The functional ecology of bat pollination in the African sausage tree Kigelia africana (Bignoniaceae)
Associate Editor: Tomás A. Carlo
Handling Editor: Gustavo Romero
Abstract
Plants often interact with a wide range of animal floral visitors that can vary in their pollination effectiveness. Flowers of the African sausage tree Kigelia africana are visited by bats and bush babies during the night and by birds during the day. We studied floral traits (phenophases, scent, color, and nectar chemistry) and the visitation frequency and pollination effectiveness of different flower visitors to determine whether K. africana is functionally specialized for bat pollination. We found that flower opening corresponds with bat activity, flowers emit scent dominated by aliphatic esters and alcohols, and that nectar is produced in copious amounts accessible to bats. Pollen deposition on stigmas was twenty-fold greater per visit by bats than it was per visit by birds, likely a result of the close morphological fit between snouts of bats and the flowers. However, bat visits appear to be rare at some sites and the delayed senescence of flowers that are open throughout the morning provides an opportunity for additional pollination by birds. We conclude that K. africana is primarily adapted for bat pollination, but is also able to exploit other animals for pollination.
1 INTRODUCTION
Specialization for pollination by particular groups of animals can result in convergent evolution in suites of floral traits (known as “pollination syndromes”) in unrelated plant species (Faegri & van der Pijl, 1979; Fenster et al., 2004; Johnson & Steiner, 2000; Rosas-Guerrero et al., 2014; Vogel, 1954). For example, flowers adapted to bat pollination or chiropterophily are usually characterized by large pale flowers that open at night (Amorim et al., 2013; Baum, 1995; Fleming et al., 2009), scent dominated by sulfur compounds (Pettersson et al., 2004), and copious amounts of nectar, often secreted in the evenings (Sazima et al., 1994). This type of floral specialization can be explained by the “most effective pollinator principle” which postulates that plants should adapt to the “most frequent and effective” pollinators within a particular region (Stebbins, 1970). However, the extent of functional specialization of flowers to particular animal groups and even the extent to which plants conform to recognizable pollination syndromes is still much debated.
The spatiotemporal availability of pollinators is highly variable, and Waser et al. (1996) argued that this should lead to the evolution of moderate to substantial generalization in pollination systems. Indeed, plants are often visited by a myriad of different functional pollinators groups and this can make it difficult to determine whether there has been functional specialization of flowers to a particular group. In these cases, data on the relative pollination importance of different visitor groups are essential to resolve the degree of specialization in a pollination system (Aigner, 2005; Diller et al., 2019; Johnson & Steiner, 2000; Wester & Johnson, 2017; Wilson, 1995). The importance of a given visitor group in a mutualism is a function of the number of visits and the amount of pollen deposited per visit (Schupp et al., 2017). In some cases, floral visitors may be abundant, yet consume floral resources without effective pollination and these interactions can be antagonistic at the expense of plant fitness (Hargreaves et al., 2009; Irwin, 2006; Irwin et al., 2010).
Although it may be expected from the theory of pollination syndromes that plants tend to be functionally specialized for pollination by a particular animal group, there is abundant evidence that many plants, even those that appear to conform to a particular floral syndrome, can be pollinated effectively by a wide range of animal groups (Ollerton, 1996; Waser et al., 1996). If there are few trade-offs in the deployment of floral traits, such that a trait that attracts one animal group does not diminish the attraction or pollination effectiveness of another animal group, then relatively generalized pollination systems should evolve easily (Aigner, 2001; Phillips et al., 2020). Support for generalist systems has been obtained from network studies incorporating all visitors instead of focusing on one single important visitor (Fang & Huang, 2013). However, one obvious shortcoming of network studies is that it is difficult to incorporate the relative importance of different visitors for pollination (Ballantyne et al., 2015; Rosas-Guerrero et al., 2014). Here, we provide an example of the use of camera traps to record the frequency of different visitors, together with experiments to record pollen deposition on a per-visit basis to test whether the functional visitor group predicted by the floral syndrome makes the most important contribution to pollination.
Pollination by vertebrates is well-developed in the tropics (Bawa, 1990) and plays an important role in transferring large quantities of pollen over long distances (Fleming et al., 2009; Fleming & Muchhala, 2008). Flowers of the African sausage tree Kigelia africana Benth. (Bignoniaceae) are considered to exhibit a bat pollination syndrome (see Fleming et al., 2009). Dwarf epauletted fruit bats (Micropterus pussilus) have been observed to visit the flowers in West Africa (Harris & Baker, 1958), but a recent study in the Kruger National Park in South Africa suggested that flowers of K. africana are pollinated mainly by opportunistic and specialist nectar-feeding birds (Namah et al., 2019). No bats were recorded on camera traps deployed by Namah et al. (2019), and very little pollination was recorded for flowers that were exposed to visitors during the night, but not during the day. Floral traits of K. africana such as spectral reflectance, flower-opening patterns, and floral scent have not been well understood. Attempts by Pettersson et al. (2004) to characterize the floral scent chemistry were largely unsuccessful. The contrasting results of these previous studies raise important questions about whether bats are effective pollinators of K. africana and whether floral traits are functionally specialized for this group of animals.
To achieve a better understanding of the functional ecology of pollination in K. africana flowers, we asked the following questions 1. What are the patterns of flower opening and do they coincide with bat activity? 2. Are the floral rewards consistent with the bat pollination syndrome? 3. Is the chemical composition of the scent emitted by flowers consistent with that reported for other bat-pollinated species? 4. What are the pollinators of Kigelia africana and which of these visitors are most frequent and effective?
2 METHODS
2.1 Study species and sites
Kigelia africana (Bignoniaceae) is the only member of its genus and is widespread throughout Africa from Chad to South Africa. The species often inhabits the banks of river courses. The trumpet-shaped, burgundy flowers appear between August and early October and are borne on long inflorescences with tough pedicels and a hard leathery calyx. The bizarre “sausage like” fruit can attain up to 500mm length and weigh up to 7kg and contain several hundred seeds. The species is known to be genetically self-incompatible and thus entirely reliant on flowers visitors for seed production (Namah et al., 2019).
We studied K. africana from early September to mid-October from 2014 to 2019 at two localities in the KwaZulu-Natal Province of South Africa. In Pietermaritzburg (lat: −29.62°, long: 30.40°), we studied 20 trees in the UKZN botanical garden and along roadsides, and at Ndumo (lat: −26.94°, long: 32.24°), we studied c. 15 trees growing alongside a river.
2.2 Flower-opening patterns
We used camera traps (Bushnell® 14 Mp NatureView Cam HD) configured in time-lapse mode to take still images (eight megapixels) of trees at intervals of 15 min to assess flower-opening times over 24-hr periods. We then tracked the development of 86 flowers from a total of 7,677 images recorded over 21 days in September-October 2018 and 2019. From the time-lapse information, we determined the timing of flower opening and senescence (floral abscission), and we calculated flowering duration (time from when a flower is fully open until it wilts and the flower abscises from the calyx).
2.3 Floral rewards and advertising traits
We took floral measurements from 10 K. africana trees in the vicinity of Pietermaritzburg. Nectar volume was measured using a 50μL capillary tube, and nectar concentration was subsequently measured using a Bellingham-Stanley refractometer (Bellingham and Stanley). Spectral reflectance for flowers from 10 individual trees in Pietermaritzburg was measured over the UV-visible range from 300 to 700nm using an Ocean Optics S2000 + spectrometer with a DT-mini light source and fiber optic probe (UV/VIS 400μm), which was held at 45° relative to the floral tissue sample. Furthermore, we measured flower depth as the straight-line distance from the base of the nectar chamber to the tip of the stigma, as this is a trait that is useful for explaining the fit between pollinator and plant morphology.
We collected 21 samples of floral scent from 15 K. africana trees in Pietermaritzburg. To assess diel effects on scent composition and emission rates, we collected 16 of the samples in the early evening and six samples during the morning. Flowers were enclosed in polyacetate bags (Kalle, Germany), and air from the bags was sucked through glass cartridges containing 1.5mg carbotrap® B (20–40 mesh; Sigma-Aldrich Co.) activated charcoal and 1.5 mg tenax® TA (60/80; Supelco™) using a PAS-500 personal air sampler (Spectrex). Sampling duration ranged from 30–180 min per sample. Samples were also taken from empty bags and used to identify (and exclude) background contaminants.
Volatiles were analyzed by coupled gas chromatography–mass spectrometry (GC-MS) using a Varian CP-3800 GC coupled to a Bruker 300 quadrupole MS. The GC was fitted with a Varian 1,079 PTV injector port modified with a ChromatoProbe thermal desorption device into which sample cartridges were placed. Volatiles samples were separated with either an Alltech EC-WAX SGE or SolGel Wax polar capillary column (30 m × 0.25 mm ID, 0.25 µm film thickness) or a Restek Rxi-5Sil MS nonpolar fused silica capillary column (30 m 9 0.32 mm ID, 0.25 μm film thickness). The GC temperature program was 40°C for 3 min, then increased to 240°C at 10°C/min, and then kept at 240°C for 12 min. The injector temperature program was 40°C for 2 min (with a 20:1 split), then increased to 200°C at 200°C/min and held for 2 min (splitless) for thermal desorption, and finally increased to 250°C at 200°C/min and held at 250°C (with a 1:100 split) for the remainder of the run. Helium (1 ml/min) was used as a carrier gas. Both mass spectrometers were operated in electron-impact ionization mode at 70 eV with the detector voltage continually adjusted by the Extended Dynamic Range (EDR) function. Compounds were identified using Varian MS Workstation (version 7.0) and NIST MS Search (version 2.3) with the NIST 2017 mass spectral library. Library identifications were confirmed by comparison of calculated linear (non-isothermal) n-alkane Kováts retention indices (Van den Dool & Kratz, 1963) with published values, and where possible, comparison of retention times and mass spectra with those of synthetic standards injected under identical conditions to samples. Absolute emission rates (in methyl benzoate equivalents) were calculated from comparison to peak areas resulting from the injection of known amounts of methyl benzoate.
We compared day versus night rates of floral scent emission using generalized linear models with a gamma distribution and log link function. We used a single average value for each tree in cases where more than one sample was taken per tree. To assess patterns of similarity in volatile composition among samples taken during the day versus at night, we square-root transformed the percentages that each compound contributed to the overall scent and calculated a similarity matrix among samples using the Bray–Curtis method. We then compared the two sets of scent profiles using the ANOSIM permutation test implemented in Primer 6.1.6.
2.4 Floral visitors
At both sites, we recorded animal visitors to Kigelia flowers using direct observations and videos obtained using motion-activated camera traps (Bushnell® 14 Mp NatureView Cam HD). We observed trees in Pietermaritzburg for a total of 22 mornings and evenings between 2014 and 2019. A single camera was run over 18 nights from 19:00 to 05:00 and over four days from 06:00 to 18:00. We observed trees at Ndumo around the clock over four days in 2019 where we deployed five cameras. From videos, we extracted the behavior and timing of activity of various visitors to flowers. These included inspections, legitimate visits (using the flower entrance), nectar robbing (piercing the side of the corolla), and nectar consumption from the calyx of wilted flowers.
2.5 Single-visit pollen deposition experiments
To compare the effectiveness of bats versus birds as pollinators of K. africana, we conducted single-visit pollination experiments over two seasons in 2018 and 2019 at the UKZN botanical garden, Pietermaritzburg. Flowers in late bud which we predicted to open during the same evening were bagged to prevent pollinators from visiting the flowers. Once flowers were open, we removed the bags to expose the flowers to a single visit by a foraging bat or bird (flowers enclosed throughout the duration of the evening, but removing bags from 05:00 in the morning and exposing flowers to birds) and then collected stigmas for later pollen counting. Since it was difficult to obtain a sufficient sample of directly observed single visits, we also used motion-triggered cameras to monitor virgin flowers. At 5a.m. the following morning before bird activity commenced, we reviewed the footage and collected stigmas from flowers that received a single bat visit (flowers that received more than one visit were excluded from analysis). The number of conspecific pollen grains on stigmas was counted using a dissecting microscope. We obtained a sample of 42 flowers that received single visits by bats or birds—17 visits by epauletted fruit bats (Epomophorus wahlbergi), 15 visits by Cape white eyes (Zosterops capensis), two visits by dark-capped bulbul's (Pycnonotus tricolor), and eight visits by amethyst sunbirds (Chalcomitra amethystina)—and also a further eight unvisited bagged flowers as control samples.
Pollen deposition from single visits was analyzed using a generalized linear model with a negative binomial error distribution and log link function. For comparisons between functional pollinator groups (birds and bats) and eight unvisited controls, we treated functional pollinator type as a fixed factor and nested species within each functional pollinator group. All post hoc comparisons involved the Sequential-Šidák procedure. All statistical analysis was implemented in SPSS version 25 (IBM Corp.) unless otherwise stated.
3 RESULTS
3.1 Flower-opening patterns
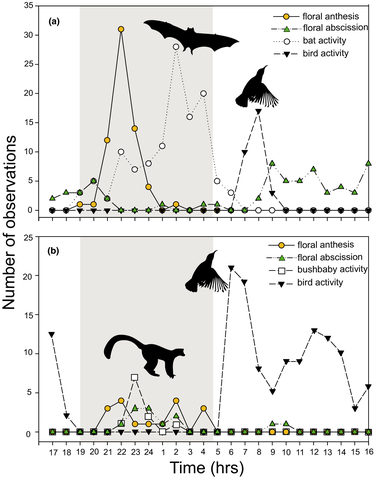
The inflorescences have long pedicels and are directed downwards. The uppermost flowers open first and a maximum of three flowers are open at a time. In Pietermaritzburg, flowers opened between 1900 hrs and 0300 hrs, with a peak at c. 2200 hrs (Figure 1a). At Ndumo, we found a similar pattern, though the sample size was small. Here, flowers opened between 2000 hrs and 0400 hrs, and the peak of flower opening was just before midnight. Flowers remained open for c. 18 hrs (median) at both localities and senesced in the late morning to late afternoon (Figure 1b).
3.2 Floral rewards and advertising traits
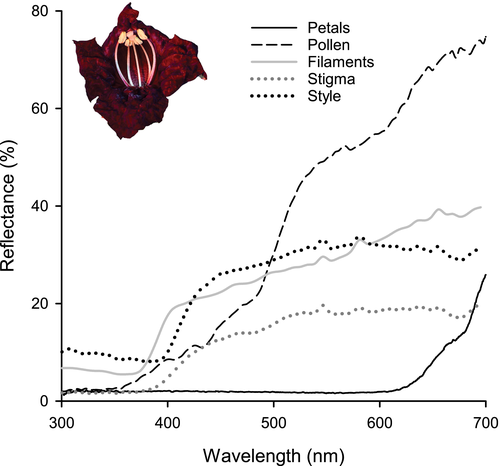
The mean (± se) depth of flowers was 59.6 ± 0.01 mm. The nectar volume was 53 ± 6.88 μl, and the nectar sugar concentration was 27.51 ± 11.27%. The corolla is dark maroon with a peak in reflectance between 500 to 600 nm (Figure 2). The filaments, pollen, style, and stigma are creamy white and thus form a striped pattern that contrasts with the corolla (Figure 3a).

The floral scent of K. africana was found to be dominated by aliphatic esters and alcohols (Table 1). We found no significant difference in the mean amount of scent emitted per flower per hour during the night versus during the following morning (35.2 ± 16.4 µg versus 7.78 ± 5.1 µg, χ2 = 2.79, p = 0.119) and also no difference in the chemical composition of scent between night and day samples (R = 0.14, p = 0.82).
| Compound class | Compound | Estimated Kovats | ID criteria | Relative amount (%) | Occurrence (n = 21 samples) |
|---|---|---|---|---|---|
| Aliphatic acids | Acetic acid | 1479 | B | 0.07 ± 0.05 | 3 |
| Aliphatic alcohols | Hexan−1-ol | 1363 | C | 0.05 ± 0.04 | 2 |
| (E)-Hex−3-en−1-ol | 1375 | D | 0.24 ± 0.12 | 4 | |
| (Z)-Hex−3-en−1-ol | 1397 | D | 2.08 ± 1.17 | 9 | |
| (E)-Hex−2-en−1-ol | 1416 | B | 1.94 ± 1.27 | 5 | |
| Oct−1-en−3-ol | 1460 | B | 0.76 ± 0.75 | 2 | |
| 1-Octanol | 1560 | D | 2.16 ± 0.79 | 11 | |
| 1-nonanol | 1660 | B | 0.13 ± 0.08 | 5 | |
| Aliphatic aldehydes | (E)-Hex−2-enal | 1239 | B | 0.68 ± 0.47 | 2 |
| Aliphatic esters | Isoamyl acetate | 1160 | B | 0.04 ± 0.04 | 1 |
| Amyl acetate | 1196 | D | 3.81 ± 0.67 | 18 | |
| Prenyl acetate | 1246 | B | 0.23 ± 0.14 | 3 | |
| Hexyl acetate | 1291 | C | 5.56 ± 0.92 | 20 | |
| (Z)-Hex−3-en−1-yl acetate | 1334 | D | 58.25 ± 3.22 | 21 | |
| (E)-Hex−2-en−1-yl acetate | 1352 | D | 17.17 ± 2.27 | 21 | |
| Hexyl formate | 1376 | B | 0.24 ± 0.12 | 4 | |
| Heptyl acetate | 1389 | B | 0.44 ± 0.16 | 9 | |
| Octyl acetate | 1434 | B | 0.02 ± 0.02 | 1 | |
| Monoterpenes | (Z)-Ocimene | 1257 | D | 1.22 ± 0.49 | 8 |
| (E)-Ocimene | 1275 | D | 1.86 ± 0.67 | 11 | |
| Camphor | 1543 | B | 0.02 ± 0.01 | 3 | |
| Linalool | 1556 | D | 0.28 ± 0.09 | 11 | |
| Unknowns | 61,101,44,47,35,46,54,63,58,57a | 1724 | 0.04 ± 0.02 | 3 | |
| 74,43,61,41,47,75,45,59,46,76 | 0.99 ± 0.40 | 10 | |||
| 43,80,79,81,41,39,77,65,67,60 | 1436 | 0.05 ± 0.02 | 4 | ||
| 55,70,69,56,97,57,41,43,68,71 | 1628 | 0.83 ± 0.26 | 13 | ||
| 57,58,44,43,30,45,31,55,39,42, | 2178 | 0.13 ± 0.12 | 5 | ||
| Unknown sesquiterpenes | 204b, 119,105,161,93,91,92,120,81,117,77 | 1545 | 0.56 ± 0.38 | 3 | |
| 204b,91,105,79,81,77,161,41,93,55,119 | 1738 | 0.16 ± 0.12 | 2 |
Note
- B = library match confirmed with comparison of retention index to published values, C = library match, retention time match and confirmation from injection of synthetic standards, D = library match and retention time match on two columns with different phases.
- a Good library match for 3-(Methylthio)propanenitrile.
- b Molecular ion.
3.3 Floral visitors
Using motion-activated cameras, we obtained 278 videos with footage of visitors. In the UKZN botanical garden, we recorded a total of 129 visitors, including 100 epauletted fruit bats, Epomophorus wahlbergi (Video S1), 18 Cape white eyes (Zosterops capensis), five spectacled weavers (Ploceus ocularis) and five dark-capped bulbul's (Pycnonotus tricolor) and one honeybee (Apis mellifera scuttelata). In addition, we estimate that we directly observed more than 300 visits to flowers by epauletted fruit bats (Epomophorus wahlbergi) during our evening observations in the botanical garden (Figure 3). At Ndumo, we recorded a total of 149 visitors using camera traps, namely a single Cape white eye (Zosterops capensis), seven spectacled weavers (Ploceus ocularis), 29 dark-capped bulbul's (Pycnonotus tricolor), 15 collared sunbirds (Hedydipna collaris), 78 grey Sunbirds (Cyanomitra veroxii), 11 thick-tailed bush babies’ (Otolemur crassicaudatus), four honeybees (Apis mellifera scuttelata), two unidentified dormouse individuals, a single unidentified hawkmoth and a single unidentified rodent.
From these observations, we recorded different types of behaviors from visitors (Figure 4). Of the 100 visits by bats captured on video, 64 individuals were filmed while flying about and inspecting the flowers and 36 while landing on inflorescences, of which 32 clearly inserted their entire snout into the flower while hanging onto the pedicels (Figure 4, Video S1). In addition, we directly observed many hundreds of additional visits by bats to the flowers and photographed many with conspicuous pollen loads (Figure 3d). Of the eleven videos of thick-tailed bush babies, three record these animals sitting on the branches of a tree, two inspecting the flowers, and six probing flowers (Figure 4c, Video S2). Other visitors such as hawkmoths did not contact the reproductive parts of the flower.
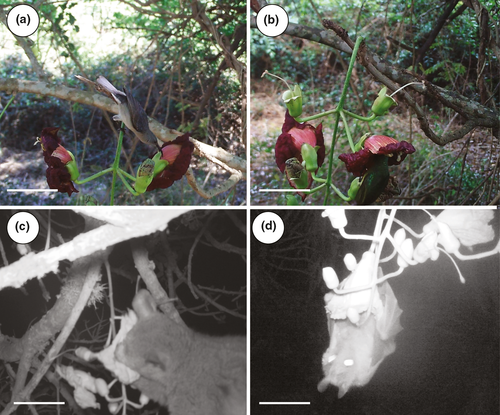
Of the total of 149 avian flower visitors recorded on video at Ndumo, 13 consumed nectar from the nectar chamber after the flower had fallen from the calyx, 40 inspected the flowers either by hovering or perching next to the flowers, 15 robbed nectar by piercing the corolla (Figure 4, Video S3), and the remaining 81 probed flowers legitimately via the entrance (Figure 4, video S4).
Activity times of flower-visiting animals corresponded with patterns of flower opening. At Pietermaritzburg, fruit bats visited flowers from 1900 hrs until 0600 hrs, with a peak in activity at 0200 hrs (Figure 1a). At Ndumo, thick-tailed bush babies visited flowers between 2200 hrs and 2400 hrs, with a peak activity at 2300 hrs (Figure 1b). Flowers were also visited in the morning by opportunistic and generalist birds at both localities. Birds often fed on nectar that remained in the calyx chamber even after the petals had fallen to the ground. At Pietermaritzburg, birds fed on nectar between 0700 hrs and 1000 hrs with a peak at 0900 hrs (Figure 1a), whereas at Ndumo, this occurred throughout the day between 0600 hrs and 1800 hrs, with a peak just after dawn at 0600 hrs (Figure 1b).
3.4 Single-visit pollen deposition experiments
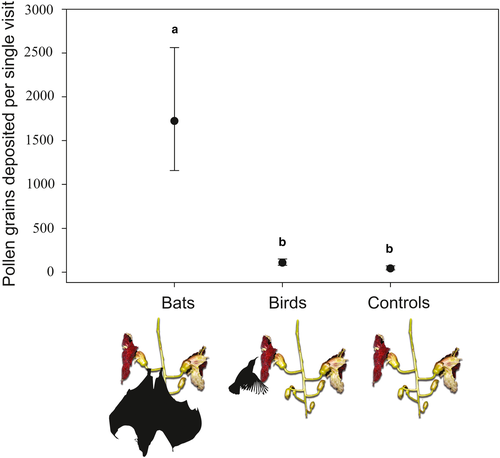
We found significant differences in pollen loads on stigmas among the treatment groups in the pollen deposition experiment (χ2 = 26.41, p < 0.001; Figure 5). Bats deposited twenty-fold more pollen grains on stigmas per visit than did birds (post hoc comparison: p < 0.01). Flowers visited once by birds did not differ from bagged control flowers in terms of the number of pollen grains deposited on the stigma (post hoc comparison: p = 0.30).
4 DISCUSSION
The evidence in this study supports the findings of Harris and Baker (1958), based on their anecdotal observations, that K. africana is primarily adapted to pollination by bats. Kigelia africana has large trumpet-shaped flowers with wide openings that accommodate the relatively broad snout of bats. Furthermore, the flowers produce copious amounts of nectar with intermediate concentrations and the scent is dominated by compounds similar to those emitted by other bat-pollinated plants. We also found that flower opening at both study localities occurs in the evenings and coincides with the start of flight activity of bats (Figure 1). The most convincing evidence for bat pollination was obtained from the single-visit experiments (Figure 5). We found that bats deposited 20-fold more pollen grains on stigmas per single visit than do birds, thus confirming that bats are the most effective pollinators of K. africana.
In addition to bats, we found that flowers of K. africana are frequently visited by birds and opportunistic non-flying mammals. (Figure 3e, Figure 4a-c). These findings are similar to those of Namah et al. (2019) who identified 12 different bird species, consisting of both opportunistic and specialist nectar feeders, and three opportunistic mammal species as visitors to K. africana in northern South Africa. Only two of the pollinators documented in their study were recorded by us. These included thick-tailed bush babies (Otolemur crassicaudatus) and dark-capped bulbuls (Pycnonotus tricolor).
Despite fruit bats being frequent visitors to flowers of K. africana in West Africa (Harris & Baker, 1958) and at our Pietermaritzburg site (Figure 3), Namah et al. (2019) failed to record any bats visiting the flowers and concluded that birds make the primary contribution to fruit production. Similarly, we did not observe bats visiting flowers at our Ndumo site and we suspected that nearby fruiting fig trees were more profitable for foraging fruit bats, but this remains to be confirmed. Given that the trees in the botanical garden in Pietermaritzburg were grown from locally sourced seed and are pollinated effectively by bats (Figure 3 and Figure 4), we think it unlikely that K. africana in southern Africa as a whole is adapted primarily for bird pollination. Rather, it seems that in places where bats are rare or have profitable alternative food sources, pollination of K. africana is effected mainly by birds. In such environments, floral adaptations for more effective bird pollination may be favored (Namah et al., 2019), though evidence from South American studies suggests that transitions from bat to bird pollination are relatively rare, perhaps on account of the high degree of floral specialization required for bat pollination (Tripp & Manos, 2008).
Unlike South America where there are specialist flower-feeding bats (Fleming & Muchhala, 2008), bat pollination systems in Africa involve fruit-feeding bats which do not have an obligate dependence on flowers. Consequently, it is thought that the low diversity of bat-pollinated plants on the African continent, which has less than 1% of all bat-pollinated angiosperms (Dobat & Peikert-Holle, 1985; Grünmeier, 1993; Pettersson et al., 2004), is largely the result of the generalist feeding behaviors of African bat pollinators compared to nectar specialist new world bats (Fleming & Muchhala, 2008).
Floral traits of K. africana are strongly suggestive of adaptation primarily for bat pollination. The flowers are suspended from long pedicels (Figure 1a) which is strongly convergent with the morphology of other species with bat-pollinated flowers or bat-dispersed fruits. In addition, flower opening coincides with nocturnal pollinator activity (Figure 1). Nocturnal anthesis is common in plants adapted for pollination by bats or which have bimodal bat and bird pollination systems (Aguilar-Rodríguez, Krömer, García-Franco, & MacSwiney, 2015; Buzato, Sazima, & Sazima, 1994; Machado, Sazima, & Sazima, 1998; Muchhala et al., 2008).
Flower senescence (when the corolla falls to the ground) tended to occur in the early to late afternoon (Figure 1), which provides a window of opportunity for birds to visit and pollinate flowers that were not previously visited by bats. Birds also consume nectar remaining in the nectar chamber after floral abscission, but this behavior is very unlikely to lead to pollination. Bird visitation to intact flowers could be advantageous when bats are absent or are unreliable visitors due to competition with plants that provide alternative food sources. Despite the abundance of bird visitors at both localities, floral anthesis at dusk should still be favored in K. africana if pollen transfer efficiency by bats is higher compared to birds (Miyake & Yahara, 1999). The dark maroon color of the flowers (Figure 2) is unusual among bat-pollinated plants and may reflect selection for traits that render flowers conspicuous to birds (Rodriguez-Girones & Santamaria, 2004). Lagomarsino and Muchhala (2019) suggested that the red flowers of Centropogon mandonis may be a secondary adaptation to pollination by birds, as bats and birds are equally important for pollination of this species, in contrast to the pale-flowered sister taxa C. brittonianus and C. incanus that are pollinated almost exclusively by bats. An alternative explanation is that the dark red flowers provide a background that contrast against the creamy white stamens and styles which may act as a visual “nectar guide” pattern for approaching bat visitors with dichromatic vision (Winter et al., 2003) (Figure 2 and Figure 3a).
The emission of scent and the large volumes of nectar in flowers of K. africana are consistent with other species known to be bat-pollinated (Amorim et al., 2013; Buzato et al., 1994; Sazima, Buzato, & Sazima, 1999; Sazima et al., 1994). Pettersson et al. (2004) described the floral scent of K. africana as “very weak and barely detectable to the human nose,” although in our experience mass-flowering K. africana trees can be detected by human olfaction at distances of about 50 meters. Pettersson et al. (2004) identified only traces of volatiles in their samples and were thus unable to properly characterize the chemical composition of the scent. This was probably because they used a solvent elution method of extracting scent which is much less sensitive than the direct thermal desorption method used here. The dominance of esters and alcohols in the scent profile of K. africana (Table 1) makes sense given that these compound classes feature prominently in the lists of compounds known to attract mammals, including bats (Borges et al., 2008; Hodgkison et al., 2007, 2013; Laska, 1990). Laska (1990) found that the short-tailed fruit bat, Carollia perspicillata, was sensitive to many of the compounds we detected in the scent of K. africana, including n-amyl acetate and isoamyl acetate. Although sulfur compounds are considered to play a role in attraction of bats (von Helversen et al., 2000), we could not firmly identify any sulfur containing compounds in the scent. Several sulfur compounds were good library matches for some of the unknown compounds, but could not be identified with certainty due to absence of Kovats values for these compounds for the polar phase columns that we used in this study. While some African bat-pollinated plants, notably the baobab Adansonia digitata, produce sulfur compounds in floral scent, sulfur compounds are not as prominent in the floral scent of Old World plants pollinated by fruit bats as they are in the floral scent of New World plants pollinated by phyllostomid bats (Pettersson et al., 2004).
Another important line of evidence for adaptation of K. africana flowers for bat pollination is demonstrated in the morphological fit between flower and pollinator morphology (Video S1). The depth of flowers, 59.6 ± 0.01 mm (mean ± SD) is comparable to the distance from the back of the head to the tip of the snout (the cyndylo-incisive length) of the fruit bat Epomorphorus wahlbergi,: male: 47.1 ± 1.9 mm (mean ± SD); female: 53.5 ± 1.1 mm (mean ± SD) (Taylor & Monadjem, 2008) and to a lesser extent the snout of the thick-tailed bush baby Otolemur crassicuadatus: 33.6 ± 1.7 mm (mean ± SD) (Kieser, 1990) (Video S2). By contrast, the narrow bills of opportunistic birds and specialist sunbirds fit the flowers poorly (Video S4). This match between bat skull morphology and Kigelia flower morphology could explain the results of the single-visit experiments which showed that bats transfer 20-fold more pollen per visit than do birds. However, birds can still be important pollinators at sites where bats are absent as visitors (Namah et al., 2019). Thick-tailed bush babies were too infrequent to conduct single-visit experiments for comparison, but we suspect that these non-flying mammals would be poor agents of cross-pollination given that trees can be spaced several hundred meters, even kilometers apart in natural settings. In a study exploring the boundaries between bat and bird pollination syndromes in Burmeistera cyclostigmata and B. tenuiflora, bats also deposited more pollen than did birds (Muchhala, 2003).
In conclusion, several lines of evidence—patterns of flower opening, nectar properties, scent profile, morphology, visitor observations, and single-visit experiments suggest that K. africana is primarily adapted for pollination by bats. Nevertheless, the extended floral anthesis means that birds can be important pollinators at some sites (Namah et al., 2019), despite their ineffectiveness on a per-visit basis (Figure 5). It is thus likely that there are only weak trade-offs between bat and bird pollination in K. africana (Aigner, 2001; Lagomarsino et al., 2017; Lagomarsino & Muchhala, 2019; Machado et al., 1998; Muchhala, 2003).
ACKNOWLEDGMENTS
This manuscript is dedicated to the memory of our colleague Keeveshnee Govender who passed away during the preparation of this paper. ELN was funded by a Claude Leon grant, and SDJ by grant 46372 from the National Research Foundation.
Open Research
DATA AVAILABILITY STATEMENT
Data available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.05qfttf1v (Newman et al., 2020).




