Interactions between stoneworts (Charales) and waterbirds
ABSTRACT
Stoneworts (Charales) are green algae that represent an important food resource for many waterbird species in Europe and elsewhere. Browsing avian herbivores (e.g. swan, goose, duck and coot species) consume Charales plant vegetative parts, by head-dipping, up-ending or diving. A lower fibre content and longer growing season may make Charales as attractive to such herbivores as sympatric submerged higher plant species in some circumstances. Charales respond to environmental stress (e.g. drought) by producing abundant diaspores, in the form of oospores (sexual) and bulbils (asexual), both rich in starch, generating abundant food for waterbirds at critical stages in their annual migratory cycles. Waterbirds feed on these by diving (e.g. common pochard Aythya ferina and red-crested pochard Netta rufina) or by filtering from the water column (e.g. dabbling duck species), ensuring dispersal of sexually produced and vegetative diaspores locally (because of predator swamping) and remotely (through endo- and ectozoochorous dispersal by long-distance migratory waterbirds). Greater invertebrate density and diversity associated with Charales canopies enhances their attractiveness over other submerged macrophyte beds to diving predators [e.g. tufted duck Aythya fuligula, common pochard and Eurasian coot Fulica atra (hereafter coot)]. Fish fry preying on these invertebrates use such vegetation as predator cover, in turn providing prey for avian piscivores such as grebes and cormorants. Abundant Charales contribute to maintaining a transparent water column due to canopy density, nutrient effects, dampening of sedimentation/remobilisation of suspended matter and nutrients and allelopathic effects on other plants (especially phytoplankton). Shallow, relatively eutrophic waters can flip between clear-water high-biodiversity (where Charales thrive) and turbid species-poor depauperate stable states (lacking Charales). Shifts between turbid conditions and rich submerged Charales beds have profound elevating effects on aquatic diversity, to which waterbirds show rapid aggregative responses, making them ideal indicator species of ecological change; in the case of Charales specialists (such as red-crested and common pochard), indicators of the presence and abundance of these plants. Large-bodied colonial nesting birds (e.g. cormorants, gulls, heron and egrets) aggregating along lake shores contribute high N and P loadings to water bodies sensitive to such external and internal inputs and can cause local eutrophication and potential loss of Charales. Despite variation from complete seasonal removal of Charales biomass to undetectable grazing effects by herbivorous birds, evidence suggests little effect of avian grazing on biomass accumulation or the stability of community composition (under otherwise stable conditions), but we urge more research on this under-researched topic. We also lack investigations of the relative foraging profitability of different Charales organs to waterbirds and the degree of viability of gyrogonites (fertilised and calcified oospores), vegetative bulbils and plant fragments after passage through the guts of waterbirds. We especially need to understand better how much the carbonate armour of these organs affects their viability/dispersal via waterbirds and urge more research on these neglected plants and their relationships and interactions with other organisms in the aquatic biota.
I. INTRODUCTION
Charophytes are ancient multicellular green algae that number ca. three hundred species worldwide, divided between seven genera Chara, Nitella, Nitellopsis, Tolypella, Sphaerochara, Lamprothamnium and Lychnothamnus based on the split between Tolypella and Sphaerochara following Soulié-Märsche (1989). Here, we use the current recognised taxonomy and nomenclature provided by Prof. Hendrik Schubert (in preparation) based upon Arbeitsgruppe Characeen Deutschlands (2016), with the scientific species names and authorities listed in the online Supporting Information, Appendix S1. Of the most common and well-studied species, most belong to the genera Chara and Nitella, although Nitellopsis obtusa ranks among the best studied of charophyte species, partly because of its success as an invasive species in North America (Larkin et al., 2018). The extant living species are all placed within the order Charales, and we use this collective descriptor throughout this review. Charales thrive mainly in fresh and brackish water, occasionally in hypersaline waters to 58 g l−1 (García & Chivas, 2006). Stoneworts can be found in all continents, including Arctic regions (Chemeris et al., 2020; Langangen et al., 2020) and Antarctica (Schubert et al., 2018). They occur in very shallow water (in the absence of wave action, to which some forms seem highly vulnerable (Torn et al., 2015; van Zuidam & Peeters, 2015) down to 65 m where light penetration allows [e.g. in Lake Tahoe, California (Spence, 1982); see also Schwarz, de Winton & Hawes, 2002]. Most Charales prefer clear nutrient-poor ‘hard’ water although often rich in carbonates (Sand-Jensen et al., 2018). Nutrient concentrations above a certain threshold are known to cause the disappearance of many forms of submerged vegetation (González-Sagrario et al., 2005; Beklioglu & Tan, 2008), particularly Charales, which are known to be among the most sensitive of aquatic plants to nutrient enrichment (Blindow, 1992a). As a result, Charales are useful indicators of the quality of hard water oligo- to mesotrophic freshwater habitats (e.g. Søndergaard et al., 2010). Despite this, many Nitella species show a broad ecological amplitude and may live in relatively soft waters, so can be used as indicator species in relation to changes in water quality in more neutral and low water pH environments (e.g. Olsen, 1944; Prescott, 1962; Bociąg, Rekowska & Banás, 2011).
The Charales have a highly distinctive plant form (thallus), distinguishable from other macrophytes (which we here define as other algae and water plants), characteristically consisting of long, cylindrical ‘stems’ composed of internodal cells and groups of small, isodiametric or flat nodal cells from which distinctive whorls of lateral cells emerge as side branches (Wood & Imahori, 1965). The growth of the main axis and the side branches is indeterminate and occurs by the ordered division of a dome-shaped apical cell, which sequentially produces internodal and nodal cells. The growth of lateral cells is determinate; indeed, the number, shape, and spatial arrangement of branchlets, bract cells, stipulodes and cortex cells (i.e. the cell types derived from the nodal cells) provide important criteria for identifying species. Charales anchor themselves to the sediment by delicate rhizoids and because their thalli can range from several centimetres to 2 m in length, can come to dominate the plant cover on the bottoms of brackish lagoons, freshwater lakes, slowly flowing rivers, and streams (Wehr, Sheath & Kociolek, 2015).
The English collective common noun ‘stoneworts’ comes from the calcium (and to a lesser extent magnesium) carbonate encrustations found on the surface of many of the species growing in alkaline conditions, which several species favour. Charales can use bicarbonates as well as carbon dioxide for photosynthesis, depositing generated calcium carbonate onto thalli surfaces as highly distinctive encrustations (Pentacost, 1984; Raven, Smith & Walker, 1986; Wetzel, 2001; Martin et al., 2003). This process has a decalcifying effect on surrounding water and, by precipitation of inorganic phosphorus incorporates this element and carbon into sediments, rendering them relatively inaccessible to biological activity (Murphy, Hall & Yesaki, 1983; Anderson & Ring, 1999; Rodrigo, Alonso-Guillén & Soulié-Märsche, 2009). There remains much to be understood about the biochemistry of these mineral encrustations, but they clearly depend on species, age and the ionic concentrations of the environment (Herbst & Schubert, 2018).
Haploid thalli reproduce sexually by producing oogonia that are fertilised by spiral-shaped male gametes with two long flagella of equal length. Only one species, Chara canescens, has been reported to reproduce parthenogenically, forming oospores in the absence of male gametangia (Braun, 1857). In most cases, the oospore develops into a ripened stage resulting from the calcification of the spiral cells surrounding the oospore, with characteristic spiral ridges depending on the species and environmental conditions (Olsen, 1944; Soulié-Märsche & García, 2015). These calcified fructifications, known as gyrogonites, allow the survival of oospores in wet or dry sediment for many years or even decades after release, if conditions are unfavourable for growth. These protective structures have also contributed to the remarkably long fossil record of the evolution of the Charophytes (Olsen, 1944; Soulié-Märsche & García, 2015). Although tough and protected for their long-term survival of periodic drought, these gyrogonites still constitute energetically attractive food items for those consumers able to dissolve their calcified husks to access the energy-rich contents. Germinating oospores undergo meiosis and the surviving cell develops into gravitropic rhizoids, which anchor the thallus in the substratum, and a delicate protonema, which grows upwards to develop into the ultimate multicellular thallus.
In many Charales species, sexual reproduction does not appear to play an important role and asexual reproduction is much more common (Casanova & Brock, 1999; Skurzyński & Bociąg, 2011). Vegetative propagation occurs readily by means of detached thallus fragments [e.g. in Chara subspinosa (Skurzyński & Bociąg, 2011) and Chara vulgaris (Rodrigo & Carabal, 2020)] or by node modifications called ‘bulbils’, which may survive adverse environmental conditions in the sediment. Note that throughout this review, we use the terms ‘oospores’ and ‘oogonia’ as they are used in the cited literature, but use the collective term ‘diaspores’ to cover all forms of sexual and asexual Charales propagules where these are not defined.
The thalli of Charales can be remarkably strong compared to angiosperm macrophytes, given that they have no specialised strengthening tissue, such as the cellulose fibres of higher plants (Schutten, Dainty & Davy, 2005). As a result, Charales often form a very dense thick matted growth in the water column, which dampens wave action, stabilises the sediment and increases water column transparency (Moore, 1986). For this reason, they can be important constituents of aquatic systems as primary producers (often the dominant source of aquatic photosynthesis; Porter, 2007) and by their nature, density, extent and biomass potentially as important sources of substrate, habitat and abundant food for other organisms, including refuges for prey species from predators (Hesselschwerdt & Wantzen, 2018).
From an avian point of view, Charales beds provide valuable habitat for epiphytic algae and invertebrates (Hawes & Schwarz, 1996; James et al., 1998; van den Berg et al., 1998a; van Donk & van de Bund, 2002), as well as structural refuges for zooplankton (Kuczyńska-Kippen, 2007) and juvenile vertebrates (e.g. fish and amphibia; Schmieder, Werner & Bauer, 2006; Pípalová, 2006; Dibble & Kovalenko, 2009). They are also the source of food for many consumers, such as beetles, amphipods and molluscs (Proctor, 1999; Elger et al., 2004) as well as fish (Lake et al., 2002). By their subaquatic nature, Charales are predominantly accessible as food and as an environment that provides a source of other associated prey to waterbird species that exploit such habitats (e.g. Schmieder et al., 2006). It is the association between these feeding waterbirds and the Charales in Europe (where we have the greatest experience) that we wish to describe in this review but also illustrate examples from elsewhere. In a Geographical Information System analysis, we found no fewer than 450 Important Bird Areas in Europe (BirdLife International, 2022) that were congruent with the habitat Oligotrophic to mesotrophic waterbodies with Characeae based on the data in Janssen et al. (2016). The European Red List of Habitats classifies this habitat type as ‘Vulnerable’, criteria A1, C/D1 (Janssen et al., 2016). Hence, it would appear that improved knowledge about the relationships between birds and Charales is necessary to enhance the conservation of both.
Herein we use a literature review to attempt to establish (i) the nutritional and energetic value of Charales to herbivorous waterbirds relative to other sympatric angiosperm macrophytes to try to understand their apparent disproportional value to supporting local waterbird densities. We then attempt to (ii) document which waterbird species consume which parts of Charales, to establish (iii) the range and nature of waterbird species showing aggregative responses to Charales-dominated systems in Europe and the relationships between the species involved and their specific feeding ecologies. From a nature conservation perspective, we look for (iv) examples of how local restoration of aquatic ecosystems has resulted in the re-establishment of local Charales-dominated communities. Given that locally abundant migratory grazing waterbirds can apparently rapidly remove all above-ground biomass of Charales in autumn, we also review (v) the documented impacts of waterbirds on the dynamics of these important food plants in aquatic systems. Finally, we consider (vi) the value of waterbirds as dispersive agents to the Charales, given the range of studies that apparently confirm endozoochorous dispersal of these iconic algal species by often long-distance migratory waterbirds.
II. METHODS
We searched the peer-reviewed literature in Web of Science using the search term ‘Chara/Charales/Charophyte and birds’. To check the effectiveness of this original search, we subsequently used the search criteria subsets ‘Chara and Pochard’, ‘Chara and Teal’ and ‘Chara and swan’, which generated no new references, giving us confidence in the original search terms in covering our area of interest. The initial search on 20 June 2022 generated a core list of 4876 references that forms the basis of this review, the vast majority of which contributed no useful, relevant information. The body texts and reference lists of the relevant articles were checked for further pertinent studies, and these were then, in turn, checked in the same way until no further relevant papers were found. Inevitably, this resulted in a majority of peer-reviewed studies, so we concede that this approach likely and regrettably excluded highly relevant reports and other ‘grey literature’.
III. TOPICAL REVIEW
(1) Sources of food for waterbirds provided by Charales
Charales plant bodies are differentiated into nodes and internodes (McCourt et al., 2017). Nodes bear regular whorls of branches and because of the very dense nature of the green biomass presented in this way within the water column, these vegetative structures form one major source of food biomass, especially for herbivorous grazing birds (e.g. Noordhuis, van der Molen & van den Berg, 2002). Such green material is attractive to waterbirds due to several intrinsic properties of the Charales. First, these species (especially those forming dense vegetation) tend to have a comparatively long growing season (Pereyra-Ramos, 1981; Nichols, Schloesser & Geis, 1985), although some species, such as Sphaerochara intricata and Nitella capillaris may be spring annuals with comparatively short growing seasons, while others may be restricted by summer desiccation. Many other Charales species continue to grow well into the autumn where this is possible, retaining green biomass into winter in some situations, whereas the above-ground parts of other sympatric flowering plant macrophytes may have disappeared by the end of August (van Wijk, 1989; van Dijk & van Vierssen, 1991). Secondly, although the surface calcium carbonate deposition results in relatively low overall caloric value [although this can be up to 12 kJ g−1 dry mass (DW); Palma-Silva, Albertoni & Esteves, 2004], the low fibre content [less than half that of other vascular submerged macrophytes growing sympatrically in British gravel pits (Fox et al., 1994; A. D. Fox, unpublished data)] makes Chara potentially relatively easy to digest by herbivorous waterbirds compared to other plant material richer in structural carbohydrates (assuming the avian gut can dissolve the calcium carbonate without high metabolic costs). The higher content of cellulose compounds in the structural tissues of higher plants are not just hard to digest but inhibit digestion in the relatively simple avian gut, characterised by high throughput rates and rapid absorption of accessible soluble proteins and carbohydrates (e.g. Prop & Vulink, 1993). In Danish brackish lakes, Chara spp. had half the structural fibre (27–36% neutral detergent fibre of ash-free dry mass) and twice the non-structural soluble (i.e. potentially easily digested) carbohydrates of other submerged macrophytes in the same systems (Ruppia cirrhosa and R. maritima; Holm, 2002). Dry mass and calcium carbonate encrustation varies among Chara species and are related to water chemistry, water depth and light penetration (Pukacz et al., 2016; Sviben et al., 2018). Intriguingly, carbonate encrustations of Charales of the same species are significantly greater in freshwater than brackish systems even when calcium concentrations and pH are similar in their environment (Herbst & Schubert, 2018), a fact that potentially could affect their attractiveness as waterbird forage in different situations. Because the dissolution of calcium carbonate during digestion requires copious sources of acid, it has also been suggested that these encrustations offer protection from herbivores, yet the evidence for this is not compelling (McConnaughey & Whelan, 1997). Moreover, as we will show below, there are numerous avian herbivores [and indeed many fish species (e.g. Pípalová, 2006; Dibble & Kovalenko, 2009)], that habitually graze upon Charales above-ground biomass, suggesting that these species at least have adapted to these features of the plant ‘defence’ systems. There remains much debate about whether Charales gain structural benefit and/or protection from herbivores by their calcified deposits. Many Charophytes grow without calcifying in soft or Ca2+-depleted water. Although carbonate encrustations present unattractive properties to consumers, such as physical hardness, acid-neutralising capacity and their displacement of other nutritious components of the food quality, it seems that researchers overrate all of these qualities. Indeed, Charales seem to constitute highly profitable forage to some herbivores (McConnaughey & Whelan, 1997). The dense, compact growth form of most Charales results in very high local densities of plant biomass, which enable head-dipping, up-ending and diving waterbirds to harvest abundant local biomass rapidly and persistently (e.g. Schmieder et al., 2006), suggesting that these are highly attractive food resources.
The asexual reproduction of Charales can occur through unusual contracted starch-filled apices of branches, where the primary branchlet segments and internodes become packed with starch grains, similar in morphology to the turions of some aquatic angiosperms (Casanova et al., 2007). Asexual reproduction also occurs more commonly through starch-filled outgrowths of the rhizoids called bulbils which may become abundant on plants and eventually are shed and become buried within the substrate, persisting throughout the year (Wood & Imahori, 1965; Casanova, 1994) and which may ultimately germinate separate from the thallus. Still more common is asexual reproduction (and hibernation) by starch-filled node cells, as in the case of Nitellopsis obtusa (Bharathan, 1987). As is the case for many starch-packed Charophyte structures, if a consumer organism is able to dissolve and free their contents from calcified structures, they are potentially highly attractive to organisms browsing such structures or dabbling in substrates where they can accumulate (for example due to water currents) in some abundance as an easy source of concentrated energy. Water depth seems to play an important role in determining the degree to which Charophytes invest in bulbil production, with deeper perennial water bodies stimulating greater investment in such vegetative reproduction whereas the same species reproduce sexually in shallow water (e.g. García, 1994; Bociąg & Rekowska, 2012; Soulié-Märsche & García, 2015). This may explain the specialisms of diving waterbirds such as common pochard Aythya ferina, red-crested pochard Netta rufina and coot Fulica atra to feed on these diaspores of the Charales (see Section III.2). According to Bonis & Grillas (2002) there had only been one investigation of bulbil density in lake sediments at the time of their review, that of van den Berg (1999), who found 5 × 103–19 × 103 m−2 of buried Chara bulbils in the top 20 cm under permanently flooded conditions in a lake in The Netherlands. Conversely, sexual reproduction seems more frequent in shallow waters (perhaps related to the threat of desiccation in ephemeral arid-zone water bodies) so studies have shown a direct relationship between oospore production and receding water depth (Casanova, 1994; Asaeda, Rajapakse & Sanderson, 2007).
The sexual reproductive structures of the Charales are also highly attractive to waterbirds. Unfertilised female oogonia consist almost entirely of starch grains (Groves & Bullock-Webster, 1920–1924), so even prior to fertilisation are an attractive source of digestible stored energy to consumer species. The zygote is again filled with starch, surrounded by smooth walls overlaid with the oospore protective calcified walls (see Fig. 2 in Soulié-Märsche & García, 2015). Oospore production can occur throughout spring, summer and autumn (Casanova, 1994). Oospore production and ‘rain’ can be spectacular (e.g. 21,520 m−2 day−1 in Nitella mucronata in open field collections; van Onsem, Rops & Triest, 2018) leading to massive oospore densities in the sediment (e.g. 1.7 × 106 oospores m−2 after 6 years of continuous Chara aspera presence in Lake Veluwe; van der Berg, Coops & Simons, 2001). Some studies have shown the production of oospores to be salinity dependent in halophytic species, with production of ripe oospores only occurring in Lamprothamnium papulosum between 20‰ and 40‰ in populations growing in a salinity gradient from fresh water (0‰) to hypersaline (60‰) (Soulié-Märsche, 1998, 2008). At higher salinities, plants invested in starch-filled gametangia, and while the oospores were not fertilised they remained attractive to predators because of their high carbohydrate content.
(2) Which waterbirds species exploit which Charales parts, when, how, and why?
The literature review, based largely on analyses of gut contents supplemented by direct observations, revealed 17 European waterbird species that feed on Charales, primarily swans and dabbling ducks, plus a few specialist diving waterbirds like common and red-crested pochard, as well as the coot (see Table 1). Charales tend to achieve maximum green biomass in late summer (at least in boreal and temperate regions), although some species continue to attain greater biomass in autumn (e.g. Fernández-Aláez, Fernández-Aláez & Rodríguez, 2002) while many species remain green through the winter, albeit at lower biomass than in summer/autumn (e.g. Sanderson et al., 2008). Charales are distributed throughout mainland Europe to northern latitudes, where they may attain some of the highest biomass values known for Charales [for example in Poland (Pereyra-Ramos, 1981) and Sweden (Blindow, 1992b)]. However, in North America, their extent and biomass tends to be greater further south (Mann, Proctor & Taylor, 1999). Many northern-breeding (i.e. Arctic and boreal forest) waterbirds arrive at temperate regions in autumn, and the range of available studies suggest that 80% of these species forage on Charales in autumn and winter (Table 1). With due prudence, given the small sample size and biased seasonal distribution, it seems possible that this is the period when most waterbirds likely exploit Charales as a food resource, given the low biomass of these plants in spring, especially in relation to the availability of other new green macrophyte biomass at this time.
| Species | Locality | Charales species | Study method | References |
|---|---|---|---|---|
| Mute swan Cygnus olor | SE Sweden (sp, s) | Chara spp. | Gut contents | Berglund et al. (1963) |
| Mute swan Cygnus olor | Lake Balaton, Hungary (s) | Chara globularis | Observation | Grúz et al. (2015) |
| Mute swan Cygnus olor | Denmark (a, w, sp, s) | Chara aspera/baltica | Gut contents | Spärck (1957) |
| Whooper swan Cygnus cygnus | Banff, Scotland, UK (w) | Chara spp. | Observation | Hewson (1973) |
| Bewick's swan Cygnus columbianus | Zuiderzee, Netherlands (w) | Chara spp. | Observation | Brouwer & Tinbergen (1939) |
| Bewick's swan Cygnus columbianus | White Sea coasts, Russia (sp) | Chara sp. | Observation | Nolet et al. (2001) |
| Bewick's swan Cygnus columbianus | Matsalu Bay, Estonia (sp) | Chara spp. (2) | Observation | Rees & Bowler (1991) |
| Bewick's swan Cygnus columbianus | Veluwemeer, Netherlands (w) | Chara aspera bulbils | Faecal analysis | Noordhuis et al. (2002) |
| Bean goose Anser fabalis | Hungary (w) | Chara spp. | Gut contents | Sterbetz (1971) |
| Wigeon Mareca penelope | Coto Donana, Spain (w) | Chara spp. | Faecal analysis | Figuerola et al. (2003) |
| Wigeon Mareca penelope | Jutland, Denmark (a) | Chara vulgaris, C. canescens | Visual observation, kleptoparasitising coot | Holm & Clausen (2009) |
| Gadwall Mareca strepera | Coto Donana, Spain (w) | Chara spp. | Faecal analysis | Figuerola et al. (2003) |
| Gadwall Mareca strepera | Bodensee, Germany (a) | Chara spp. | Visual observation, kleptoparasitising coot | Berthold (1961) |
| Gadwall Mareca strepera | Tisza floodplain, Hungary (a) | Chara spp. | Gut contents | Sterbetz (1970) |
| Eurasian teal Anas crecca | Coto Donana, Spain (w) | Chara spp. | Faecal analysis | Figuerola et al. (2003) |
| Eurasian teal Anas crecca | Camargue, France (a, w) | Chara spp. | Faecal analysis | Pirot (1981) summarised in Green et al. (2002a) |
| Eurasian teal Anas crecca | Camargue, France (a*, w, sp*) | Chara spp. | Oesophagus and gut contents | Tamisier (1971) |
| Eurasian teal Anas crecca | Denmark (unknown) | ‘oospores of Charophytes’ and ‘young oogonia’ | Gizzard contents | Olsen (1944) |
| Mallard Anas platyrhynchos | Camargue, France (a, w) | Chara spp. | Faecal analysis | Pirot (1981) summarised in Green et al. (2002a) |
| Mallard Anas platyrhynchos | Coto Donana, Spain (w) | Chara spp. | Faecal analysis | Figuerola et al. (2003) |
| Mallard Anas platyrhynchos | Götzu Delta, Turkey (s, a) | Chara vulgaris | Faecal analysis | Green (1998); Green & Selva (2000) |
| Northern pintail Anas acuta | Coto Donana, Spain (w) | Chara spp. | Faecal analysis | Figuerola et al. (2003) |
| Northern pintail Anas acuta | Camargue, France (a, w) | Chara spp. | Faecal analysis | Pirot (1981) summarised in Green et al. (2002a) |
| Northern shoveler Spatula clypeata | Coto Donana, Spain (w) | Chara spp. | Faecal analysis | Figuerola et al. (2003) |
| Northern shoveler Spatula clypeata | Camargue, France (a, w) | Chara spp. | Faecal analysis | Pirot (1981) summarised in Green et al. (2002a) |
| Garganey Spatula querquedula | Camargue, France (a, w) | Chara spp. | Faecal analysis | Pirot (1981) summarised in Green et al. (2002a) |
| Garganey Spatula querquedula | Tisza floodplain, Hungary (a) | Chara spp. | Gut contents | Sterbetz (1970) |
| Marbled duck Marmaronetta augustirostris | Götzu Delta, Turkey (s, a) | Chara spp. | Faecal analysis | Green & Selva (2000) |
| Red-crested pochard Netta rufina | Camargue, France (w) | Chara spp. | Gut contents | Allouche et al. (1988) |
| Red-crested pochard Netta rufina | Lake Constance, Germany/Switzerland (a, w) | Chara spp. | Gut contents | Szijj (1965b) |
| Red-crested pochard Netta rufina | Ismaninger Reservoir and fish ponds, Germany (s, a) | Chara vulgaris | Observation | Köhler et al. (2009) |
| Pochard Aythya ferina | Denmark (w) | Chara spp. | Gut contents | Madsen (1954) |
| Pochard Aythya ferina | SE England and Northern Ireland (w) | Chara spp. | Gut contents | Olney (1968) |
| Pochard Aythya ferina | Lake Constance, Germany (w) | Chara spp. | Gut contents | Szijj (1965a) |
| Pochard Aythya ferina | Coto Donana, Spain (w) | Chara spp. | Faecal analysis | Figuerola et al. (2003) |
| Ferruginous duck Aythya nyroca | Hungary (a. w, sp) | Chara spp. | Gut contents | Sterbetz (1969) |
| Coot Fulica atra | Denmark (unknown) | ‘Fragments of Charophytes with sexual organs’ | Gut contents | Olsen (1944) |
| Coot Fulica atra | Jutland, Denmark (a) | Chara vulgaris, C. canescens | Visual observation | Holm & Clausen (2009) |
| Coot Fulica atra | Coto Donana, Spain (w) | Chara spp. | Faecal analysis | Figuerola et al. (2003) |
| Coot Fulica atra | Lake Geneva, Switzerland (w) | Chara spp. | Gut contents | Madon (1935) |
- * Most important August–September and February–March.
Herbivorous waterbirds gain access to submerged (but often near-surface) Charales by browsing green biomass on the surface, by head-dip feeding below the surface or by upending to gain access from the surface. Herbivorous dabbling ducks are reticent to dive, so access green biomass on the surface and up-end in shallow water to access standing crop below the surface, while having limited access beyond these levels. Both gadwall Mareca strepera and Eurasian wigeon Mareca penelope are quintessential surface-feeding ducks that never dive (Table 1). Despite this, both species have been recorded kleptoparasitising diving ducks (especially red-crested pochard) and coot and longer-necked species (such as swans) that bring submerged macrophytes to the surface (Hudson, Pierce & Taverner, 1960), including Chara species (e.g. Bodensee, Germany; Berthold, 1961). Berthold (1961) described the Charales vegetation as growing in deeper water, i.e. in situations inaccessible to surface-feeding ducks without the assistance of diving and long-necked waterbird species upon which they could kleptoparasitise. Finally, specialist diving herbivores such as coot, red-crested and common pochard access biomass by active diving below the water surface (see Fig. 1), while the former two species are also known to dive to access abundant diaspores where these occur.

Receding water levels may also change accessibility of Charales to waterbirds. Many Charales are adapted to thrive and reproduce in ephemeral shallow water lakes in arid zones, which may dry out later in the season. For example, in the shallow saltwater coastal lagoons of South Australia, Lamprothamnium papulosum invests heavily in bulbil and oospore production, but vegetative growth becomes increasingly stunted as salinity increases, failing to grow at all above 60‰ (Delroy, 1974). During late spring and summer, these same saltwater lagoons provide vital feeding areas for many species of waterbirds, but especially three dabbling duck species (grey teal Anas gibberifrons, chestnut teal A. castanea and mountain duck Tadorna tadornoides) displaced from ephemeral desiccated freshwater feeding areas elsewhere in Australia. These birds harvest the superabundant bulbils and oospores accumulated in shallow waters at this time because of receding water levels and it seems highly likely that this cycle of events supports similar patterns of seasonal production and avian exploitation in lagoons in Europe and elsewhere.
Among the diving duck species, the red-crested pochard is often considered an obligate Charales specialist (e.g. Allouche, Roux & Tamisier, 1988; Cramp & Simmons, 1977), almost entirely confined to clear-water lakes with abundant Charales, especially Nitellopsis obtusa (van der Winden et al., 1994; Ruiters, Noordhuis & van den Berg, 1994). In Camargue, southern France, red-crested pochard feed in winter on both the vegetative parts (stems and side shoots) and oospores (Allouche et al., 1988). In Austria and Switzerland, the occurrence and abundance of Charales (related to water quality) are essential factors influencing habitat use by the red-crested pochard (Aubrecht & Winkler, 1997; Schneider-Jacoby, 1998–1999; Keller, 2000a,b). There, females of the species dive to bring green biomass to the surface to consume and share with their ducklings during the breeding season (see Fig. 2). Diving for food is energetically costly relative to surface foraging for green plant biomass, so there may be energetic benefits from mate-feeding by red-crested pochards recorded in the Netherlands (Fig. 3), another subject worthy of further study. The common pochard is a fresh and brackish water diving duck that has long been known to specialise on feeding on Charales (e.g. Stevenson & Southwell, 1890; Ticehurst, 1932; Szijj, 1965a; Dementiev & Gladkov, 1952; Cramp & Simmons, 1977). This is especially the case in autumn and winter, when plant material occurred in all sampled birds, with Charales constituting up to 86% of total food volume in 22 common pochard examined in the UK (Olney, 1968). Among the dabbling ducks, which sieve water through the lamellae of their bills to extract animal and vegetable matter from the water column, species such as Eurasian teal Anas crecca, mallard Anas platyrhynchos, northern pintail A. acuta, northern shoveler Spatula clypeata, garganey Spatula querquedula and marbled duck Marmaronetta angustirostris have all been shown to concentrate diaspores of Charales (e.g. Cramp & Simmons, 1977; Pirot, 1981; Green, Figuerola & Sánchez, 2002a), often in very large amounts – Olsen (1944) reported a dissected gizzard from a Eurasian teal containing 10.5 cm3 ripe Charales oospores, also taken in great abundance in the Camargue (Tamisier, 1974). To what extent these oospores have been actively selected from the water column or have become concentrated in the gut by the resistance of such structures to decomposition compared to the green parts remains unknown and in need of confirmation. However, all of these duck species are omnivores that specialise in filtration of seeds and small invertebrates from the water column and show few traits of being herbivorous, suggesting active filtration of oospores as a source of food. Diving ducks submerging to forage on bottom sediments likely undertake similar sieving of the substrate to obtain diaspores concentrated within the benthos. While it seems highly likely that surface-grazing herbivorous species, such as swans, and diving species, such as common and red-crested pochard, regularly (and unselectively) consume large quantities of green above-ground production from Charales, it is the case that, at the same time, they coincidentally take in other Charales diaspores that are energetically more profitable, potentially elevating the attractiveness of these species over other submerged macrophytes.

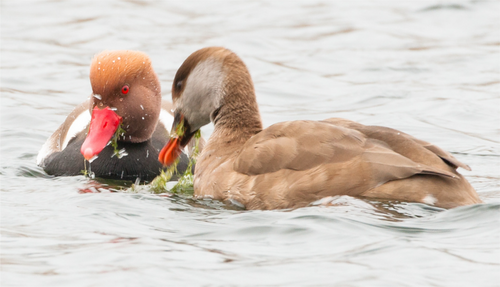
Perhaps surprisingly, despite a wealth of studies, we still know relatively little about the true breadth of the diet of many Anseriformes. There are very few reports of geese feeding on Charales, with the notable exception of the bean goose Anser fabalis in Hungary (Table 1). However, this is likely partly the result of the relative lack of feeding studies of geese on natural and semi-natural wetlands. There has been a major increase in the numbers of geese feeding on agricultural habitats in Europe during the last 50 years, due to the greater profitability of feeding on food resources available there (Fox et al., 2017; Fox & Madsen, 2017; Fox & Abraham, 2017), effectively isolating many current generations of geese from formerly exploited natural habitats. The current concentration of geese foraging on terrestrial vegetation and the large number of wild goose dietary studies in recent years on such habitats makes it unlikely that we underestimate the degree to which geese currently feed on Charales, although we cannot rule out that this may have been different in the pre-agricultural past.
All three European swan species – mute swan Cygnus olor (sedentary in a large part of distribution area) and the more migratory whooper Cygnus cygnus and Bewick's C. columbianus, have been reported as grazers of submergent aquatic vegetation, frequently including green biomass of Charales (see Table 1). Mute swans feed on Charales throughout the annual cycle when biomass is sufficient and accessible (e.g. Holm, 2002) and have been reported doing so even as an introduced alien in North America (Bailey, Petrie & Badzinski, 2008). While both whooper and Bewick's swans are typically winter consumers, they are also apparently dependent upon overwintering Chara material at spring staging areas (such as Matsalu Bay in Estonia) to replenish diminished energy and nutrient stores en route to breeding areas (Rees & Bowler, 1991; Nolet et al., 2001). This may be especially critical for staging Bewick's swans in late springs in the Russian White Sea when sea ice covers more profitable Zostera marina beds and substrates containing Stuckenia pectinata tubers (such as in 1996). This is because the Charales grew in places where freshwater streams rapidly melted the ice in coastal ecosystems when no other green vegetation was available (Nolet et al., 2001). To compensate for the relatively low availability of such forage when restricted by ice during the vernal thaw, the swans consume large amounts of biomass and therefore have the potential to make a major impact on the local standing crop. The coot is unusual for being an omnivore that also shows an herbivorous feeding habit, feeding on many submerged macrophytes including Charales (Cramp & Simmons, 1977) and their oospores (Olsen, 1944) throughout their range (including Nitella in North Africa; Madon, 1935).
Although some authors imply that Charales are not important to American waterbirds (e.g. Anderson, 1958), they have long been reported to contribute to the diet of at least 14 different species there (e.g. McAtee, 1915; see also Knapton & Pietrie, 1999). Trumpeter swans Cygnus buccinator have been reported feeding on Chara spp. in the Yellowstone Ecosystem (Squires & Anderson, 1995). Chara spp. have also been reported in the oesophageal contents of northern pintail, northern shoveler and blue-winged teal Anas discolor in Mexico (Thompson, Sheffer & Balassarre, 1992). McAtee (1915) reported finding 1100–15,000 oospores in the guts of many dissected ducks, so this is a food source that appears important to several species, as well as to the invasive alien mute swan (Bailey et al., 2008) and young white-winged coot Fulica leucoptera in Argentina (Nores & Cerana, 2010). Non-breeding American flamingoes Phoenicopterus ruber ruber also feed on the tubercules and oogonia of Chara fibrosa on the Yucatan Peninsula of Mexico (Schmitz et al., 1990; Arengo & Baldassarre, 2002).
(3) Waterbird aggregative responses
Several studies have shown relationships between either biomass or extent of submerged Charales and the numbers of feeding-specialist waterbirds that have been reported in the literature to exploit these plants as source of food. For instance, van der Winden et al. (1994) and Ruiters et al. (1994) linked the red-crested pochard expansion in range and increase in abundance to improvements in freshwater lake quality and the concomitant expansion in the distribution and abundance of the Charales. Similarly, increasing numbers of moulting red-crested pochard on the Ismaninger reservoir and fishponds, Germany, fed on Chara vulgaris and several Potamogeton/Stuckenia species, which have spread following reductions in water column dissolved nutrients at the site, associated with the restoration of a clear water column (Köhler et al., 2009). In the Cotswold Water Park, England, densities of wintering common pochard on multiple flooded gravel pits of uniform depth, all characterised by dense Charales cover (mostly Chara globularis) were related to the extent of open water area, but these relationships were reduced on lakes with bank-side access or water-based recreation compared to nature reserves with restricted access, suggesting a numerical response modified by human disturbance (Fox et al., 1994). Hargeby et al. (1994) found correlations between the biomass and extent of Chara tomentosa stands and numbers of mute swans, coot and dabbling ducks in a Swedish lake following the natural recovery of submerged macrophytes. At the Dutch Lake Veluwe (3400 ha), after a period of severe eutrophication, the aquatic vegetation, mainly Chara aspera beds, recovered and began to increase gradually. The development of the vegetation coincided with a strong decrease in the bream Abramis brama population, probably the result of introducing a commercial bream fishery there during 1993–1997, which reduced fish biomass by ca. 65%, reducing sediment disturbance and enhancing transparency in the water column (Noordhuis et al., 2002). Despite abundant Potamogeton beds at the site in the 1980s, waterbirds only really returned in large numbers following the re-establishment of Chara. Of all correlations between filamentous algae, narrow-leaved Potamogeton/Stuckenia, zebra mussel Dreissena polymorpha and Chara biomass, eight of 12 herbivorous and omnivorous waterbirds (predicted to benefit from the presence of Chara) showed significant positive correlations with Chara biomass during 1987–1998, with mute swan (virtually absent before the return of Chara) and coot showing the strongest relationships (Noordhuis et al., 2002). Northern pintail and red-crested pochard numbers were correlated with Chara biomass and all of the recoveries in waterbird numbers were linked to improvements in water transparency, responding positively to increases in densities and extent of Dreissena and Chara and decreases in filamentous algae and narrow-leaved pondweeds, mainly Stuckenia pectinata (Noordhuis et al., 2002). Holm & Clausen (2006) found herbivorous waterbirds choose to forage at greater densities at one site where the more energetically profitable Chara species were superabundant compared to another adjacent site with the less-profitable Ruppia cirrhosa, in waterbodies that otherwise differed little (e.g. in water depth or nutrient status) from each other. Following drastic changes in water quality, Chara species disappeared from many Lithuanian lakes in the 1980s. In this region, Švažas, Stanevičus & Čepulis (1997) linked marked declines in breeding mute swan populations in SW Lithuania to the extinction of the Charales, their most important food plants, as was the case for coot reductions during post-breeding and autumn staging for the same reasons (Stanevičius, 1999). Likewise, when an invasive fish species, the common carp Cyprinus carpio was introduced to the Tablas de Daimiel National Park (Spain), exclosure and other experiments showed that enhanced levels of phosphorus and the presence of these invasive herbivorous fish (and not the alternative hypothesis of the presence of herbivorous waterbirds) were responsible for the loss of Chara meadows (Laguna et al., 2016). These submerged macrophyte beds formerly supported a year-round abundance of red-crested pochard, a species that completely disappeared following the loss of Chara. Armitage, Holloway & Rehfisch (2000) found that high densities of coot, mute swan and common pochard fed extensively on Chara papillosa, to the extent that there were significant positive correlations between Chara height and the densities of all three species at Hickling Broad, East Anglia, UK. Numbers of herbivorous mute swan, coot and dabbling ducks breeding at Lake Krankesjön, Sweden, during a period when conditions flipped between a clear and a turbid water column, were very highly correlated with biomass and area of Chara tomentosa, which thrived in years characterised by improved water transparency (Hargeby et al., 1994).
While we predict numerical responses of species known to feed on the green biomass and diaspores of Charales to increase with the extent and biomass of these plants, it is important to remember that Charales beds themselves also offer refuge to abundant invertebrate biomass seeking refuge from predatory fish (e.g. Diehl, 1992; Marklund, Blindow & Hargeby, 2001). Invertebrate biomass in Chara beds was more diverse and seven times greater than that simultaneously sampled in Stuckenia pectinata stands and areas of bare substrate from the same lake (Hargeby et al., 1994). The presence of such biomass associated with Chara beds presents a food resource for omnivorous waterbirds that might also be predicted to show relationships with changes in Charales biomass, especially since invertebrate species show diurnal movement cycles within such beds (Kuczyńska-Kippen, 2001) that increase availability. At the same time, very dense Chara growing to the surface reduces invertebrate availability to dabbling and diving ducks and thus contributes to their reduced use of such areas, as was found by Marklund (2000) for omnivorous mallard and tufted duck. These experiences may explain why omnivorous duck species benefit from the restoration of beds of Charales vegetation without showing specific numerical responses to the extent of the submerged macrophytes. This was the case in the study of Noordhuis et al. (2002), where mallard showed no relationship to Chara biomass and tufted duck increases were more related to increasing extent of Dreissena (a favoured prey species that also benefited from clear water conditions at Lake Veluwe) than Chara per se.
Freshwater fish use macrophyte beds (including Charales) for shelter and refuge, spawning, nesting and nursery sites, and in some cases as direct and indirect sources of food (Petr, 2000). However, a number of factors complicate the relationships between piscivorous waterbirds and Charales. For instance, the abundance and size class distribution of different fish species vary enormously with multiple factors, many of which are unrelated to submerged macrophytes, but piscivorous birds (fast-pursuit visual foragers) generally need a clear water column and an abundance of fish of adequate biomass, regardless of species. So, interpreting changes in piscivorous bird abundance can be challenging without a detailed understanding of the fish species composition, abundance and size-class distribution. For instance, studies at Lake Tåkern have shown that piscivorous species such as great cormorant Phalacrocorax carbo and goosander Mergus merganser were more abundant in years with limited Chara growth (Milberg et al., 2002). However, while the same authors speculated that dense submerged plants presented both a refuge to fish and a mechanical obstacle to avian fish pursuit, they also explained that the great cormorant had shown major expansions in range and numbers that also affected local abundance. A third piscivorous species in their study, the great crested grebe Podiceps cristatus, showed no strong correlation with submerged macrophyte abundance. Ironically, highly turbid anoxic waters can kill fish stocks and attract numerous heron and egret species, as well as cormorants for short periods of feeding when dead and dying fish are abundant (as in the case of over-manured fishponds in the Czech Republic, A. D. Fox, personal observations).
(4) Contributions of Charales to maintaining water clarity
Charales form a common component of the littoral zone in clean, unpolluted oligo- to moderately eutrophic water bodies (McCourt et al., 2017). However, with increasing eutrophication, Charales often become out-competed by submerged angiosperm macrophytes [e.g. Potamogeton/Stuckenia species (Ozimek and Kowalczewski, 1984; Pieczyńska, Ozimek & Ryback, 1988; Blindow, 1992a)], all of which ultimately completely disappear from extremely turbid lakes. It is however, possible to reverse this process, restoring Charales communities following lake restoration measures (Blindow, 1992b; Simons et al., 1994; van den Berg et al., 1999). This can include applying biomanipulation techniques, such as removal of planktivorous and benthivorous fish (Bernes et al., 2015) or reduction of nutrients (especially phosphorus; Richter & Gross, 2013). Once stabilised, Charales can form very dense underwater canopies, often greater than in beds of vascular aquatic macrophytes, which are highly effective at trapping sediment and restricting resuspension, especially of soluble nutrients, a major source of internal nutrient loading in shallow lakes (Søndergaard, Kristensen & Jeppesen, 1992; van der Berg et al., 1998a; Casanova, de Winton & Clayton, 2002; Schneider et al., 2015). In doing so, they restrict aqueous sources of nutrients to planktonic algae and the periphyton, the growth of which they also inhibit through allelopathic secretions (e.g. Forsberg, Kleiven & Willén, 1990; van Donk & van de Bund, 2002; Berger & Schagerl, 2003; Mähnert, Schagerl & Krenn, 2017; Złoch et al., 2018). Several Chara species overwinter as green material, suggesting accumulation of plant biomass beyond one growing season, although it is known that some Charales can efficiently take up nutrients through their rhizoids (e.g. Wüstenberg, Pörs & Ehwald, 2011; Raven, 2013). Charales have been reported to decompose more slowly than their vascular plant counterparts prolonging nutrient (including carbon and phosphate) storage in plant biomass compared to other plant communities (Kufel & Kufel, 2002). In addition, Charales precipitate abundant calcite during periods of intensive photosynthesis, leading to major carbonate accumulation (Pełechaty et al., 2013) which can immobilise phosphorus within the crystalline structure and lock this and carbon into the sediment (e.g. Murphy et al., 1983). Generally, the relative abundance and spatial distribution of Charales is correlated to water clarity and concentrations of total phosphorus and chlorophyll a (e.g. Hutorowicza & Dziedzicb, 2008), often associated with distinct clear/turbid water phases in the lifetimes of lakes, to which waterbirds respond in relation to the food abundance available from Charales (e.g. Hargeby et al., 1994; Moreno-Ostos et al., 2007, 2008). The effects described above often mean that once restored (usually through measures to severely restrict phytoplankton abundance), a clear water column and dense established Charales beds represent a naturally low-trophic-status stable state, which is discrete from the previous turbid system (Scheffer et al., 1993; Jeppesen et al., 1998) that was far less attractive to feeding waterbirds (e.g. Noordhuis et al., 2002). It is not just the dense standing crop of green Charales biomass that attracts feeding herbivorous waterbirds. Charales oospore densities were 6–15 times greater in clear-water ponds compared with those in turbid waters from a study of Belgian ponds (van Onsem & Triest, 2018) and so more likely to attract specialist filter-feeding waterbirds actively foraging on diaspores. The presence of Chara during such changes has a major effect on the biomass, diversity and community composition of the associated invertebrate fauna. For example, Chara beds were dominated by chironomids and gastropods whereas amphipods dominated the community in the rooted-angiosperm beds (Hanson, 1990; van der Berg et al., 1997), so affecting the aquatic and waterbird community composition responding to such changes in food supply.
As described above, after a period of severe eutrophication, the aquatic vegetation of Lake Veluwe, in the Netherlands, began to recover, showing dramatic increases in Chara cover and enabling colonisation by invasive zebra mussels. The presence of both forms (i.e. a dense charophyte vegetation canopy and an abundant filter-feeding bivalve) contributed to creating a clear water column and supporting the further rapid expansion of macrophyte beds dominated by Chara aspera (Lammens et al., 2004). The presence of such previously absent food sources consequently attracted large numbers of specialist waterbird species exploiting Chara as a food resource, or sources of food associated with dense Chara beds (Noordhuis et al., 2002; see Section III.3).
(5) Avian effects on Charales
Bird herbivory may have contrasting effects on the standing crop of growing aquatic plants. Some authors reported negligible effects on living vegetation (e.g. Kiørboe, 1980; Perrow et al., 1997; Marklund et al., 2002), while others reported a strong negative impact of herbivory by waterfowl on plant biomass (e.g. Lauridsen, Jeppesen & Andersen, 1993; Hilt, 2006; Hidding et al., 2009), but clearly the season and levels of grazing affect plant abilities to use compensatory growth to make up for biomass lost to grazing. Bird herbivory may also induce changes in relative abundance of different species making up the aquatic vegetation (e.g. van Donk & Otte, 1996; Weisner, Strand & Sandsten, 1997; Santamaría, 2002; Rodríguez-Villafañe, Bécares & Fernández-Aláez, 2007), so herbivorous waterfowl may potentially shape the species composition of aquatic plant communities in shallow wetlands by selecting more palatable species over others. Hidding et al. (2010) showed no effect of below-ground herbivory in spring by whooper and Bewick's swans, combined with summer grazing by waterfowl and fish on the biomass and macrophyte community composition (dominated by Chara aspera, with subdominant Stuckenia pectinata and Najas marina) present in a shallow Baltic estuary, Matsalu Bay, in Estonia during 1 year. However, S. pectinata was more abundant in plots closed to spring and summer herbivores, N. marina was more abundant in grazed plots, whereas Chara spp. biomass remained unaffected. Below-ground propagules of both C. aspera and S. pectinata were likely consumed by swans but the authors considered that since C. aspera bulbils were so superabundant this may have compensated for the losses.
A study on Lake Constance showed that feeding waterbirds (mainly coot, common and red-crested pochard) extensively depleted Charales biomass in shallower areas (<1 m water depth) in early winter, while deeper regions were only used later (Schmieder et al., 2006). At the end of winter, herbivorous waterbirds had almost completely removed available Charales biomass down to 2 m water depth. Enclosure experiments showed plant senescence had a negligible influence on Charales biomass loss until early February. Despite this major impact on winter biomass, avian herbivores likely had limited influence on subsequent Charales regeneration because of the production of innumerable oospores that contributed to the subsequent formation of dense Charales meadows at depths down to 4 m every year (Schmieder et al., 2006). In a study of the diet of summering and moulting waterbirds (dominated by coot) at the same site, comparison of vegetation in exclosure cages (protecting macrophytes from avian grazing) with unprotected grazed sites showed that herbivorous waterbirds depleted over 40% of Charales biomass at 1.5 m depth, although no effect was evident at greater depths (>2 m). Available food consisted mostly of Chara spp. (350 g m−2), since available animal food items were negligible, yet dense Chara beds persisted at the site despite such annual heavy grazing pressure (Matuszak et al., 2012). Long-term studies of the submerged macrophytes at Lake Krankesjön, Sweden also strongly suggest that waterbird herbivory on Chara spp. has no long-term effects on species composition, persistence or biomass of these macrophytes at this site (Hansson et al., 2010). Seventeen large-scale waterbird exclosure plots monitored over 2 years at Lake Botshol, Netherlands showed no significant increase in Charales biomass in the absence of grazing compared with the controls during transition from turbid to clear water and high Chara biomass, demonstrating that light limitation was the main factor controlling the collapse and return of Charales there (Rip, Rawee & de Jong, 2006). Charales biomass at Lake Veluwe, Netherlands was greater inside bird exclosures than outside as a result of overwintering waterbird exploitation, mostly after the end of the growing season, but this too did not affect the annual persistence of Charales at the site as a whole (van den Berg et al., 1998b). To what extent herbivorous birds may selectively exploit overwintering diaspores (including bulbils produced by Chara aspera) also remains unclear (van den Berg et al., 1998b). Migrating swans grazing on Chara spp. in spring did not seem to affect biomass later in the season in Matsalu Bay, Estonia, thought due to compensatory growth in summer when avian herbivory is limited and light attenuation high (Hidding, 2009).
At Lake Veluwe, a community of dabbling, up-ending and diving herbivores completely removed above-ground Chara biomass after arrival, large numbers of autumn-arriving birds concentrating conspicuously in parts of the lake where Chara was the dominant plant cover and hence provided an abundant food supply (Noordhuis et al., 2002). Ironically, reduction in biomass of some other submerged macrophytes through waterbird herbivory may actually be beneficial to Charales, as in the case of shallow lakes in the Netherlands and southern Sweden, where avian herbivory (especially by Bewick's swans depleting Stuckenia pectinata tubers which affects subsequent regrowth; Van Vierssen, Hootsmans & Vermaat, 1994) was shown to induce a shift in macrophyte dominance from S. pectinata to Chara spp. and Myriophyllum spicatum (van Wijk, 1989).
Outside of Europe, the experience is similar, autumn exclosure experiments carried out on Lake Erie (Canada/USA) showed that, compared to ducks and abiotic factors, two large herbivorous waterfowl (tundra swan Cygnus columbianus and Canada goose Branta canadensis) had no additional impact on annual above- or below-ground biomass of Chara vulgaris at the site (Badzinski, Ankney & Petrie, 2006). The population density of black swans Cygnus atratus feeding on Nitella hookeri at Hawksbury Lagoon, New Zealand correlated with plant biomass, but plant biomass removal by grazing was less than replacement by regrowth. However, nutrient recycling and bioturbation affected suspended solids mobilisation, phytoplankton abundance and light attenuation, which adversely affected plant abundance (Mitchell & Waas, 1996).
Existing studies suggest that Charales are not normally affected by avian herbivory (in the sense of suffering reduced annual persistence), even when autumn and winter grazing in shallow waters completely removes all biomass before the start of the following growing season. This effect may be because most intensive grazing in the observed systems tends to occur outside the main growth season (spring/summer/autumn) when densities of grazing avian herbivores are generally lower or absent. The effect is likely also mitigated by the release of very large numbers of sexual and asexual diaspores into the environment each season (e.g. Schmieder et al., 2006), which despite harvest by avian and other predators still maintains stock levels in substrates sufficient to maintain biomass and the dominance of Charales in the subsequent submergent plant community.
Indirect impacts of avian foraging on Charales should not be neglected. Bonis & Grillas (2002) claim that burial of oospores deeper than 2 cm inhibits their germination. Swans not only graze on macrophyte green parts, but also dig for tubers in shallow substrates, simultaneously removing thalli and potentially burying oospores to inhibit regrowth, but also bringing buried oospores towards the sediment surface to germinate. Such impacts are especially likely in sites where Charales grow interspersed by pondweeds such as Stuckenia pectinata or close to common reed Phragmites australis stands (A. Stīpniece, personal observation).
When water nutrient levels are not sufficiently low to prevent algal blooms, consumption of Charales by birds may trigger a change from the clear-water, macrophyte-dominated state to the turbid state discussed above (Van Donk & Gulati, 1995; Weisner et al., 1997). Occasionally waterbirds themselves can contribute to eutrophication of waterbodies, enhancing water turbidity and leading to the extinction of submerged macrophytes, potentially including Charales. This is especially the case where large-bodied colonial nesting birds (such as cormorants, gulls, herons and egrets) aggregate along lake shores to contribute high N and P loadings to water bodies sensitive to such external and internal inputs, although we could find no cases involving Charales specifically (see review in Klimaszyk & Rzymski, 2016).
(6) Waterbirds as a means of dispersal of Charales
Many researchers have been fascinated by the ability of Charales to colonise new and novel aquatic habitats often very rapidly as such opportunities become available (e.g. Wade, 1990). Many have been convinced that waterbirds are a principal agent of dispersal (e.g. Ridley, 1930; Olsen, 1944; Proctor, 1962), as for instance in the colonisation of the Faroes, Iceland and Greenland from freshwater habitats further south (Langangen, 1996; Langangen, Hansen & Mann, 1996; Hrafnsdottir et al., 2019). The presence of Chara species on Svalbard (Langangen et al., 2020) has been considered totally dependent upon their transport there by geese, in the absence of any migratory duck species regularly using these polar areas with links to suitable Charales habitats further south. Malone (1966) reported that two out of five 6-month-old female mallards fed to repletion on Chara sp. on one occasion regurgitated 2.5 cm balls of loosely compacted food 45 min later, which had not been altered by compaction in the gizzard nor the digestive process. Proctor (1962) found no viability for Charales thallus fragments after passing through a duck. In the same study, Proctor (1962) removed intact oospores of six Chara species from the lower digestive tracts posterior to the opening of the caeca, in 16 of 47 shot waterbirds, of which 40–60% germinated from eight of these birds. Although Charalambidou & Santamaría (2005) found oogonia of Chara species intact in faeces of mallard, teal and coot (demonstrating an ability to pass through the guts of these waterbird species and potentially to being transported elsewhere by them), they did not measure propagule germination rate, so the degree to which they escaped digestion and remained viable remains unknown. In contrast to previous studies of Chara spp. (e.g. Proctor, 1962), Brochet et al. (2010b) found low germination rates and viability among Chara oospores. Nevertheless, they reported that teal oesophagi can be full of thousands of Chara oospores (among the smallest of identifiable prey regularly found in this species; Tamisier, 1971), which constituted the most abundant taxon amongst viable diaspores in the lower gut of teal shot in the Camargue (Brochet et al., 2010a). Oogonia of Chara (C. vulgaris, C. globularis and C. aspera) were most abundant in the rectum of sampled teal (60% of all intact propagules), suggesting that small size favours internal transport through the gut of this species (Brochet et al., 2010a). Two Charales oogonia have even been found in the faeces of a wading bird, the common redshank Tringa totanus (Lovas-Kiss et al., 2019), so perhaps we should look further afield to judge the full extent of potential dispersal by birds. Oospores can survive desiccation and rewetting over periods from 4 years (Proctor, 1967) to 50–150 years in ghost ponds (Alderton et al., 2017), so are likely extremely long-lived and resistant to desiccation, further enabling their survival during transit by birds to colonise new habitats (Wade, 1990). Waterbirds can migrate relatively fast, so there is at least the theoretical potential for long-distance dispersal. Brent geese Branta bernicla have been recorded flying 2600–3500 km in less than 94 h (Clausen & Bustnes, 1998; Green et al., 2002b), while a Eurasian teal was tracked migrating 1285 km in less than 24 h (Clausen et al., 2002). However, it is generally considered that retention times are relatively short within the avian alimentary canal, ranging from an estimated 6 h in the mute swan to as little as 1.7–1.9 h in Eurasian wigeon and greylag geese Anser anser (Clausen et al., 2002). The same authors considered it likely that many species would deliberately eject most if not all contents of the alimentary canal prior to departure on migration (to reduce payload flight costs). It may also be the case that migrating waterbirds potentially alter their gut architecture to reduce body mass and metabolic maintenance costs (as is the case for shorebirds; see Battley & Piersma, 2005) and/or at least eject faeces in flight well before arrival at final destinations to avoid carrying unnecessary mass in flight. This does not exclude the possibilities of small diaspores persisting in the caeca (although this is not normally a site of entrapment of small seeds passing through the avian gut; Kleyheeg, Claessens & Soons, 2018) and folds of the alimentary canal. Figuerola et al. (2010) found that smaller ingested angiosperm seeds showed longer avian gut retention times, higher survival and germination rates than larger seeds, so the very small size of Charales diaspores may also be advantageous.
It is well known that seeds with hard integuments or of small size are more capable of passing through the avian intestinal tract and are more likely to germinate when ejected (Krefting & Roe, 1949; deVlaming & Proctor, 1968; Lovas-Kiss et al., 2020). In this respect, the small size and the potential protective function provided by calcification of oospores suggest that these diaspores theoretically have some advantages over angiosperms for both passing through the guts of waterbirds and being viable on ejection. Although this review has found a rich literature on this subject, there remains enormous potential for field and (more particularly) manipulative captive studies on waterbirds to assess the levels of ingestion/ejection of viable Charales diaspores of different species and levels of calcification after passage through the gut of a variety of waterbird species.
IV. DISCUSSION
This review has confirmed that the Charales are an important food source for a few specialist and more generalist waterbird species in Europe, and potentially elsewhere. These primarily comprise browsing herbivores that consume the often-abundant vegetative parts of these plants, by head-dipping, up-ending (swans and dabbling ducks) or diving (diving ducks and coot). Other waterbirds using Charales as a food supply are more specialist consumers, exploiting the more energy-rich oospores and bulbils produced in great abundance by some Charales species. The low structural cellulose fibre and high soluble carbohydrate content of Charales, together with the specialisms of some herbivorous waterbirds to feed on them, implies that they are as nutritionally attractive to avian herbivores as sympatric aquatic higher plants, if not more so, yet this was not found to be the case in some studies (e.g. Langhelle, Lundgren & Marklund, 1996; Hidding et al., 2010). We contend that low fibre/high soluble carbohydrate content is key here, as a high fibre content is associated with reduced digestibility in avian herbivores, in a way that is not necessarily the case for ash content generally. A high ash fraction in angiosperms reflects indigestible structural fibres that inhibit digestion, whereas that in Charales (up to 70% ash content) reflects largely the calcification material which is potentially dissolvable. We suggest that the pH of the avian gut (ca. pH 4 generally depending on diet, but in mallard may be as low as pH 2; Ziswiler & Farner, 1972) would dissolve the calcification on Charales, so dry mass ash content does not necessarily equate to poor food quality in the same way as in angiosperms, as long as there is no biological/energetic or other cost associated with disposing of calcium and carbonate ions in the gut. Investigating these aspects of herbivorous waterbird digestion represents another important area for future research. The nutritional demands of herbivorous waterbirds also change across the annual cycle in relation to physiological need, with greater emphasis on deriving energy for stores prior to migration, investment in reproduction and moult, and as a buffer against severe winter weather. Protein-rich food becomes more essential prior to egg-laying in females and for ducklings to support growth, explaining shifts in food from plants to macroinvertebrates in many duck species (e.g. Noyes & Jarvis, 1985, Swanson, Meyer & Adomaitis, 1985). Studies of sympatric non-breeding garganey, white-faced whistling duck Dendrocygna viduata and fulvous whistling duck D. bicolor in the Senegal Delta, West Africa found the sedentary white-faced whistling ducks fed on Chara spp. in November and January, whereas the sedentary fulvous whistling ducks and migratory garganey (which migrate north to Europe in spring) only did so in March (Tréca, 1981a,b, 1986). Clearly, we need to compare the relative attractiveness of Charales as food with other available food items in the context of specific seasonally changing waterbird energy and nutrient needs throughout the annual cycle. We also assert that what is clearly missing from the current literature is a comparative assessment of the energetic and nutritional content of different parts of the thalli, starch-filled apices of branches, bulbils, gametangia and oospores of different Charales species throughout the growing season under different environmental conditions to relate seasonal profitability to herbivorous waterbirds to observed patterns in their exploitation. Such an analysis would contribute greatly to our understanding of why different species of waterbirds seem to forage intensively on Charales at particular sites at specific times of the year and potentially precisely how and why they exploit this potentially rich food resource when they do so.
In the case of dabbling ducks, it would appear that a diversity of species successfully filter Charales oospores and bulbils from the water column, sometimes in vast quantities. The response of Charales to environmental stress, producing abundant oospores and bulbils (e.g. under drought conditions in ephemeral shallow waters), may enhance the availability of these diaspores to waterbirds in shallow lakes at critical times in the annual cycle. It is less clear to what degree this harvesting of abundant diaspores is anything other than accidental while seeking other food items (although this seems highly unlikely). More manipulative captive-feeding experiments to determine the feeding profitability of foraging on such small energy-rich items would be incisive in confirming the importance of Charales diaspores to dabbling ducks where they occur.
Most of the identified literature sources referred to birds consuming stoneworts in autumn and winter after the growth season or in spring on migration in northern latitudes where winter ice cover protected green above-ground biomass. By contrast, feeding by moulting waterbird concentrations, where sometimes large densities of birds consume Charales in the middle of the growth season, was not reported (this may simply be an issue of underreporting, as certainly moulting mute swans do so; see Holm, 2002). Despite often very high rates of removal of Charales biomass by herbivorous birds during the annual cycle, some evidence suggests that these grazers may have relatively little effect on the stability of community composition (under otherwise stable conditions), but we urge more research on all these topics. It is evident, however, that consumption by waterbirds can, under some circumstances, have a major effect on the Charales biomass standing crop measured by researchers. These factors become especially important under conditions of climate change, when changes in annual wintering waterbird distributions (Pavón-Jordán et al., 2019) may expose Charales to prolonged exposure to grazing in more northern areas. Hence, Charales beds protected in former times by winter ice cover in colder climates could persist as spring green biomass for exploitation by staging waterbirds during the prelude to breeding, whereas the current lack of ice cover now permits winter consumption by the same or other herbivore species, denying birds a key feeding resource later in spring (see Fox, Nielsen & Petersen, 2019). Climate change (higher temperatures and/or reduced precipitation) could reduce water levels and increase birds' access to stonewort stands previously more protected by water depth. Clearly investigation of the effects of enhanced levels of pressure from seasonal grazing of green biomass and harvest of diaspores and their impacts on Charales growth, biomass and competitive ability under a range of environmental conditions represents a rich avenue for future research.
The literature provides evidence that several species of waterbird travel long distances in their annual cycle. Because many of these species forage within dense Charales beds and consume large numbers of Charales diaspores, they are potential long-distance endo- and ectozoochorous dispersers of these species, as well as potentially dispersing diaspores locally. We see this as a particularly exciting field for future study and experimentation and urge more investigations to determine the viability of oospores and vegetative bulbils after full passage through the guts of waterbirds to add to the existing literature and especially to understand the extent to which the carbonate armour of these organs contributes to this protection.
Several case studies have shown the tendency of shallow, relatively eutrophic waters to flip between clear-water, high-biodiversity and turbid, species-poor stable states. Charales thrive in the former and the literature shows they respond rapidly to improvements in water clarity. Indeed, Charales may contribute to these transitions, as well as maintaining a transparent water column once established, due to the physical density of their canopy, nutrient effects, and dampening of sedimentation/remobilisation of sediment and nutrients (Schneider et al., 2015) as well as having potential allelopathic effects on other plants, particularly phytoplankton communities (van Donk & van de Bund, 2002). Because the change from turbid conditions to rich submerged Charales beds has such profound effects on other features of aquatic diversity, waterbirds also show rapid aggregative responses to such regime changes, making them potentially ideal indicator species of ecological change. The literature confirms numerical responses to Charales biomass and area among specialist feeders on these plants (e.g. red-crested and common pochard) and more generalist herbivores known to exploit them (e.g. swans, gadwall, wigeon and coot) as we would predict. Although other species, such as omnivorous dabbling (e.g. mallard and teal) and diving ducks (e.g. tufted duck) invariably show some abundance benefits of increasing Charales biomass, relationships are less clear (because of their more indirect trophic relationships to other organisms associated with Charales) and require better investigation. Likewise, responses of piscivorous birds to changes in Charales biomass are variable and difficult to interpret and so also would repay further research. Finally, given the established importance of the rare and declining Charales habitat within the existing Important Bird Area and Natura 2000 protected area network, we urge a pan-European research and monitoring programme integrating data on levels of nutrients and chlorophyll a, and on submerged macrophyte, invertebrate and fish biomass associated with the waterbird communities that interact with them to improve their effective conservation management.
V. CONCLUSIONS
- (1)
This literature review showed that the thalli, asexual and sexual diaspores of a range of Charales species are consumed by waterbirds and in some cases appear to be highly significant sources of energy and nutrition, particularly in autumn and winter to dabbling and diving ducks and swans.
- (2)
Charales featured in the stomach contents of a large range of surface-feeding, diving and up-ending waterbird species. Several species exploit Charales as a local source of abundant food when available (including dabbling ducks, diving ducks, swans and coot) but only a few seem to be specialist feeders (e.g. red-crested pochard).
- (3)
As a result, several waterbird species show numerical or aggregative responses to increased Charales biomass and extent, while other avian species benefit because of indirect benefits of clear-water-column conditions that are associated with Charales abundance in shallow lake ecosystems.
- (4)
Previous studies collectively document the contributions of Charales to maintaining water clarity, with knock-on beneficial effects for a variety of waterbird species and biodiversity targets, a process to which waterbirds can contribute.
- (5)
Despite substantial and sometimes total removal of above-ground Charales biomass by avian (and other) herbivores in autumn/winter, these plants persist in aquatic systems over decades, suggesting that the abundance of diaspores overcomes the annual loss of standing crop to such grazers.
- (6)
Despite major interest in the topic of aquatic plant dispersal by waterbirds and numerous studies, we lack rigorous studies of the nutritional and energetic value of Charales and their diaspores to waterbirds, especially relative to other submerged macrophytes. We also urgently require manipulative studies of the ability of Charales diaspores to maintain their viability after progression through the alimentary canal of migrating waterbirds and hence their ability to disperse over large biogeographical distances, and over barriers such as mountain ranges and oceans, to colonise new suitable habitats under conditions of rapid climate change.
ACKNOWLEDGEMENTS
We sincerely thank the participants of the 23rd Meeting of the Group of European Charophytologists (GEC) 16–19 August 2022, held in Riga, Latvia for their contributions to a presentation on this subject and our employers for the time to compile this review. Many thanks to Prof. Hendrik Schubert at the University of Rostock for his friendly and understanding guidance on the taxonomy and nomenclature of the Charophytes and for providing the definitive current taxonomy. We are also deeply grateful to two anonymous referees and to the editors for their suggestions and improvements to the manuscript.




