0.83 | 0.83 | |||||||||
| R2adj | 0.55 | 0.83 | 0.83 | |||||||||
| Hand-cross-pollination | Hand-self-pollination | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Σ wi | Averaged | MAM† | Σ wi | Averaged | MAM£ | Σ wi | Averaged | MAM | Σ wi | Averaged | MAM | |
| MAT | 0.56 | −0.72 | −0.78** | 0.31 | −0.66 | – | 0.10 | −0.06 | – | 0.14 | −0.08 | – |
| MAP | 0.09 | +0.14 | – | 0.08 | −0.10 | – | 0.13 | −0.25 | – | 0.19 | −0.31 | – |
| MAT seas | 0.51 | +0.74 | – | 0.64 | +0.64 | +0.59* | 0.13 | −0.29 | – | 0.18 | −0.31 | – |
| MAP seas | 0.06 | −0.21 | – | 0.04 | −0.12 | – | 0.29 | +0.47 | – | 0.47 | +0.50 | – |
| MAT anomaly | 0.14 | −0.32 | – | 0.53 | +0.58 | – | ||||||
| MAP anomaly | 0.59 | +0.54 | +0.51* | 0.19 | −0.29 | – | ||||||
| AICc | −6.997 | −8.84 | ||||||||||
| Moran's I | ≤0.27NS | ≤0.22NS | ||||||||||
| CN | 1 | 1.5 | ||||||||||
| R2 | 0.61 | 0.82 | ||||||||||
| R2adj | 0.61 | 0.80 | ||||||||||
Notes
- The standardized regression coefficients are reported for ordinary least square (OLS) regression and reported for both an averaged model based on weighted wi and minimum adequate models (MAMs) (Diniz-Filho et al., 2008). For all MAMs, we give AICc, the Condition Number (CN), Moran's I (significance tested using 5 distance classes and applying a permutation test with 10,000 iterations), and coefficients of determination (R2 and R2adj ). We did not assign any MAM if all variables in the best-fit model were non-significant. Notice that historical climate stability is represented by temperature and precipitation anomaly between 21,000 years ago and now. As these two estimates of climate stability were strongly intercorrelated (Table S2), we separately modeled temperature anomaly (grey columns) and precipitation anomaly (white columns) effects on the output of each pollination experiments. The results are qualitatively the same if using temperature and precipitation velocity as estimates of climate stability (results not shown).
- NS non-significant. †One model was equally fit (i.e., ∆AICc ≤ 2) containing the following variables: 1) MAT seas. £two models were equally fit: 1) MAT; 2) MAT Seas.
- ** p < 0.01; *p < 0.05.
For all analyses, MAP, MAP anomaly, MAP velocity and MAT velocity were Log10-transformed, pollination visitation frequency was square root transformed, and all proportional measures (i.e., pollination mode variables and large bee predictors) were arcsine-square root transformed. All other variables were left untransformed. All analyses were conducted using the software Spatial Analysis in Macroecology, SAM 4.0 (Rangel et al., 2010).
3 RESULTS
3.1 Pollination and mating system variation
The main flower visitors and potential pollinators of C. americana flowers were bees of different sizes (more details in Rech et al., 2018). Beetles were also recorded at all populations, but they only ate anthers and copulated on the flowers, with little, if any, importance as pollinators. In eight out of ten populations, flies and wasps were also recorded as flower visitors; however, they were visiting with a very low frequency; only in Jatai, Caldas Novas and Santarém did they perform more than 1% and never more than 5% of total visits. During their visits, they ate pollen directly from the anthers (flies) and did not always touch anthers and stigmas (flies and wasps).
In all populations, cross-pollinated flowers set more fruit than self-, natural- or autogamously pollinated flowers (Table 2). Cross-pollination (measured by fruit set) was negatively correlated with self-pollination (r = −0.87, p = 0.009). Fruit set from cross- and self-pollination were more contrasting in the southern and more similar in the northern populations, showing that out-crossing decreases from south to north (Table 2). The analysis of fruit weight according to pollination test and site showed that only pollination treatment was important, with hand cross-pollination producing heavier fruit than self-, natural- or autogamously pollinated flowers (Figure 2, Table 3). This tells us that the populations that were studied are pollen limited and therefore that the reproductive success of plants is more likely to be influenced by climate variables, if those variables in turn affect pollinator numbers.
| Ama | Faz | BV | Stm | Cui | Man | Poc | Nxav | Cnov | Jat | |
|---|---|---|---|---|---|---|---|---|---|---|
| Cross-pollination | 0.52 | 0.54 | 0.33 | 0.66 | 0.66 | 0.83 | 0.81 | 0.79 | 0.82 | 0.73 |
| Hand selfing | 0.53 | 0.29 | 0.22 | 0.06 | 0.13 | 0.37 | 0.08 | 0.17 | 0.43 | 0.20 |
| Autogamous self | 0.21 | 0.24 | 0.23 | 0.05 | 0.06 | 0.06 | 0.05 | 0.02 | 0.29 | 0.20 |
| Natural pollination | 0.32 | 0.15 | 0.23 | 0.28 | 0.65 | 0.48 | 0.62 | 0.63 | 0.72 | 0.34 |
Notes
- At the region of Roraima—BV: Boa Vista, Faz: Fazenda Bamerindus, Ama: Amajari; Pará region—Stm: Santarém; Mato Grosso region—Cui: Cuiabá, Man: Manso, Poc: Poconé, Nxav: Nova Xavantina, and Goiás region—Jat: Jatai, Cnov: Caldas Novas.
| Model | ∆AIC value | Degrees of Freedom |
|---|---|---|
| Pollination treatment | 0.0 | 4 |
| Null model | 6.3 | 3 |
| Pollination treatment + Site | 6.4 | 5 |
| Site | 12.3 | 4 |
| Pollination treatment + Site +Interaction | 15.0 | 6 |
Note
- Individuals were considered random factors.
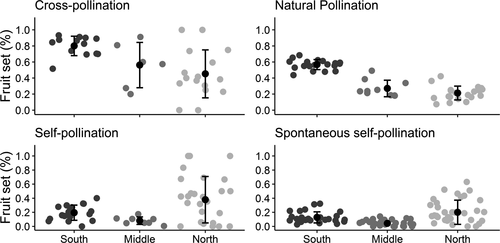
The results of pollination tests in C. americana were best explained by the full model including region, pollination treatments, and the interaction between them (Table 4). Considering only the additive effects of region and reproductive system makes the model nearly as likely as including only the reproductive system regardless of region, reinforcing that these factor are interacting. The reproductive system of the species was structured on a regional scale, and although there are differences among populations inside a region, differences among regions were greater. Although there is a strong difference in the reproductive tests among regions, it is possible to see that the level of autogamous pollination is highly variable among individuals within a given region and, even in the North region it is possible to find some individuals with very low fruit set inside bagged inflorescences (Figure 3).
| Model | ∆AIC | Degrees of Freedom |
|---|---|---|
| Full | 0.0 | 13 |
| Full without interaction | 1,523.9 | 7 |
| Only reproductive system | 1,545.7 | 5 |
| Only region | 4,813.5 | 4 |
| Null | 4,846.8 | 2 |
Notes:
- The full model included region (South, Middle, and North) and reproductive systems (cross-, self-, autogamous, and natural pollination) as fixed factors, the interaction between them and individuals and sites (replication) as random factors. “Full without interaction” was similar to the full model except for the interaction between fixed factors. “Only reproductive system” did not considered region, while “Only region” did not considered reproductive system, and the null model is only the intercept and the random factors (individual and population).
3.2 Historical and current climate analyses
Both current and past climate influenced the reproductive system of C. americana. Natural pollination was highly related to the yearly seasonality, that is, current fluctuations in temperature. This was significant both in non-spatial and spatial correlation analysis, and alone explained 91% of the variation in natural pollination (Table 5). Natural pollination was also positively related to visitation by large bees (79%), and negatively correlated to mean annual temperature (63%) and mean annual temperature velocity (76%). Autogamous pollination was higher in areas with more temperature anomaly, that is, historical climatically unstable areas.
| Pollination tests | ||||
|---|---|---|---|---|
| Hand-cross | Hand-self | Spontaneous-self | Natural | |
| MAT | −0.78† | +0.00NS | +0.19NS | −0.63† |
| MAP | −0.22NS | −0.24NS | −0.16NS | −0.50NS |
| MAT seas | +0.78† | −0.24NS | −0.36NS | +0.91 * |
| MAP seas | −0.36NS | +0.51NS | +0.62† | −0.23NS |
| MAT anomaly | −0.37NS | +0.59NS | +0.74† | −0.23NS |
| MAP anomaly | +0.72† | −0.32NS | −0.49NS | +0.47NS |
| MAT velocity | −0.65† | +0.07NS | +0.06NS | −0.76† |
| MAP velocity | +0.68† | −0.50NS | −0.52NS | +0.45NS |
| Pollinator richness | +0.52NS | −0.30NS | −0.47NS | +0.54NS |
| Pollinator visitation frequency | +0.40NS | −0.13NS | +0.09NS | +0.17NS |
| % Large bee visits, incl. honey bee | +0.70† | −0.37NS | −0.53NS | +0.79† |
| % Large bee visits, natives only | +0.84† | −0.15NS | −0.25NS | +0.79† |
Note
- Statistically significant relationships are marked in bold.
- * p < 0.05 both when using non-spatial statistics and when significance level is based on degrees of freedom corrected for spatial auto-correlation using Dutilleul's (1993) method; †p < 0.05 when using non-spatial statistics, but non-significant when using Dutilleul's (1993) method; NS non-significant.
4 DISCUSSION
The current pollination mode and mating system of C. americana in the Brazilian savannah is the result of both historical and contemporary factors. Quaternary climate instability has clearly influenced the level of autogamous self-pollination in populations, whereas contemporary temperature seasonality and proportion of large bee visitation determined the level of cross-pollination. This indicates that autogamous self-pollination is likely to occur in areas that have experienced higher climate variability that subjected populations to local extinctions and re-colonization events. This has occurred many times in the past, as pollen records indicate in the northern (Rodrigues & Absy, 2006) and southern edge of C. americana distribution in Brazil (Salgado-Labouriau et al., 1997). In agreement with this, genetic data on the phylogeography of C. americana indicated recent expansion in most populations (Canuto, 2011). Most of the literature on Brazilian savannah biogeography agrees that its area varied considerably during the Quaternary (Adrian Quijada-Mascareñas et al., 2007; Anhuf et al., 2006; Pennington & Ratter, 2006; Werneck, 2011), and this has impacted the mating systems of C. americana populations.
Higher levels of autogamy in the northern populations were the results of a weaker restriction to self-pollen germination and a shorter distance between stigma and anthers (low herkogamy), probably in response to mismatches to pollinator distributions during historical fluctuations in climate (Rech et al., 2018). The occurrence of autogamous self-pollination as a reproductive assurance mechanism has been suggested in many other plant species (reviewed in Eckert et al., 2006). For natural pollination, current temperature and the proportion of the total visits carried out by large bees were more important factors. Moreover, the proportion of large bees was correlated to several historical and current climate variables (MAT, MAT seasonality and velocity), preventing us from separating the effect of temperature on pollinators or, alternatively, direct temperature effects on natural pollination.
Most of the studied populations in central Brazil (Populations 5, 6, 7, and 8—Figure 1) occur in a geologically old savannah area (Terribile et al., 2012), where the longer distance pollen flow mediated by large bees and climate stability may be acting to promote the reproduction of individuals better able to cross-pollinate (Koski et al., 2018; Sirois-Delisle & Kerr, 2018). Considering that cross-pollination produces heavier—and presumably higher quality—fruit, the progeny from this fruit will be expected to outcompete or survive longer periods of unfavorable conditions than the ones from self-pollination (Coomes & Grubb, 2003). However, increased dispersal is selected when there is local adaptation to climate instability, thus, self-fertilization may be favored between expansion and contraction of the range margins by providing reproductive assurance (Hargreaves & Eckert, 2014). In line with this rationale, the two populations in the southern edge of the Brazilian savannah (Caldas Novas and Jatai) both showed moderate levels of autogamous self-pollination, consistent with recent colonization events followed by east and south expansion of savannah limits (Salgado-Labouriau et al., 1997; Souza et al., 2017). In addition, the high levels of cross-pollination are supported by a greater proportion of large bee pollination found in southern populations. Hand pollination of plants in the population from Jatai (pop 9, Figure 1) resulted in high fruit set, while natural pollination was low. We suspect that this may be due to the large numbers of honey bees (Apis mellifera), which were responsible for around 90% of the flower visits, as this species is often a poor pollinator for many plant species (Rech et al., 2018; Westerkamp, 1996).
A gradient of pollinator species richness and abundance reducing from south to north was previously reported for woody plants in Brazilian savannahs (Bridgewater et al., 2004). There is a suggestion that this pattern, which contrasts to the expected tendency of increasing diversity toward the Equator, could be related to climatic instability in the past (Werneck et al., 2012). Our results for pollinator richness also point out the importance of historical climate for the number of bee species (see Table S11). This reversed latitudinal pattern of diversity is also found in other invertebrate groups, such as ants (Vasconcelos et al., 2018). Therefore, perhaps the patterns observed for woody plant species diversity in Brazilian savannah could also be applicable to other groups of organisms, such as the ones that interact with plants (pollinators, seed dispersers and herbivores), as observed in some systems (Chen et al., 2019; Moreira et al., 2018; Schemske et al., 2009).
Although the absence of biotic pollination may reduce plant species distribution in isolated environments (Lord, 2015), higher cross-pollination in cooler and more seasonal places is in accordance with the pattern of global bee diversity, which peaks in subtropical areas with higher seasonality (Michener, 2007, Ollerton, 2017). Reinforcing the idea of the mediating role of bees to promote cross-pollination, both hand-self- and autogamous pollination showed no relationship with any of the variables related to the pollinators. Moreover, cross- and natural pollination were related to the proportion of large bees, and not to pollinator species richness and visitation frequency, indicating that not all visitors are equally good pollinators and not all proxies are equally realistic for pollinator quality (Popic et al., 2013; Sakamoto & Morinaga, 2013). Moreover, it was already experimentally shown that functional complementarity is far more important than the simple increment in pollinator species number (Fründ et al., 2013).
In conclusion, our results indicate that historical instability in climate has favored autogamy, while pollinators are currently modulating the level of cross-pollination. Although the direct impact of historical climate on pollinator communities should be examined in future studies, this association of historical climate instability to autogamy suggests a reproductive assurance strategy that may have benefitted the species during unstable conditions in the past (Rech et al., 2018). This strategy could be a key factor explaining why C. americana is one of the most conspicuous and widely distributed woody species in Neotropical savannahs (Ratter et al., 2003). We also corroborate here the already proposed effect of high functional diversity of pollinators buffering influences of climate dynamics, since places with more species of large-sized bees were more likely to remain functional when the environment changed and provide current higher levels of cross-pollination (Bartomeus et al., 2013). Although there are many aspects of pollination and historical climate relationships to be clarified, our results support the idea that historical climate dynamics are fundamental in determining pollination mode (level of autogamy), suggesting that plant–pollinator interactions may be even more sensitive to climate instability than species themselves (Bartomeus et al., 2013).
ACKNOWLEDGMENTS
ARR was supported by FAPESP (Proc. 2009/54591-0), CAPES, CNPq, Unicamp, and Santander Universities. BD was supported by the Carlsberg Foundation and thanks the Danish National Research Foundation for its support of the Center for Macroecology, Evolution and Climate. GJB is grateful for a postdoctoral fellowship awarded by CAPES/PNPD/UFVJM (process number 88887.352134/2019-00). JCS considers this work a contribution to his VILLUM Investigator project “Biodiversity Dynamics in a Changing World” funded by VILLUM FONDEN (grant 16549). JO is grateful to FAPESP for a visiting researcher grant (Proc. 2013/14442-5). We also thank a huge number of people that helped with fieldwork logistics when covering large distances in Brazil. This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001. MS thanks CNPq for the support (grant 302781/2016-1).
DISCLOSURE STATEMENTS
The corresponding author confirms on behalf of all authors that there have been no involvements that might raise the question of bias in the work reported or in the conclusions, implications, or opinions stated.
AUTHOR CONTRIBUTION
ARR, JO, and MS conceived the study. ARR and GB involved in data curation. ARR designed the methodology, involved in project administration, and wrote the original draft of the manuscript. BD, LRJ, BS, and J-CS involved in formal analysis. ARR, MS, and JO involved in funding acquisition. MS and JO supervised the study. ARR, JO, LRJ, GJB, BD, BS, and MS wrote, reviewed, and edited the manuscript.
Open Research
DATA AVAILABILITY STATEMENT
Data are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.xwdbrv1cd (Rech et al., 2020).




