Spatiotemporal regulation of maternal mRNAs during vertebrate oocyte meiotic maturation
ABSTRACT
Vertebrate oocytes face a particular challenge concerning the regulation of gene expression during meiotic maturation. Global transcription becomes quiescent in fully grown oocytes, remains halted throughout maturation and fertilization, and only resumes upon embryonic genome activation. Hence, the oocyte meiotic maturation process is largely regulated by protein synthesis from pre-existing maternal messenger RNAs (mRNAs) that are transcribed and stored during oocyte growth. Rapidly developing genome-wide techniques have greatly expanded our insights into the global translation changes and possible regulatory mechanisms during oocyte maturation. The storage, translation, and processing of maternal mRNAs are thought to be regulated by factors interacting with elements in the mRNA molecules. Additionally, posttranscriptional modifications of mRNAs, such as methylation and uridylation, have recently been demonstrated to play crucial roles in maternal mRNA destabilization. However, a comprehensive understanding of the machineries that regulate maternal mRNA fate during oocyte maturation is still lacking. In particular, how the transcripts of important cell cycle components are stabilized, recruited at the appropriate time for translation, and eliminated to modulate oocyte meiotic progression remains unclear. A better understanding of these mechanisms will provide invaluable insights for the preconditions of developmental competence acquisition, with important implications for the treatment of infertility. This review discusses how the storage, localization, translation, and processing of oocyte mRNAs are regulated, and how these contribute to oocyte maturation progression.
I. INTRODUCTION
Infertility is a global health issue affecting millions of people of reproductive age worldwide (Mascarenhas et al., 2012; Dyer, 2009; Boivin et al., 2007). Over the last two decades, with significant advancements in assisted reproduction technologies (ART), such as in vitro fertilization (IVF) coupled with embryo transfer, an increasing number of people are seeking ART treatment. IVF success is generally reported on the basis of live birth rate, which is calculated as the number of live births per 100 embryo transfers. The live birth rate from the first cycle of IVF with fresh embryo transfers in women under 35 years of age is still low, at around 29–33% (Toftager et al., 2017; Calhaz-Jorge et al., 2016). Oocyte quantity and quality are believed to be rate-limiting to the success of IVF. There still is room for optimization of current ART treatment strategies at several levels, mainly through a better understanding of the processes yielding a developmentally competent egg.
Oocyte developmental competence is usually defined as the ability of a female gamete to mature into an egg that has the potential to be fertilized and support embryo development and pregnancy. This gamete property comprises some crucial and complex biological transitions, including remodelling of the female gamete to accept and integrate the male pronucleus, nuclear reprogramming to form the totipotent zygote, gamete genome inactivation, and embryonic genome activation. Oocyte developmental competence is gained through multiple sequential and interrelated events. Oocytes enter meiosis I in the embryonic gonads and remain arrested at the prophase I stage of meiosis. The oocytes then develop and grow in the ovarian follicles to acquire developmental competence. Growing and fully grown oocytes display distinct nuclear configurations. In growing oocytes, most chromatins are dispersed and decondensed. As the oocyte grows, the foci of chromatin cluster and eventually surround the nucleolus. The growing oocytes are transcriptionally active, allowing them to accumulate maternal transcripts that are essential for oocyte maturation and early embryo development. However, transcription declines and eventually halts towards the end of oocyte growth. Fully grown oocytes contain all the necessary transcripts for completing meiotic maturation. Based on their chromatin organization, fully grown oocytes from antral follicles can be classified as surrounded nucleolus (SN, chromatin forming a ring around the nucleolus) oocytes or non-surrounded nucleolus (NSN, chromatin has a diffuse pattern) oocytes (Tan et al., 2009; Monti et al., 2013). Most SN oocytes are competent regarding meiotic resumption but NSN oocytes have limited meiotic competence. Following meiotic resumption, oocytes complete meiosis I and are arrested again at metaphase II until fertilization. After IVF, embryos originating from NSN oocytes usually arrest at the two-cell stage, while SN-derived embryos develop to the blastocyst stage (Monti et al., 2013). Overall, transcriptionally silent SN oocytes that have accumulated all essential transcripts have higher developmental potential compared to transcriptionally active NSN oocytes.
Because global transcription ceases after oocyte growth and remains silent until embryonic genome activation, oocyte maturation and fertilization largely depend upon posttranscriptional regulation of already synthesized transcripts. Fully grown oocytes harbour a large pool of maternal messenger RNAs (mRNAs) that likely correspond to more than half of all the protein-coding genes in the genome of mice and humans (Evsikov et al., 2006; Wang et al., 2004; Sha et al., 2020b,a; Wu & Dean, 2020; Liu et al., 2016; Yu et al., 2016). A large proportion of mRNA transcripts in oocytes are stored in a dormant state, selectively recruited to translate at the appropriate time, and degraded in a timely manner to coordinate oocyte maturation events. These posttranscriptional regulation events are controlled by trans-acting factors that interact with cis-acting elements within mRNAs. More than 200 RNA-binding proteins (RBPs) have been identified in oocytes (Conti & Franciosi, 2018). Rapidly developing genome-wide techniques have greatly expanded our knowledge of global translation changes at different stages of oocyte maturation and the potential regulatory mechanisms involved (Chen et al., 2011; Luong et al., 2020; Yang et al., 2020c). Cytoplasmic polyadenylation is a key mechanism for translational activation and the differential translational activation of different mRNAs likely involves a combinatorial code of different cis-acting elements and multiple RBPs (Charlesworth et al., 2006; Pique et al., 2008). In addition, timely elimination of maternal mRNAs is also crucial for proper oocyte-to-zygote transition. Increasing evidence indicates that multiple mechanisms, involving RBPs (Zhao et al., 2020, 2022; Dumdie et al., 2018; Sha et al., 2018), posttranscriptional modifications (Chang et al., 2018; Morgan et al., 2017; Ivanova et al., 2017; Zhao et al., 2017), and small RNAs (Yang et al., 2019; Roovers et al., 2015; Liu et al., 2017a; Stein et al., 2015), account for maternal mRNA stabilization and destabilization during oocyte maturation. Despite these exciting findings, a comprehensive understanding of the regulation of mRNA fate during oocyte maturation is yet to be established. In this review, we discuss various mechanisms of spatial and temporal posttranscriptional regulation of maternal mRNAs and highlight their roles in oocyte meiotic maturation. To appreciate fully the critical role of posttranscriptional regulation in oocyte maturation, we first outline the remarkable series of molecular and cellular events that the oocyte undertakes as it undergoes the two meiotic divisions – all in the complete absence of transcription.
II. KEY MOLECULAR AND CELLULAR FEATURES OF OOCYTE MEIOTIC MATURATION
In vertebrates, fully grown oocytes are arrested at the diplotene stage of prophase I, which is characterized by a large nucleus called the germinal vesicle (GV). GV breakdown (GVBD) is the first morphological sign of the resumption of meiosis. Following GVBD, the meiosis I spindle is assembled and homologous chromosomes align along with chiasmata. Once all chromosomes are attached to the spindle microtubules, the homologous chromosomes are segregated, and the oocyte is asymmetrically cleaved. After the first polar body is emitted, the oocytes arrest at metaphase II until reactivated by fertilization. This process is summarized in Fig. 1.
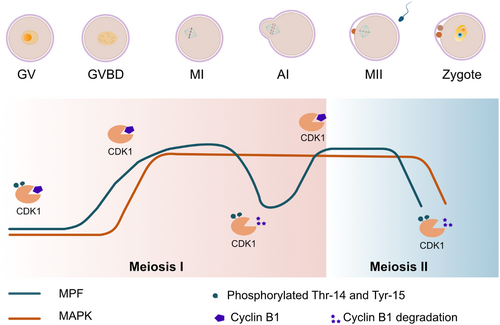
(1) Synthesis and modification of cell cycle regulators
The arrest, resumption, and progression of oocyte meiosis are determined by the coordinated protein synthesis, modification, and degradation of cell cycle regulators. Meiosis resumption and progression depend on an increase in M-phase promoting factor (MPF) kinase activity, the main driver of mitosis and meiosis. MPF is a complex of cyclin-dependent kinase 1 (CDK1), also known as p34 kinase cell division control (CDC) 2 and its partner B-type cyclins (Polanski et al., 1998; Murray, Solomon & Kirschner, 1989; Choi et al., 1991; Ledan et al., 2001; de Vantery et al., 1997; Kanatsu-Shinohara, Schultz & Kopf, 2000). During prophase I arrest, MPF is indirectly kept inactive due to high concentrations of intraoocyte cyclic AMP (cAMP) and active protein kinase A (PKA) in vertebrates (Gilchrist et al., 2016; Maller, Butcher & Krebs, 1979). CDK1 is phosphorylated (inactivated) by the inhibitory kinases WEE1B (also referred to as WEE2) (Parker & Piwnicaworms, 1992; Han et al., 2005). Cyclin B1 is constantly degraded by the anaphase promoting complex (APC), an E3 ubiquitin ligase, together with its activator CDC20 homolog (CDH) 1 (Reis et al., 2006). Hormonal stimulation or removal of oocytes from their follicular environment (in mammals) leads to a drop in cAMP levels and PKA inactivation, which, in turn results indirectly in MPF activation (Gilchrist et al., 2016; Maller et al., 1979). CDK1 is dephosphorylated (activated) shortly before meiotic resumption by phosphatase CDC25B (Coleman & Dunphy, 1994; Lincoln et al., 2002). Meanwhile, cyclin B1 levels increase through new protein synthesis and reach a plateau at the end of metaphase I (Ledan et al., 2001). At the onset of anaphase I, cyclin B1 is rapidly degraded by the APC together with its activator CDC20 (Glotzer, Murray & Kirschner, 1991; Yu et al., 2015). Importantly, the establishment of metaphase II arrest requires a second wave of cyclin B1 synthesis (Clarke & Masui, 1983; Hashimoto & Kishimoto, 1988) (Fig. 1). Hence, meiosis progression is regulated by oscillations in the activity of MPF.
CDK1 protein synthesis rises in mouse oocytes prior to the resumption of meiosis (Kanatsu-Shinohara et al., 2000; de Vantery et al., 1997; Levasseur et al., 2019). Three forms of cyclin B1 mRNAs carrying different lengths of 3′ untranslated regions (UTRs) exist in mouse oocytes (Pique et al., 2008). The cyclin B1 mRNAs with short 3′ UTRs are constantly translated at low levels (Yang et al., 2017). The cyclin B1 mRNAs with intermediate and long 3′ UTRs are repressed in GV oocytes and activated after GVBD. Increased cyclin B1 protein synthesis is characterized by ribosome association (Han et al., 2017; Sousa Martins et al., 2016). Reducing cyclin B1 levels in oocytes leads to defects in chromosome alignment and hence, missegregation events (Levasseur et al., 2019).
Cyclin B2 also plays important roles in the regulation of the meiotic cell cycle and works largely redundantly with cyclin B1 in mouse oocytes (Li et al., 2018; Brandeis et al., 1998; Daldello et al., 2019; Gui & Homer, 2013). However, unlike cyclin B1, cyclin B2 is present at higher levels at the GV stage and remains relatively stable during metaphase I, while there is a slight increase in translation upon GVBD (Han et al., 2017). Cyclin B3 has recently been reported to be required for metaphase to anaphase transition during meiosis I in mouse oocytes, however, its role is distinct from that of cyclin B1 and cyclin B2 (Li et al., 2019b; Karasu et al., 2019; Zhang et al., 2015).
In addition to the synthesis of cyclins, timely synthesis of some other cell cycle regulators is also essential for meiosis progression. Early mitotic inhibitor-1 (Emi1, an APCCDH1 inhibitor) is synthesized in GV oocytes to regulate MPF activity (Marangos et al., 2007). Cdc20 mRNA is recruited for translation to activate the APC during late metaphase I in mouse oocytes (Chen et al., 2011). Moreover, the synthesis of Emi2 (also called Erp1, an APCCDC20 inhibitor) is required for the reestablishment of MPF activity during metaphase II arrest (Fujioka et al., 2016; Madgwick et al., 2006; Shoji et al., 2006; Ohe et al., 2007). In addition, the c-mos proto-oncogene product (Mos), a mitogen-activated protein kinase (MAPK)-kinase–kinase (MAPKKK) that is expressed exclusively in the oocyte, is necessary for the activation of MAPK during oocyte maturation. Mos protein synthesis is activated at prometaphase in mouse oocytes (Gebauer et al., 1994; deMoor & Richter, 1997). Cyclins and CDK1 proteins in mouse GV oocytes are sufficient for meiotic resumption, and new protein synthesis is not required at this stage (Fulka et al., 1986). By contrast, the formation of the spindle and progression to metaphase II require active protein synthesis. However, the above findings on the synthesis of cyclins and CDK1 proteins upon mouse oocyte meiotic resumption could not be applied to other organisms, such as pigs and Xenopus (Richter, 2007). In addition, global translation is continuously active throughout oocyte and preimplantation development in mice, while the specific mRNAs that are recruited to the translation machinery alter at different stages (Chen et al., 2011; Luong et al., 2020; Zhang et al., 2022).
(2) Meiotic spindle assembly and function require protein synthesis
Following the resumption of meiosis I, the chromosomes in oocytes become condensed and the meiotic spindle is assembled. Unlike mitosis, the mammalian oocyte does not have the typical centrioles and astral microtubules for spindle formation (Severance & Latham, 2018; Duan & Sun, 2019). Instead, bipolar meiotic spindle assembly depends on the self-organization of numerous acentriolar microtubule organizing centres (MTOCs) (Schuh & Ellenberg, 2007; Combelles & Albertini, 2001; Messinger & Albertini, 1991). During chromosome congression, the homolog pairs are fully assembled in bivalents and remain tethered by the cohesin complexes (Fig. 2). The spindle assembly checkpoint (SAC) components monopolar spindle (MPS) 1, budding uninhibited by benzimidazoles (BUB) 1–3, and mitotic arrest deficiency (MAD) 1–3 localize to unattached kinetochores and inhibit APC activity, thereby preventing premature chromosome segregation (Yu, 2002; Shah & Cleveland, 2000; De Antoni et al., 2005). The nuclear division control (NDC)80 kinetochore protein complex and Aurora B/C kinases direct the kinetochore–microtubule attachments (Sanders & Jones, 2018; Vallot et al., 2018). Once all kinetochores are attached and stabilized, the SAC is inactivated, and the APC is activated (Kops & Shah, 2012). The activated APC causes securin and cyclin B1 destruction, thereby activating separase. It is followed by separase-mediated cleavage of cohesin complexes on the chromosome arms (Li et al., 2019a; Gorr et al., 2006; Petronczki, Siomos & Nasmyth, 2003), however, cohesins on centromeres are protected from cleavage by protein phosphatase 2A (PP2A) and shugoshin2 (Sgo2) (Rattani et al., 2013; El Yakoubi et al., 2017). Subsequently, the homologous chromosomes are segregated (Fig. 2). Various mRNAs are localized onto the meiotic spindle to allow for the local translation and control of meiotic progression (Susor et al., 2015, 2016; Vinot et al., 2004; Dumont et al., 2007; VerMilyea et al., 2011; Romasko et al., 2013). For example, the mRNAs for components of the spindle [such as targeting protein for Xenopus plus end-directed kinesin-like protein (TPX) 2, kinesin family member (KIF) 11, and spindle apparatus coiled-coil protein (SPDL) 1], proteins involved in the SAC (such as BUB1B and BUB3), proteins involved in kinetochore–microtubule attachments (such as NDC80 and KIF18B), and APC activator CDC20 are recruited at the appropriate time to translation machinery during these processes (Chen et al., 2011, 2013; Yang et al., 2020c).
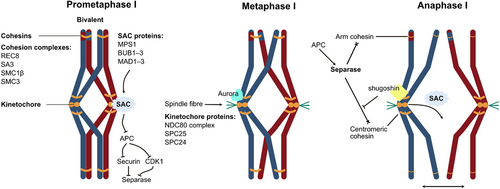
(3) Metaphase II arrest requires protein synthesis
Following the completion of meiosis I, the oocytes are arrested at metaphase II until reactivated by fertilization. Restoration of MPF activity and securin is critical for metaphase II arrest. Timely protein synthesis is essential for the restoration of cyclin B1 and securin during metaphase II arrest (Fan & Sun, 2019), and high MPF activity and securin responsible for metaphase II arrest are maintained by the cytostatic factor (CSF). CSF stabilizes MPF activity and securin by inhibiting APC ubiquitin ligase activity (Tung et al., 2005; Sako et al., 2014). Emi2 is an important CSF component, which maintains MPF activity by stabilizing cyclin B1 through inhibiting APC activity (Fujioka et al., 2016; Madgwick et al., 2006; Shoji et al., 2006; Ohe et al., 2010; Tang et al., 2010; Sako et al., 2014). Emi2 levels are low during oocyte meiotic progression, presumably to allow the APC to be active and permit passage through meiosis I (Madgwick et al., 2006). Interestingly, Emi2 synthesis begins around the end of meiosis I (Ohe et al., 2007; Tung et al., 2007). Thus, the establishment of cyclin B1, securin, and Emi2 by protein synthesis is an important mechanism for maintaining metaphase II arrest.
Taken together, oocyte meiotic maturation depends on the maintenance of optimal protein levels of key regulators through timely protein synthesis and regulated protein destruction to orchestrate various cellular events of oocyte maturation. The temporally regulated synthesis of these proteins is dependent solely on the posttranscriptional regulation of pre-existing maternal mRNAs, such as translational activation, mRNA stability, and subcellular localization. We consider these processes in more detail below.
III. GENESIS AND STORAGE OF MATERNAL mRNAS IN OOCYTES
During the period of oocyte growth, mammalian oocytes transcribe and accumulate maternal mRNAs required for subsequent maturation and embryonic development. However, in some organisms such as Drosophila, maternal mRNAs are transcribed by cells supporting the oocyte, and then are transferred to the oocyte (Wilsch-Brauninger, Schwarz & Nusslein-Volhard, 1997). High rates of transcription in growing mammalian oocytes generate large quantities of maternal RNAs (Stitzel & Seydoux, 2007). Among these accumulated RNAs, more than 60% are ribosomal RNAs (rRNAs) and about 19% are mRNAs that likely correspond to more than half of all the protein-coding genes in the genome of mice and humans (Evsikov et al., 2006; Wang et al., 2004; Sha et al., 2020b,a; Wu & Dean, 2020; Liu et al., 2016; Yu et al., 2016). In addition to mRNAs which are translated shortly after transcription, a large class of maternal mRNAs (30–45%) are stabilized in various ribonucleoprotein (RNP) complexes in a dormant state (Bettegowda & Smith, 2007; Balagopal & Parker, 2009).
These RNP complexes are often organized as membrane-less RNA–protein condensates known as cytoplasmic granules. One of the most common types of RNP granules is the processing bodies (P-bodies), which are sites of mRNA repression, decapping, and degradation. P-bodies were revealed by the presence of mRNA decapping enzyme 1A (DCP1A); thus, these RNP granules are also called DCP1-bodies. In mice, P-body-like structures are found in early oocytes arrested at prophase I, where they reside in the perinuclear region and at the cell cortex (Swetloff et al., 2009; Flemr et al., 2010). Immunostaining using antibodies against P-body components Argonaute 2 (AGO2), DCP1A, DEAD (Asp-Glu-Ala-Asp) box helicase 6 (DDX6), and enhancer of mRNA decapping 4 (EDC4)/trinucleotide repeat-containing gene 6 protein (TNRC6) showed bright spots of colocalizing signals in early oocytes from 2- and 12-day-old mice (Flemr et al., 2010). This indicates that P-bodies are present in growing mouse oocytes. Interestingly, P-bodies appeared larger in the smallest oocytes. However, P-bodies are lost in fully grown GV oocytes, MII eggs, and early embryos (Flemr et al., 2010). In another study, through exogenous overexpression of enhanced green fluorescent protein (EGFP)–DCP1A, P-body-like foci of two different sizes were observed in mouse GV oocytes (Swetloff et al., 2009). Overexpression of EGFP–DCP1A might result in non-physiological formation of P-bodies, which therefore appear different from the endogenous P-bodies detected by immunostaining. Considering that overexpression of EGFP alone did not lead to the formation of foci, differences in properties of endogenous and artificially expressed DCP1A proteins likely account for the above inconsistencies. In fact, endogenous DCP1A protein levels are low in GV oocytes (Flemr et al., 2010). Artificially overexpressing DCP1A proteins in GV oocytes might disturb the disassembly of DCP1A granules. Nevertheless, similar to the results from immunostaining, EGFP–DCP1A foci disappear during meiotic maturation (Swetloff et al., 2009). Although the mechanistic explanation for the loss of P-bodies in fully grown oocytes is unknown, it could be related to the function of the microRNA (miRNA) pathway, as miRNA-mediated mRNA repression is very inefficient in fully grown mouse oocytes (Ma et al., 2010).
Subcortical aggregates (SCAs) are another type of RNP complexes found in fully grown mouse oocytes (Flemr et al., 2010). The SCAs contain maternal mRNAs and RBPs such as DDX6, cytoplasmic polyadenylation element-binding protein 1 (CPEB1), and mouse Y-box protein 2 [MSY2, also known as Y box protein 2 (YBX2)]. During oocyte growth, the loss of P-bodies is observed and some of their components including CPEB1 and DDX6 become localized to the SCAs (Flemr et al., 2010). Another P-body component, like-Sm (LSm) domain-containing protein 14 homolog A (LSM14A) was localized to the subcortical region, indicating that LSM14A is a potential additional SCA component (Swetloff et al., 2009). Although the mechanism for the loss of P-bodies during oocyte growth is unclear, it could be related to the silencing of miRNA functions in fully grown oocytes (likely mouse oocyte specific). Nevertheless, SCAs are likely one potential strategy for maternal mRNA storage. These aggregates disperse during oocyte maturation, consistent with translational activation of maternal mRNAs that occurs during this time (Flemr et al., 2010). In contrast to the typical P-bodies, the SCAs lack the decapping enzyme DCP1 implicated in mRNA degradation (Flemr et al., 2010). This may indicate the functioning of SCAs in maternal mRNA storage and translational repression but not degradation. During oocyte maturation, the SCAs are disassembled, presumably to release stored maternal mRNAs from repression. The function of the SCAs is similar to stress granules, which are another type of RNP granules known to sequester and repress mRNAs during stress (Buchan & Parker, 2009). However, human antigen R (HuR) protein [also known as embryonic lethal abnormal vision (ELAV) like protein 1 (ELAVL1)], a component of stress granules, is not found in SCAs, thus excluding the possibility that SCAs are stress granules (Flemr et al., 2010). SCAs are in fact similar to the conserved germline helicase 1 (CGH-1, an ortholog of human DDX6) granules observed in Caenorhabditis elegans oocytes (Boag et al., 2008; Noble et al., 2008). In these granules, a subset of maternal mRNAs are bound to CGH-1 proteins, leading to the stabilization and translational repression of maternal mRNAs. These granules are thought to be an atypical type of P-bodies. Unlike typical P-bodies, these granules lack decapping activity.
The subcortical maternal complex (SCMC), which assembles during oocyte growth and localizes to the oocyte cortex, is critical for cytoplasmic partitioning and early embryogenesis (Li, Baibakov & Dean, 2008; Bebbere et al., 2021; Zhu et al., 2015). Upon its discovery in mouse oocytes (Li et al., 2008), four proteins were identified as components of the SCMC: nucleotide-binding oligomerization domain, leucine-rich repeat and pyrin domain-containing protein 5 (NLRP5; also known as MATER), KH domain containing protein 3 (KHDC3; also known as FILIA), oocyte expressed protein (OOEP; also known as FLOPED), and transducin-like enhancer of split6 (TLE6). Since then, further components have been attributed to the SCMC, such as peptidyl arginine deiminase 6 (PADI6), NLRP2, NLRP4f and zinc finger BED domain-containing protein 3 (ZBED3) (Mahadevan et al., 2017; Gao et al., 2018). All these proteins are maternally expressed and some, if not all, carry RNA-binding domains (RBDs). Recent studies linked the SCMC with maternal mRNA regulation regarding localization, translation, and stability. In mouse oocytes, MSY2-bound mRNAs localize to cytoplasmic lattices (CPLs) through PADI6, a component of the SCMC (Liu et al., 2017b). The oocyte CPLs are a fibrillar matrix containing proteins and RNAs, which store components of the translation machinery including ribosomes. In padi6 null oocytes and embryos, CPLs are disrupted, and de novo protein synthesis is dysregulated (Yurttas et al., 2008). These data indicate the SCMC might function in delivering or depositing maternal mRNAs to the oocyte CPLs. mRNA localization in oocytes has been of long-standing interest and RNA fluorescent in situ hybridization (FISH) is emerging as a powerful tool for RNA distribution analysis and quantification. Interestingly, RNA FISH analysis showed that Gdf9 mRNAs seemed to localize preferentially to the subcortical region of the one-cell embryo (Xie, Timme & Wood, 2018). However, whether Gdf9 mRNAs colocalize with any components of SCAs or the SCMC remains unknown. In another study, the distribution of mRNAs for deleted in azoospermia-like (Dazl) and β-actin was investigated but none showed a subcortical localization pattern (Jansova et al., 2018). RNA FISH revealed that cyclin B1 mRNAs form granules in a dormant state and are asymmetrically distributed in zebrafish (Danio rerio) and mouse oocytes (Kotani et al., 2013). Similarly, Jansova et al. (2021) showed that translationally dormant Cyclin B1 and Mos mRNAs form cloud-like structures in fully grown GV oocytes with consequent abundant translation at the centre of the MII oocytes in mice. However, these RNA granules or cloud-like structures might be distinct from SCAs and the SCMC, because they are mainly distributed in the cytoplasm whereas SCAs and the SCMC are in the subcortical region. Overall, very little is known about the localization of RNAs in the SCMC.
In early Xenopus oocytes, some maternal mRNAs are localized and translationally repressed in the mitochondrial cloud, also known as the Balbiani body, which contains mitochondria, Golgi, endoplasmic reticulum as well as germinal granules (Chang et al., 2004; Wilk et al., 2005). Within the Balbiani body, repressed mRNAs are organized in or around the germinal granules. The Balbiani body entraps and localizes maternal mRNAs to the vegetal cortex during stage I and II of oogenesis, which determines the polarity of the oocyte. The Balbiani body was also found in mouse oocytes of primordial follicles but disperses as follicles begin to grow (Pepling et al., 2007). The Balbiani body is likely to play a role in early oocytes but not in fully grown and maturing oocytes.
To prevent premature activation of the developmental program, stored maternal mRNAs are translationally repressed. There are several mechanisms that repress maternal mRNA translation. One of the most important is deadenylation, during which their poly(A) tails are shortened immediately after they are exported into the oocyte cytoplasm (Huarte et al., 1992). The immature oocytes contain translationally dormant mRNAs with short poly(A) tails of about 20–40 nucleotides (Bachvarova & Paynton, 1988; Yang et al., 2020c). They are activated for translation by elongation of the poly(A) tail at proper developmental time points. For further details, see Section V.2.
IV. TIMING OF MATERNAL mRNA TRANSLATIONAL ACTIVATION
Due to the absence of transcription, critical steps in oocyte maturation and early embryogenesis rely exclusively on the sequential translation of the deposited maternal mRNAs. The resumption of oocyte meiosis represents the first point of activation of maternal mRNAs. In vertebrates, meiosis resumes upon ovulation, which is induced by hormone stimulation – gonadotropin in mice and progesterone in Xenopus. One of the earliest transcripts to be translationally activated upon oocyte maturation is Mos. Mos mRNA is activated early after GVBD in mouse oocytes (Chen et al., 2011; Luong et al., 2020). Activation of this kinase initiates a subsequent burst of translation of mRNAs encoding factors required for oocyte maturation such as cyclin B1 (Sagata et al., 1989). The initial activation of translation of several key cell cycle regulators such as Mos and cyclin B1 takes place before MPF activation and GVBD in Xenopus, which is necessary for meiotic resumption (Richter, 2007). However, cyclins and CDK1 proteins in mouse GV oocytes are thought sufficient for meiotic resumption without new protein synthesis (Fulka et al., 1986).
Recently, several genome-wide analyses have revealed a global switch in maternal mRNA translation coinciding with oocyte meiotic resumption (Chen et al., 2011; Luong et al., 2020; Yang et al., 2020c). Using a ribosome tagging (RiboTag) coupled with RNA-sequencing (RNA-Seq) strategy, Luong et al. (2020) recently investigated the temporal correlation between maternal mRNA translation and the different steps in mouse oocyte meiosis. The genome-wide analysis revealed that mRNAs active in GV oocytes become repressed during meiotic resumption (1722 transcripts), whereas mRNAs repressed in GV oocytes become activated (1537 transcripts) (Luong et al., 2020). This global switch of translation pattern was demonstrated to start around the exit from prophase I, at the time of GVBD. They further showed that this global switch in translation pattern was dependent on CDK1 activation. Similarly, Yang et al. (2020c) recently measured 6497 mRNAs and identified 5% of them as showing a rapid increase in polysome association during Xenopus oocyte maturation. Of note, there appears to be a delay between the increase in polysome association and the increase in protein levels. For example, western blot experiments showed that protein-level increases were modest at 3 h after GVBD, when the mRNAs showed high levels of polysome association; however, robust increases in protein levels were observed at 20 h after GVBD (Yang et al., 2020c). The imaging of nascent translation of endogenous Mos and cyclin B1 mRNA in live oocytes may provide more detailed insight into the exact timing of the initial translation burst.
Using an adapted mRNA tail [3′ UTRs and poly(A) tail regions] sequencing (Tail-seq) method, Yang et al. (2020c) recently demonstrated that the global translation activation during oocyte meiotic resumption is coupled with poly(A) tail elongation and that poly(A) tail length changes precede translational changes. The poly(A) tail elongation is mediated by cytoplasmic polyadenylation, which is mainly regulated by U-rich cis-acting cytoplasmic polyadenylation elements (CPEs) near the polyadenylation signal (PAS) and associated CPEB1 protein (Richter, 2007). Notably, 95% of translationally activated transcripts contain at least one CPE in the 3′ UTR (Luong et al., 2020). However, CPEs and associated CPEB1 are not the only clues for differential translation activation of mRNAs. The timing of translation activation of individual mRNAs is likely determined by a combinatorial code of different cis-acting elements and associated trans-acting factors and is regulated by multiple mechanisms (Charlesworth et al., 2006; Pique et al., 2008; Yang et al., 2020c).
V. TRANSLATIONAL REGULATION OF MATERNAL mRNAS
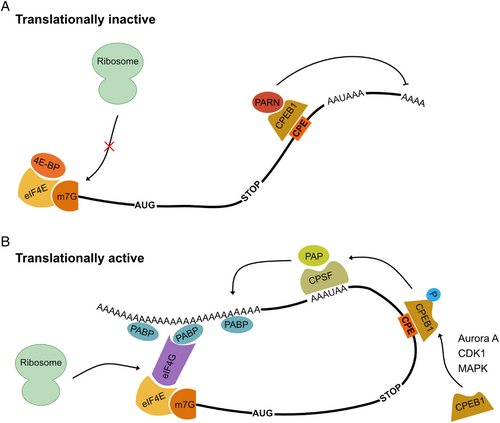
(1) Translational regulation by the complex at the 5′ UTR
Newly transcribed pre-mRNAs typically undergo splicing, 7-methylguanosine (m7G) capping at the 5′ end, and polyadenylation at the 3′ end. The latter two are relevant to translational initiation. In the cytoplasm, the 5′ cap-binding protein eukaryotic translation initiation factor (eIF) 4G (eIF4G) interacts with both eIF4E (Table 1) bound on the 5′ cap and poly(A)-binding protein (PABP) that associates with the poly(A) tail at the 3′ end (Sonenberg & Hinnebusch, 2009). By these interactions, the mRNA forms a steady closed-loop structure (Fig. 3). eIF4G and eIF4E together with eIF4A form the cap-binding complex eIF4F, which binds to the 40S ribosomal subunit to allow translation initiation. Formation of the eIF4F complex, particularly the interaction of eIF4G with eIF4E on the 5′ cap, is the rate-limiting step for translation initiation. The accessibility of eIF4E is regulated by eIF4E phosphorylation and eIF4E-interaction proteins, such as eIF4E-binding protein (4E-BP) and the eIF4E transporter (4E-T in humans, Clast4 in mice) (Susor et al., 2015). 4E-BP is a major regulator of the eIF4E and eIF4G interaction (Table 2). Upon resumption of oocyte meiosis, 4E-BP1, which is the only form of eIF4E-binding protein present in mouse and bovine oocytes, becomes phosphorylated at several sites resulting in its release from eIF4E, allowing eIF4F complex formation (Jansova et al., 2017; Susor et al., 2015; Severance & Latham, 2017). Seven phosphorylation sites (Thr-37, Thr-46, Ser-65, Thr-70, Ser-83, Ser-101, and Ser-112) of mouse 4E-BP1 have been reported (Qin, Jiang & Zhang, 2016), and at least three of these sites (Thr-37, Thr-46, and Thr-70) can be phosphorylated by mechanistic target of rapamycin (mTOR) (Table 2) (Jansova et al., 2017; Susor et al., 2015; Severance & Latham, 2017). It was first demonstrated in rat somatic cells that, upon phosphorylation by alpha serine/threonine-protein kinase (Akt, also known as protein kinase B or RAC-protein kinase) and downstream mTOR, 4E-BP dissociates from eIF4E and the free eIF4E then binds to eIF4G to form the eIF4F complex on the mRNA for protein synthesis (Gingras et al., 1999). Similarly, it was confirmed that the Akt–mTOR–eIF4E axis is activated at the onset of GVBD, followed by translational activation in mouse oocytes (Han et al., 2006; Kalous et al., 2006; Susor et al., 2015). Oocyte meiotic spindles are associated with spindle-enriched mRNAs and the translational initiation complex to promote spindle formation. 4E-BP1 phosphorylation was reported to play an important role in the translational activation of spindle-enriched mRNAs and meiotic spindle formation (Romasko et al., 2013). 4E-BP1 phosphorylated on Ser-65 is localized at the spindle poles, and 4E-BP1 phosphorylated on Thr-70 localizes on the spindle during oocyte meiosis (Jansova et al., 2017). Overexpression of a dominant negative 4E-BP1 mutant in mouse oocytes obstructs translation and causes spindle abnormality. mTOR-mediated 4E-BP1 phosphorylation on Thr-70 appears to be required for spindle formation. mTOR inhibition by Rapamycin during meiosis I disrupts the spindle structure and causes defects in chromosome alignment (Susor et al., 2015). However, mTOR inhibition by Torin 1 did not cause any spindle defects (Severance & Latham, 2017). This lack of phenotype might be caused by the inefficiency of mTOR inhibition by Torin 1.
| Name | Binding elements | Function | Species | References |
|---|---|---|---|---|
| eIF4E | m7G cap | Translational initiation | Mouse | Susor et al. (2015) |
| PABP1 (PABPC1) | Poly(A) (>12 nt) | Translational initiation and mRNA stability | Mouse; Xenopus |
Brook et al. (2009); Ozturk & Uysal (2017); Ozturk et al. (2015, 2016); Uysal et al. (2019) |
| ePABP (ePAB) | Poly(A) (>12 nt) | Translational initiation and mRNA stability; predominant PABP in oocytes | Human; mouse; Xenopus |
Ozturk et al. (2017); Seli et al. (2005); Brook et al. (2009); Ozturk et al. (2015, 2016); Guzeloglu-Kayisli et al. (2008); Uysal & Ozturk (2019) |
| CPEB1 | CPE (UUUUA[A]U) | Polyadenylation regulation | Mouse; Xenopus |
Pique et al. (2008); Dai et al. (2019); Mendez et al. (2000b); Tay & Richter (2001) |
| CPEB2 | Unknown | Unknown | ||
| CPEB3/4 | uridines in secondary structure | Polyadenylation regulation | Mouse; Xenopus |
Huang et al. (2006); Igea & Mendez (2010) |
| DAZL | DBE (UU[G/C]UU) | Translational activation | Human; mouse; pig; Xenopus | Fu et al. (2015); Chen et al. (2011); Brook et al. (2009); Liu et al. (2009) |
| PUM1 | PBE (UGUANAUA) | mRNA localization and translational repression | Human, mouse | Jansova et al. (2018); Mak et al. (2016, 2018) |
| PUM2 | PBE (UGUANAUA) | Translational repression | Mouse Xenopus |
Ota et al. (2011a); Padmanabhan & Richter (2006); Lin et al. (2018) |
| Staufen (STAU1/2) | dsRNA region | mRNA transport | Mouse; zebrafish; Xenopus | Takahashi et al. (2018); Lasko (2012); Cao et al. (2016); Santis et al. (2015) |
| Musashi (MSI) | MBE ([G/A]U1–3AGU) | Translational activation | Mouse; Xenopus |
Sutherland et al. (2015); Cragle et al. (2019); Charlesworth et al. (2006); Arumugam et al. (2012) |
| MSY2 | (UCCAUCA) | mRNA stabilization | Mouse; Xenopus |
Yang et al. (2005); Medvedev et al. (2011, 2008) |
| ZAR1/2 | ([A/U]UU[A/G]UCU) | mRNA stabilization; translational activation/repression | Mouse; Xenopus |
Rong et al. (2019); Yamamoto et al. (2013); Charlesworth et al. (2012) |
| ZAR1L | Unknown | Translational repression | Xenopus | Heim et al. (2022) |
| ZFP36L2 | ARE (AUUUA) | CCR4–NOT adaptor | Mouse | Ball et al. (2014); Dumdie et al., 2018; Sha et al. (2018) |
| BTG4 | none | CCR4–NOT adaptor | Mouse | Doidge et al. (2012); Winkler (2010) |
| PABPN1L | Poly(A) | BTG4 adaptor | Mouse | Zhao et al. (2020) |
- ARE, AU-rich element; BTG4, BTG anti-proliferation factor 4; CCR4–NOT, carbon catabolite repression 4 (CCR4)–negative on TATA-less (NOT) deadenylase complex; CPE, cytoplasmic polyadenylation element; CPEB1, cytoplasmic polyadenylation element binding protein 1; DAZL, deleted in azoospermia-like; DBE, DAZL biding element; dsRNA, double-strand RNA; eIF4E, eukaryotic translation initiation factor 4E; ePABP, embryonic poly(A)-binding protein (also known as ePAB); m7G, 7-methylguanosine; MBE, Musashi binding element; MSY2, mouse Y-box protein 2; PABPC1, cytoplasmic PABP 1; PABPN1L, nuclear PABP 1 like protein; PBE, Pumilio protein binding element; PUM1/2, Pumilio protein 1/2; ZAR1/2, zygote arrest protein 1/2; ZAR1L, ZAR1 like protein; ZFP36L2, Zinc finger protein 36-like 2.
| Name | Function | References |
|---|---|---|
| 4E-BP1 | Blocks eIF4E and eIF4G interaction | Jansova et al. (2017); Susor et al. (2015); Severance & Latham (2017) |
| mTOR | Phosphorylates 4E-BP1 | Jansova et al. (2017); Susor et al. (2015); Severance & Latham (2017) |
| PAPα | Poly(A) polymerase | Jiang et al. (2021) |
| PAN2/PAN3 | deadenylase | Wiederhold & Passmore (2010); Schoenberg & Maquat (2012) |
| CCR4–NOT | Deadenylase complex | Wiederhold & Passmore (2010); Schoenberg & Maquat (2012) |
| PARN | Deadenylase | Wiederhold & Passmore (2010); Schoenberg & Maquat (2012) |
| TUT4 (ZCCHC11) | Terminal uridylyl transferases (TUTases) | Chang et al. (2018); Morgan et al. (2017); Sha et al. (2020b) |
| TUT7 (ZCCHC6) | Terminal uridylyl transferases (TUTases) | Chang et al. (2018); Morgan et al. (2017); Sha et al. (2020b) |
| YTHDF2 | Destabilize m6A-modified mRNAs | Wang et al. (2014); Zhao et al. (2017); Ivanova et al. (2017) |
- 4E-BP1, eukaryotic translation initiation factor 4E binding protein 1; CCR4–NOT, carbon catabolite repression 4 (CCR4)–negative on TATA-less (NOT) deadenylase complex; mTOR, mechanistic target of rapamycin; PAPα, poly(A) polymerase α; PARN, poly(A)-specific ribonuclease; TUT4/7, terminal uridylyl transferases 4/7; YTHDF2, YTH-domain family protein 2; ZCCHC6/11, CCHC-type zinc finger protein 6/11.
In addition to mTOR, CDK1 might also be involved in 4E-BP1 phosphorylation. The inhibition of CDK1 by Roscovitine showed significant suppression of 4E-BP1 phosphorylation in mouse oocytes (Jansova et al., 2017). Moreover, Severance & Latham (2017) reported that polo-like kinase 1 (PLK1) phosphorylates 4E-BP1 on Ser-112 at the mouse meiotic spindle. PLK1 inhibition by BI2536 supresses 4E-BP1 phosphorylation and disrupts normal spindle formation and function (Severance & Latham, 2017). However, Jansova et al. (2017) reported that adding PLK1 inhibitor BI2536 to the oocytes had no effect on 4E-BP1 phosphorylation. This discrepancy might be caused by the different concentrations of PLK1 inhibitor BI2536 and the different treatment time used in the two studies: BI2536 was added to oocytes at a concentration of 500 nM for 7 h in Severance & Latham (2017) whereas 100 nM BI2536 was added for 2 h in Jansova et al. (2017).
In summary, the spatiotemporally regulated formation of the translational initiation complex at the 5′ UTR is a key step for maternal mRNA translation and is crucial for oocyte meiotic maturation. Notably, 4E-BP1 phosphorylation at several sites is required for this step.
(2) Translational regulation by cytoplasmic polyadenylation
Over the last three decades, many studies have demonstrated that cytoplasmic polyadenylation is one of the most important mechanisms for the control of the timely activation of maternal mRNAs during oocyte maturation (Vassalli et al., 1989; Oh et al., 2000; Paynton, Rempel & Bachvarova, 1988; Salles et al., 1992; Simon, Tassan & Richter, 1992; Huarte et al., 1992). The interaction of PABP on the poly(A) tail with eIF4G located on the 5′ cap maintains the closed-loop state of the mRNA (Fig. 3), which increases its translational efficiency and protects mRNA from degradation because the two ends of the mRNA become inaccessible to exonucleases. Recent genome-wide analyses revealed a global switch in maternal mRNA translation upon oocyte meiotic maturation and demonstrated that the poly(A) tail length of mRNAs was positively correlated with translational activity as oocytes progressed through meiosis (Chen et al., 2011; Luong et al., 2020; Yang et al., 2020c). For example, translationally repressed cyclin B1 mRNA, encoding the regulatory subunit of MPF, has a short (20–40 nt) poly(A) tail and is kept translationally dormant in oocytes (Cao & Richter, 2002). The timing of cyclin B1 mRNA translational activation is determined by different poly(A) tail length in 3′ UTRs (Tay, Hodgman & Richter, 2000). A threefold increase in the length of the poly(A) tail was observed when it is translationally activated (Yang et al., 2017; Sousa Martins et al., 2016). Cytoplasmic polyadenylation and subsequent translation of mos mRNA is necessary for MAPK and CDK1 activation upon GVBD and is required for MII arrest of mouse oocytes (Gebauer et al., 1994; Okeefe et al., 1989).
(a) cis-Acting elements within the 3′ UTR regulate cytoplasmic polyadenylation
The timing of poly(A) elongation of different mRNAs is determined by the specific arrangement of elements within the 3′ UTR, such as CPEs (Richter, 1999), DAZL-binding elements (DBEs) (Collier et al., 2005; Jenkins, Malkova & Edwards, 2011), and PASs as well as Pumilio-binding elements (PBEs) (Pique et al., 2008; Dai et al., 2019; Charlesworth, Meijer & de Moor, 2013; Weill et al., 2012). About three decades ago, it was demonstrated that the mRNA of tissue plasminogen activator (tPA) was translationally activated, and the poly(A) tail was lengthened during mouse oocyte maturation (Huarte et al., 1987, 1992; Strickland et al., 1988; Vassalli et al., 1989). This elongation of the poly(A) tail was found to be dependent on an element in the 3′ UTR, which later was recognized as CPE, a consensus U-rich sequence located in the 3′ UTR (UUUUAA or UUUAAU) (deMoor & Richter, 1997; Simon et al., 1992). Since then, numerous mRNAs that become translationally activated during oocyte maturation have been identified (Chen et al., 2011; Luong et al., 2020; Yang et al., 2020c), and CPEs confirmed to be essential for translation activation in most cases (Dai, Newman & Moor, 2005; Gebauer et al., 1994; Gershon, Galiani & Dekel, 2006; Murai et al., 2010; Tay et al., 2000; Tremblay et al., 2005; Yang et al., 2010; Mendez et al., 2000a). By analysing polysome occupancy on mRNAs (as a marker of translational activity) in mouse oocytes, it was discovered that, among the 7600 mRNAs analysed, approximately 17% had a more than twofold increase in polysome association and another 20% had decreased polysome occupancy during the GV–MII transition (Chen et al., 2011). Similar results were obtained in a recent genome-wide analysis of translation switch during prophase I to metaphase I transition (Luong et al., 2020). Further analysis of the 3′ UTR region showed that several elements, such as CPEs and DBEs, were enriched in polysome-associated mRNAs such as mos and cyclin B1 mRNAs (Chen et al., 2011; Han et al., 2017; Luong et al., 2020; Yang et al., 2020c). About 95% of translationally activated transcripts have at least one CPE in the 3′ UTR, whereas mRNAs lacking either of these elements had less polysome occupancy. These data indicate that CPEs and other elements in the 3′ UTR play essential roles for translation activation and that the number of CPEs appears to have an important effect on the early or late polyadenylation of different mRNAs. For example, the early adenylating mos mRNA has a single CPE while the late adenylating cyclin B1 mRNA contains three CPEs. Removing one of the cyclin B1 CPEs results in early adenylation (deMoor & Richter, 1997). In line with this, it was reported that two CPEs were required for repression, but one CPE was sufficient for activation (Luong et al., 2020). Conversely, another study reported that a single CPE was necessary for repression (Dai et al., 2019). It appears that the function of CPEs is determined by the 3′ UTR context and other associated regulators. Moreover, the positions of CPEs in the 3′ UTR might also influence the cytoplasmic polyadenylation (Pique et al., 2008; Weill et al., 2017). CPE density in the 100 nt 5′ UTR of the PAS site (AAUAAA or AUUAAA) is significantly higher in translationally activated mRNAs (Luong et al., 2020; Yang et al., 2020c).
In addition to CPEs, the PAS in the 3′ UTR is also required for polyadenylation. Disruption of the PAS in a reporter mRNA that contained the cyclin B1 3′ UTR impeded poly(A) tail elongation, and this reporter mRNA translated less efficiently than control mRNA (Tay et al., 2000). The PAS recruits cleavage and polyadenylation specificity factor (CPSF), a complex of four polypeptides, which then cleaves the mRNA at the polyadenylation site. CPSF also recruits poly(A) polymerases (PAPs) to catalyse the addition of adenosine nucleotides to the 3′ ends of mRNAs (Fig. 3). Mutation of the PAS-binding domain in CPSF4, an important subunit of the CPSF complex responsible for PAS binding, resulted in severe defects in meiosis (Dai et al., 2019). Thus, the loading of CPSF complex to PAS is essential for cytoplasmic polyadenylation of mRNAs in oocytes.
(b) PAPs are responsible for cytoplasmic polyadenylation
Several cytoplasmic PAPs have been identified, including PAP-associated domain-containing 4 [PAPD4, also known as germ line development 2 (GLD2)], PAPD5 [also known as terminal nucleotidyltransferase 4B (TENT4B)], and PAPD7 (TENT4A) (Laishram, 2014; Burns et al., 2011). GLD2 is translationally repressed until after maturation, and knockout of the gene did not block cytoplasmic polyadenylation during mouse oocyte maturation (Nakanishi et al., 2006, 2007), indicating GLD2 is not necessary or not the only PAP responsible for mRNA polyadenylation during oocyte maturation, at least in mice. A recent study reported that PAPD5 and PAPD7 produce a mixed poly(A) tail with an intermittent non-adenosine nucleotide that protects mRNAs from rapid deadenylation (Lim et al., 2018). Hence, they are likely not responsible for mRNA polyadenylation during mouse oocyte maturation. There are at least three nuclear-localized PAPs identified in mammalian cells: PAPα, PAPβ, and PAPγ (Laishram, 2014). PAPβ is only found in the testes (Lee et al., 2001). PAPγ exhibits monoadenylation activity toward small RNAs and is active during tumorigenesis (Topalian et al., 2001). Very recently, Jiang et al. (2021) identified PAPα as the elusive enzyme that catalyses cytoplasmic polyadenylation of maternal mRNAs in mouse oocytes (Table 2). Inhibition of PAPα activity through overexpression of a PAPα mutant impaired cytoplasmic polyadenylation and translation of maternal mRNAs, thus preventing meiotic progression. PAPα was primarily localized in the nucleus in fully grown oocytes but was shuttled to the cytoplasm after GVBD. Upon oocyte meiosis resumption, activated CDK1 and extracellular-signal regulated kinase (ERK) 1/2 MAPK cooperatively phosphorylate three serine residues (537, 545 and 558) of PAPα, thereby increasing its activity. Interestingly, activated PAPα stimulated polyadenylation and translation of its own mRNA (Papola) in a positive feedback manner, and thus its activity and levels were significantly amplified. PAPα seems to be primarily responsible for cytoplasmic polyadenylation during oocyte maturation. It is plausible that nuclear-localized PAPα might be released into the cytoplasm upon GVBD, and it then triggers the cytoplasmic polyadenylation of maternal transcripts.
(c) CPEBs regulate cytoplasmic polyadenylation
CPEB1 (also referred to as CPEB in early studies) is an mRNA-binding protein and zinc finger-containing protein originally identified in Xenopus oocytes (Table 1). It is a key factor that regulates cytoplasmic polyadenylation-induced translation of maternal mRNAs through binding to the CPEs in the target mRNAs (Hake & Richter, 1994; Hake, Mendez & Richter, 1998). Although all the factors required for cytoplasmic polyadenylation are present in oocytes, they become active only from the onset of maturation. Phosphorylation of CPEB1 at specific sites is a critical step for poly(A) lengthening and subsequent translational activation of maternal mRNAs (Tay et al., 2003, 2000; Atkins et al., 2005, 2004; Mendez et al., 2000b; Su & Eppig, 2002; Hake & Richter, 1994; Paris et al., 1991; Hodgman et al., 2001). CPE plays dual roles, namely, translational repression or activation, which are determined by the phosphorylation status of CPEB1 bound to it (deMoor & Richter, 1997; Tay et al., 2000; Luong et al., 2020). Most of the molecular details of this process have been revealed using oocytes from Xenopus and mice. In mice, CPEB1 binds to the CPE in cyclin B1 mRNA and regulates its polyadenylation and translation (Tay et al., 2000). Precluding CPEB1 binding to the CPE in cyclin B1 mRNA delays its cytoplasmic polyadenylation as well as the transition from GVBD to MII. CPEB1 is phosphorylated at MI during mouse oocyte maturation, which correlates with the activation of cyclin B1 mRNA translation. These results indicate that phosphorylation of CPEB1 switches CPEB1 activity from a translational repressor to a translational activator (Fig. 3).
It is thought that CPEB1 phosphorylation occurs in at least two waves: before GVBD (referred to as early phosphorylation) and after GVBD (referred to as late phosphorylation). Late CPEB1 phosphorylation was first discovered in Xenopus oocytes by western blots using antibodies. CPEB1 was present in immature oocytes as a single species, whereas upon GVBD an additional slow-migrating form of CPEB1 was observed, which was deemed the phosphorylated form of CPEB1 (deMoor & Richter, 1997; Hake & Richter, 1994; Paris et al., 1991). Early phosphorylation of CPEB1 before GVBD was later observed by in vivo labelling using 32P in progesterone-treated Xenopus oocytes (Mendez et al., 2000a). Of note, this early phosphorylation did not alter the mobility of CPEB1 in the sodium dodecyl-sulphate (SDS)-polyacrylamide gel (PAGE), which might explain why it was not observed in earlier studies using antibodies. In line with this, early phosphorylation of CPEB1 was observed in mouse oocytes before GVBD only when detected by Phos-tag gel but not by western blots using antibodies (Uzbekova et al., 2008; Yang et al., 2010; Han et al., 2017).
Early phosphorylation of CPEB1 in oocytes might involve multiple kinases (Table 3). Ser-174 is a key site for the early phosphorylation of Xenopus CPEB1. Phosphorylation of this site is necessary and sufficient to induce early cytoplasmic polyadenylation, for example, mos mRNA polyadenylation in Xenopus oocytes (Mendez et al., 2000a,b). Eg2 (an Aurora A serine/threonine kinase) was identified as a key kinase that accounts for early phosphorylation of CPEB1 on Ser-174 during Xenopus oocyte maturation (Mendez et al., 2000a,b). In mice, Thr-171 was identified as an essential Aurora-catalysed phosphorylation site of CPEB1 in oocytes (Tay et al., 2003). Hodgman et al. (2001) claimed that mouse Eg2 also catalyses CPEB1 phosphorylation. In fact, their data showed that the mouse MI oocyte extract can phosphorylate recombinant Xenopus CPEB1 on Ser-174, but they did not present strong evidence to show this effect is caused by Eg2. Conversely, blocking Aurora A kinase through microinjection of Aurora A-specific antibodies in Xenopus oocytes does not block meiotic resumption (Castro et al., 2003). Blocking of Aurora A kinase by this antibody looks specific and efficient because the injected oocytes are arrested at metaphase I after GVBD and this effect is abolished by co-injection with an excess of recombinant Aurora A protein. In line with this, through blocking Aurora kinases using ZM447439, it was shown that Aurora kinases are not required for Xenopus CPEB1 phosphorylation (Keady et al., 2007). Similarly, inhibition of Aurora kinase using MLN8237 or VX680 has no effect on CPEB1 phosphorylation and meiotic resumption in mouse, bovine, and porcine oocytes (Han et al., 2017; Uzbekova et al., 2008; Komrskova et al., 2014). In addition, active Aurora A is undetectable in early-stage Xenopus oocytes prior to CDK1 activation when using an antibody that recognizes phosphorylation-activated Aurora A (Keady et al., 2007). Furthermore, CDK1 was reported to be necessary and sufficient to trigger the phosphorylation activation of Aurora A kinase in Xenopus oocytes (Maton et al., 2003). Thus, Aurora A is unlikely to be activated before CDK1 activation and seems dispensable for CPEB1 phosphorylation and meiotic resumption (Meneau et al., 2020). These opinions challenge the previously proposed concept that Eg2 Aurora A kinase mediates early CPEB1 phosphorylation in oocytes. Taken together, Aurora A seems not to be the key kinase responsible for early phosphorylation of CPEB1 during oocyte meiosis, at least not before CDK1 activation.
| Phosphorylation stage | Kinase | Phosphorylation site | Reference |
|---|---|---|---|
| Early stage (before GVBD) | Aurora A | Xenopus CPEB1 (Ser-174); mouse CPEB1 (Thr-171) |
Mendez et al. (2000a,b); Tay et al. (2003) |
| CDK1 | Unknown | Padmanabhan et al. (2006) | |
| MAPK | Xenopus CPEB1 (Thr-164, Ser-184, and Ser-248) | Keady et al. (2007) | |
| Late stage (after GVBD) | CDK1 | Xenopus CPEB1 (Ser-210); mouse CPEB1 (unknown) |
Hake et al. (1994); Paris et al. (1991); deMoor et al. (1997); Mendez et al. (2002); Cao et al. (2020); Han et al. (2017) |
- CDK1, cyclin-dependent kinase 1; GVBD, germinal vesicle breakdown; MAPK, mitogen-activated protein kinase.
Interestingly, it was reported that early CPEB1 phosphorylation requires RINGO/Spy, a cyclin B1-like cofactor that activates CDK1 (Padmanabhan & Richter, 2006). RINGO/Spy might be involved in the early activation of CDK1, which, in turn, triggers CPEB1 phosphorylation. A later study reported that CPEB1 can be phosphorylated by MAPK on three residues (Thr-164, Ser-184, Ser-248) in Xenopus oocytes before GVBD (Keady et al., 2007) (Table 3). MAPK is demonstrated to bind directly and phosphorylate CPEB1 on Thr-164, Ser-184, and Ser-248 residues, but not on Ser-174. They also reported that this early MAPK activation occurred prior to and independent of Mos synthesis. Consistently, low levels of active ERK1/2 MAPK were also detected in early mouse and bovine GV oocytes (Cao, Jiang & Fan, 2020; Wehrend & Meinecke, 2001; Uzbekova et al., 2008). Cao et al. (2020) demonstrated that ERK1/2 MAPK activity is essential and likely sufficient to induce CPEB1 phosphorylation and promote cyclin B1 and Mos mRNA polyadenylation in mouse oocytes.
Late phosphorylation of CPEB1 partially shares kinases with early CPEB1 phosphorylation (Table 3). Ser-210 was reported to be crucial for CDK1-mediated late CPEB1 phosphorylation and partial destruction of the protein in Xenopus oocytes (Mendez, Barnard & Richter, 2002). Early studies indicated that CDK1 directly mediates CPEB1 phosphorylation which was detected by a mobility shift of CPEB1 in Xenopus oocytes. This phosphorylation is dependent on Mos synthesis, which indirectly activates MAPK and CDK1 (Hake & Richter, 1994; Paris et al., 1991; deMoor & Richter, 1997). However, extracts from oocytes where mos translation was destroyed were still able to catalyse early phosphorylation of recombinant CPEB1 without mobility shift, whereas they failed to catalyse the later phosphorylation with mobility shift (Mendez et al., 2000a). Mos synthesis thus seems only required for late phosphorylation of CPEB1 but not early phosphorylation. Similarly, in mouse oocytes, CPEB1 phosphorylation requires CDK1 activity (Cao et al., 2020; Han et al., 2017). When CDK1 activity is blocked with its inhibitor roscovitine, CPEB1 phosphorylation and translational activation of Mos and cyclin B1 mRNAs are prevented at the MII stage.
Overall, CDK1 and ERK1/2 MAPK mediate late CPEB1 phosphorylation and plausibly early phosphorylation as well. However, the exact nature of the initial CPEB1 phosphorylation remains elusive and needs to be reassessed in oocytes from different species. Phosphorylation at different sites by different kinases at specific time points could be responsible for the differential regulation of polyadenylation and, consequently, translation of different subsets of maternal mRNAs. Phosphorylation mediated by different kinases may result in different outcomes, for example, in addition to activating CPEB1, ERK1/2 MAPK also trigger the degradation of CPEB1; whereas CDK1-mediated phosphorylation does not lead to CPEB1 degradation (Cao et al., 2020).
In addition to CPEB1, other three CPEBs have been identified in vertebrates, designated CPEB2–4 (Table 1). CPEB1 and CPEB2–4 interact with different RNA motifs and have unique molecular functions (Huang et al., 2006). Different CPEBs could be responsible for the differential regulation of polyadenylation and, consequently, the translation of different subsets of maternal mRNAs at a specific stage. CPEB3 and CPEB4 RBDs are 95% identical. CPEB3/4 do not interact with the CPEs but, instead, CPEB3/4 recognizes a secondary structure and interacts with uridines that are single-stranded as well as double-stranded stems (Table 1) (Huang et al., 2006). Work in Xenopus oocytes showed that CPEB1 generates a positive loop by activating the translation of Cpeb4 mRNA, which, in turn, replaces CPEB1 and drives the transition from metaphase I to metaphase II (Igea & Mendez, 2010). CPEB1 and CPEB4 are differentially regulated by phase-specific kinases, generating the need for two sequential CPEB activities to sustain cytoplasmic polyadenylation during oocyte meiosis progression. Similarly, the translation of Cpeb3 and Cpeb4 mRNAs was up-regulated in mouse oocytes during maturation (Chen et al., 2011). However, injection of antisense morpholino oligonucleotides specific to Cpeb3 and Cpeb4 in mouse GV oocytes had no significant effect on meiosis I progression (Chen et al., 2011). This cannot rule out the possibility that CPEB3/4 might play a role in late CPE-mediated polyadenylations in meiosis II. It is possible that different CPEBs cooperate in cytoplasmic polyadenylations at the different stages of meiosis progression.
(d) Musashi (MSI) regulates cytoplasmic polyadenylation
Apart from CPEBs, other RBPs also are likely involved in the regulation of cytoplasmic polyadenylation and translation activation. For example, the RBP Musashi (MSI) is required for polyadenylation-induced translation of the early-class mRNAs such as Mos (Table 1) (Charlesworth et al., 2006). MSI1 binds its target mRNAs such as cyclin B1 through MSI-binding elements (MBEs) and induces the remodelling of the RNA structure, therefore revealing neighbouring CPEs and stimulating translation during oocyte maturation in Xenopus (Weill et al., 2017). MSI1 can be released from the MBEs when phosphorylated by CDK1.
(e) DAZL regulates cytoplasmic polyadenylation
Recently, Yang et al. (2020c) found that a substantial fraction of polyadenylated mRNAs show no change in translation or are translationally repressed, while the majority of polyadenylated mRNAs are translationally activated. The major difference between the two groups of mRNAs is that a higher density of U-rich sequence elements, including CPEs, was found in 100 nt 5′ UTRs of the PAS site in translationally activated mRNAs. These data indicate that these U-rich elements and RBPs interacting with these elements are critical for translational regulation. Interestingly, it has been reported that RBP DAZL recognizes U-rich sequences and regulates mRNA translation in mouse oocytes (Table 1) (Chen et al., 2011; Yang et al., 2020a; Sousa Martins et al., 2016). The consensus DBE was identified as a U-rich region (UU[G/C]UU) (Venables, Ruggiu & Cooke, 2001). Genome-wide analysis of ribosome loading onto mRNAs suggests that DAZL functions as a translational repressor in GV and as an activator after GVBD, which is dependent on the context of the 3′ UTR of target mRNAs. In addition, DAZL cooperates with CPEB1 in translational regulation of a fraction of mRNAs. For example, if the CPE element is mutated, the increased translation of a Oosp1 short 3′ UTR reporter caused by DAZL element deletion is prevented (Yang et al., 2020a). Conflicting with the above data, a recent study conducted by Fukuda et al. (2018) suggested that DAZL is dispensable for oocyte maturation and oocyte-specific Dazl knockout mice produced a normal number of pups. One possible explanation for this inconsistency could be the different genetic backgrounds of the mice used in these experiments. A mixed background (ICR and C57BL/6N) was used in this study whereas a pure C57BL/6 background was used in other studies. In line with this, it has been reported that the penetrance of the phenotypes in Dazl-deletion mice is sensitive to the genetic background (Lin & Page, 2005). Very recently, an elegant study conducted by Sharma et al. (2021) investigated the binding and dissociation kinetics of DAZL at its binding sites in cells and revealed that DAZL resides at individual binding sites for only seconds or shorter periods whereas the binding sites remain DAZL-free for a much longer time. DAZL binds to many mRNAs in clusters of multiple proximal sites (Sharma et al., 2021). Moreover, the effect of DAZL on ribosome association correlates with the cumulative probability of DAZL binding in these clusters. These findings indicate that the binding of DAZL to its target mRNAs is highly dynamic and that the density of binding sites within the target mRNAs might influence the cellular roles of DAZL. These findings also agree with the proposal that a high density of U-rich sequence elements is likely essential for the translational activation of a subset of polyadenylated mRNAs in oocytes (Yang et al., 2020c). Nevertheless, the available data about the precise mechanisms by which DAZL promotes target mRNA translation remain controversial. Collier et al. (2005) found that DAZL action is weakened when the target mRNAs are already polyadenylated in Xenopus oocytes, so it is proposed that DAZL promotes translation by enhancing recruitment of PABP1 [also known as cytoplasmic PABP 1 (PABPC1)] and embryonic PABP [(ePABP, also known as ePAB)] to mRNAs with short (not elongated) poly(A) tails (Table 1) (Collier et al., 2005; Jenkins et al., 2011). In mouse oocytes, the translation of Dazl (self-target) was confirmed still to occur when its mRNA is partially deadenylated (Chen et al., 2011) and artificially polyadenylated reporter translation in a DAZL mutant did not change significantly (Yang et al., 2020a). Conversely, in zebrafish, DAZL was found to promote lengthening of the poly(A) tail (Takeda et al., 2009). Moreover, studies in mice show that DAZL cooperates with CPEB1 to regulate maternal mRNA translation (e.g. Tex19.1 mRNA) during meiosis (Sousa Martins et al., 2016). Whether the translation-promoting action of DAZL is exerted by lengthening the poly(A) tail or by recruiting PABPs remains to be investigated. It is likely that DAZL assembles different complexes with other RBPs on the 3′ UTR of an mRNA, ultimately causing activation or repression of translation.
(f) PUM proteins regulate cytoplasmic polyadenylation
PUM1 and PUM2 Pumilio RBPs also are likely involved in direct and/or indirect regulation of cytoplasmic polyadenylation and translational activation (Table 1). Co-immunoprecipitation (Co-IP) experiments show that PUM1 and PUM2 both interact with key factors that regulate cytoplasmic polyadenylation and translation, such as CPEB1, Maskin, Symplekin, CPSF, poly(A)-specific ribonuclease (PARN), GLD2, and DAZL, on their target mRNAs in oocytes (Ota, Kotani & Yamashita, 2011a). PUM1 also targets Cdk1 mRNA and regulates its translation in mouse oocytes (Mak et al., 2018). PUM1 loss leads to inappropriate repression or degradation of Cdk1 mRNAs during oocyte maturation. Intriguingly, PUM1 is not required for translational repression of cyclin B1 mRNA in GV oocytes but is required for the timing control of translational activation in mouse and zebrafish oocytes (Kotani et al., 2013). PUM2 was reported to repress ERK2 MAPK translation in human embryonic stem cells by binding to their 3′ UTRs (Lee et al., 2007). This function of the MAPK cascade is yet to be confirmed in oocytes. Padmanabhan & Richter (2006) reported that PUM2 represses RINGO mRNA translation by binding directly to PBEs in the 3′ UTR and concurrently by interacting with DAZL and ePAB proteins in Xenopus oocytes. Blocking PUM2 leads to RINGO translation and downstream events, such as CPEB1 phosphorylation and cyclin B1 synthesis. Conversely, by using antibodies that discriminate between PUM1 and PUM2, Ota et al. (2011a) showed that PUM1 rather than PUM2 binds to RINGO mRNA in Xenopus oocytes. This inconsistency may be caused by the different antibodies used. Regardless of these discrepancies, injection of either anti-PUM1 N-terminus or anti-PUM2 N-terminus antibodies accelerates GVBD under progesterone stimulation (Ota et al., 2011a). This can be explained by translational activation of cyclin B1 mRNA, RINGO mRNA, and other unidentified mRNAs upon blocking PUM. In addition, PUM1 and PUM2 were found to be phosphorylated during oocyte maturation (Ota, Kotani & Yamashita, 2011b). Nemo-like kinase (NLK), an atypical MAPK, was demonstrated to be activated by Mos during oocyte maturation and responsible for the phosphorylation of PUM1 and PUM2. It was reported that PUM1 was phosphorylated at the N-terminus, and it preceded translational activation of its target mRNAs such as cyclin B1 mRNA in zebrafish oocytes (Saitoh et al., 2018). Although the biological function of PUM1 and PUM2 phosphorylation is unclear, the concurrence of PUM1 phosphorylation and PUM1 dissociation from CPEB1 and cyclin B1 mRNAs suggests the possibility that their phosphorylation is needed for translational activation of their target mRNAs.
(3) Translational regulation by mRNA localization
Subcellular localization of mRNAs into granules has been proved to play fundamental roles not only in the storage of dormant mRNAs but also in timing control of translational activation in maturing oocytes. An RNA FISH assay revealed that cyclin B1 mRNAs were localized in granules and that they are asymmetrically distributed in the cytoplasm of mouse and zebrafish oocytes (Kotani et al., 2013). The number of cyclin B1 mRNA granules decreases in prometaphase I and they completely disappear in metaphase II. Notably, mRNA copy number is not reduced during these stages, indicating that the decrease in the number of granules is caused by granule disassembly rather than mRNA degradation. Moreover, using a reporter of cyclin B1 nascent translation, Kotani et al. (2013) showed that cyclin B1 mRNAs with shorter poly(A) tails are deposited in RNA granules in a dormant state, and may be inaccessible to the translational machinery. By contrast, upon translational activation, cyclin B1 mRNAs are polyadenylated and dissembled from granules, ultimately becoming accessible to translational machinery.
mRNA localization to granules is controlled by multiple mechanisms. It has been reported that actin filament polymerization is crucial for the subcellular distribution of cytoplasmic components during oocyte meiosis. The depolymerization of actin filaments induced by cytochalasin B causes failure in cyclin B1 mRNA granule formation and premature translation of cyclin B1 mRNA (Kotani et al., 2013). However, the actin filament does not harbour the RNA-binding property and there should be certain RBPs that load mRNAs to the actin filament. The RBP PUM1 was demonstrated to play a key role in cytoplasmic localization and granule formation of cyclin B1 mRNA in mouse and zebrafish oocytes (Kotani et al., 2013). Although no canonical PBE (UGUANAUA) has been identified in the cyclin B1 3′ UTR, two alternative PBEs (UGUA) are present in its 3′ UTR. A Co-IP experiment using anti-PUM1 antibodies shows that PUM1 interacts directly with cyclin B1 mRNA in both mouse and zebrafish oocytes (Kotani et al., 2013). Moreover, disruption of the alternative PBEs not only prevents the formation of cyclin B1-containing granules, but also leads to precocious translational activation of cyclin B1. Furthermore, exogenous overexpression of the PUM1 N-terminus (binding domain of PBE) delays granule disassembly and the translational activation of cyclin B1 during oocyte maturation, possibly because the high level of exogenous PUM1 N-terminus competes with the endogenous PUM1 (Kotani et al., 2013). Thus, the formation of mRNA granules mediated by depolymerization of actin filaments and PUM1 binding is likely necessary for maintaining their repression state until the proteins are needed in maturing oocytes. A major focus of future studies will be to clarify whether translational status is the cause or consequence of mRNA granule assembly/disassembly and to discover the mechanisms that mediate these processes in maturing oocytes. In addition to cytoplasmic localization, mRNAs also are abundantly present in the nuclei of fully grown (transcriptionally silent) mouse and human oocytes (Jansova et al., 2018). Given that ribosome components are not present in the nucleus, the mRNAs are likely translationally silent and may be stored in the nucleus to be released and translated after GVBD.
mRNA localization to certain destinations also plays a key role in the spatial regulation of translation (localized translation) and asymmetric protein distribution in maturing oocytes. Localized translation was reported to occur close to the mitotic spindle microtubules (Blower et al., 2007). Such localized translation has also been observed during oocyte meiosis. In Xenopus oocytes, CPEB1 translation is localized to the meiotic spindle, which proved to be crucial for spindle stability and chromosome segregation by activating spindle-localized translation of CPE-regulated mRNAs (Eliscovich et al., 2008). In zebrafish oocytes, upon translational activation, cyclin B1 mRNAs are transported along microtubules toward the animal pole (where the meiotic spindle will be localized) to support local translation. The transport of cyclin B1 mRNAs is driven by the motor protein Kinesin-1 (Takahashi, Ishii & Yamashita, 2018). What organization of microtubules is required for cyclin B1 mRNA transport and which other RBPs are involved in this process are still unclear.
The findings from different organisms indicate that cis-acting elements in the 3′ UTR are necessary to direct mRNA localization through interaction with specific RBPs (Micklem et al., 2000; Takahashi et al., 2018; Andreassi & Riccio, 2009). RBP Staufen1 (STAU1) is reported to mediate the movement of zebrafish cyclin B1 mRNA, Xenopus vg1 mRNA, and Drosophila oskar mRNA in oocytes by cross-linking mRNAs to tubulin microtubules (Table 1) (Takahashi et al., 2018; Lasko, 2012). It is well documented that a double-stranded (ds) RNA-structure in the 3′ UTR is required for Staufen-mediated localization of mRNAs across different species (Micklem et al., 2000; Wickham et al., 1999). Staufen proteins were found to contain multiple dsRNA-binding domains (dsRBDs) that mediate their binding to dsRNA regions in target RNAs (Stjohnston et al., 1992). Staufen proteins also contain a tubulin-binding domain (TBD) that mediates their interaction with the tubulin cytoskeleton (Wickham et al., 1999). These two types of domains work cooperatively to mediate mRNA localization. STAU1 and STAU2 are expressed during oocyte maturation in different species, including humans, mice, pigs, and cattle (Wickham et al., 1999; Santis et al., 2015; Brevini et al., 2007; Calder, Madan & Watson, 2008; Cao et al., 2016). The subcellular localization of Staufen proteins changes during the meiotic progression from GV to metaphase II; in particular, STAU2 is localized to the meiotic spindle (Santis et al., 2015; Cao et al., 2016). Removal of STAU2 leads to defects in spindle formation, chromosome alignment, and microtubule–kinetochore attachment in oocytes (Cao et al., 2016). Staufen proteins mediate the transport of mRNAs along the cytoskeleton and anchoring to the specific destinations in the cytoplasm where local translation takes place, such as in association with endoplasmic reticulum or meiotic spindle (Takahashi et al., 2018; Wickham et al., 1999). The functions of the cytoskeleton and microtubule motors in mRNA localization have been comprehensively reviewed elsewhere (de Heredia & Jansen, 2004; Carotenuto & Tussellino, 2018; Goldman & Gonsalvez, 2017; Oh & Houston, 2017), and so will not be discussed here.
In addition, the localization of CPE-containing mRNAs to proximity with the plasma membrane is likely an important early process for cytoplasmic polyadenylation. CPEB1 has been shown to associate with plasma membranes through interaction with amyloid beta precursor-like protein (APLP) transmembrane proteins (Cao et al., 2005). In progesterone-stimulated Xenopus oocytes, most of the CPE-containing mRNAs shifted to the fraction of the sucrose gradient that contained APLP1. In addition, the injected CPE-containing mRNA was detected on membranes, whereas the CPE-lacking mRNA was not (Cao et al., 2005). In line with these results, maternal mRNAs and CPEB1 aggregates (SCAs), which also contain other RBPs such as DDX6 and MSY2, were found in fully grown mouse oocytes (Flemr et al., 2010). The localization of CPE-containing mRNAs to the membranes is likely driven by entrapment by the CPEB1-APLP1 complex. CPEB1 associated with plasma membranes might be the earliest fraction to be phosphorylated following hormone stimulation because this signalling process is thought to begin at the cell surface. mRNAs, CPEB1, and the other factors could be concentrated near the membrane-associated receptors for hormones (progesterone in Xenopus), and a wave of polyadenylation could begin locally and then subsequently spread throughout the oocyte.
Overall, mRNA localization to different regions in oocytes likely plays a fundamental role not only in translational repression and maternal RNA storage but also in the spatial and temporal activation of maternal mRNA translation, which coordinates the process of oocyte meiotic maturation. The interaction networks among mRNAs, RBPs, cytoskeleton, and different microtubule motors involved in the subcellular localization of different maternal mRNAs are intricate. Future studies are needed to reveal mRNA-specific scenarios: which components are involved in this process, how they interact, and where the mRNAs are localized. The development of new methods for mRNA detection in live and fixed cells at single-molecule resolution will allow the tracking and quantification of single mRNAs in a three-dimensional (3D) manner to decipher the cooperation between RBPs and motor proteins to regulate the transport of mRNAs.
VI. REGULATION OF MATERNAL mRNA STABILITY
(1) Transition from mRNA stability to instability
(a) Characterization of degraded maternal mRNAs
The stabilization and storage, translational activation, and degradation of maternal mRNAs are a series of sequential events that collectively regulate maternal mRNA processing during oocyte maturation. Programmed maternal mRNA degradation is critical for the reprogramming of gene expression during the oocyte-to-zygote transition. Aberrant or impaired degradation of certain classes of transcripts could be deleterious to oocyte quality, impacting developmental competence. Maternal mRNAs are gradually degraded from the onset of oocyte maturation. The destruction of transcripts during oocyte maturation is a selective rather than promiscuous process (Schellander, Hoelker & Tesfaye, 2007). In mice, among the 8081 poly(A)-containing maternal mRNAs [fragments per kilobase of exon model per million reads mapped (FPKM) >2] selected and analysed, approximately 60% are degraded from the GV stage to the two-cell embryo stage (Sha et al., 2020b). Similarly, approximately 79% of poly(A)-containing maternal mRNAs are degraded from the GV stage to the eight-cell embryo stage in humans (Sha et al., 2020a). Of note, a significant number of maternal mRNAs in mouse oocytes were relatively stable until the late two-cell stage but were degraded at the four-cell stage (Sha et al., 2020b). The degradation of maternal mRNAs during the oocyte-to-embryo transition can be outlined into two phases: (i) degradation accelerated after meiotic resumption and (ii) degradation accelerated after fertilization. mRNAs in the first wave of degradation usually have short 3′ UTRs and are deadenylated during oocyte maturation. By contrast, mRNAs in the later wave of degradation tend to have long 3′ UTRs, maintain long poly(A) tails, and are translationally active during oocyte maturation (Sha et al., 2020b). The degradation of these mRNAs is accomplished by two modes. The first mode is dependent on maternal transcripts (called M-decay), while the second mode relies on zygotic transcription (called Z-decay) (Yartseva & Giraldez, 2015; Sha et al., 2020b). Through RNA-seq, Luong et al. (2020) revealed that the first wave of maternal mRNA degradation in mouse oocytes is detected as early as the exit from MI. Consistently, by quantifying the poly(A) mRNAs using RNA FISH with an oligo(dT) probe, Wu & Dean (2020) demonstrated that there was a modest decrease in poly(A)-containing mRNAs from GV to GV+3h (MI) and then a sharp decrease upon MI-to-MII transition in mouse oocytes. A drawback of the oligo(dT) probe-based RNA FISH quantification is that the signal will be affected by both the polyadenylation and the degradation of poly(A)-containing mRNAs, and thus the result cannot fully reflect overall mRNA degradation. By using single-oocyte RNA-seq, Wu & Dean (2020) identified 56 mRNAs that decreased in abundance from GV to MI and 11,065 mRNAs that decreased from MI to MII. Of note, the total number of degraded mRNAs from GV to MII reported by Wu & Dean (2020) is much greater than that reported by Sha et al. (2020b), although both studies used poly(A)-based RNA-seq to analyse the oocyte transcriptome profile. Sha et al. (2020b) showed that among the 8081 maternal transcripts analysed, 2028 transcripts were degraded from GV to zygote. This might be caused by the different procedures and criteria used in the two studies. In the study performed by Sha et al. (2020b), 10 oocytes/embryos were used per RNA-seq sample, and only mRNAs with FPKM >2 at the GV stage were selected and analysed. An mRNA was considered as degraded if it showed a significant decrease >twofold. By contrast, Wu & Dean (2020) performed single-oocyte RNA-seq and likely analysed more mRNAs (possibly the whole oocyte transcriptome). In this study, an mRNA was considered as degraded if it showed a decrease with an adjusted P < 0.01. Nevertheless, these data indicate that modest maternal mRNA degradation occurs upon meiotic resumption and is accelerated during the MI-to-MII transition.
To investigate zygotic activation (ZGA)-dependent maternal mRNA degradation (Z-decay) during the mouse oocyte-to-embryo transition, Sha et al. (2020b) treated zygotes with the RNA polymerase II inhibitor, α-amanitin, to inhibit transcription, and newly transcribed RNAs were labelled with 5-ethynyl uridine (EU). α-amanitin-treated zygotes cannot develop beyond the two-cell stage. Interestingly, α-amanitin treatment blocked the degradation of half of transcripts (1064/2243) that were degraded from fertilization to the two-cell stage. In addition, α-amanitin treatment blocked the degradation of 199 of 375 transcripts that were continuously degraded from the GV to the two-cell stage (Sha et al., 2020b). These transcripts are considered to be degraded by the Z-decay pathway.
(b) MSY2 is involved in the mRNA stability to instability transition
Oocyte maturation is accompanied by a transition from maternal mRNA stability to instability. RNA degradation machinery and RBP MSY2 (also called YBX2) play critical roles in this transition (Table 1). MSY2 is one of the most abundant proteins in oocytes and it persists until the two-cell stage (Yu, Hecht & Schultz, 2001). It was suggested that most mRNAs are bound by MSY2 in oocytes. MSY2 is essential for oocyte competence acquisition, and Msy2 knockout causes infertility in female mice, with far fewer mRNAs detected in the oocytes (Medvedev et al., 2008). This suggests that MSY2 could be involved in the regulation of maternal mRNA stability. A reporter complementary RNA (cRNA) injected into Msy2-null oocytes is less stable compared to that injected into wild-type oocytes. Additionally, co-injection of Msy2 mRNAs restores the stability of the reporter cRNA, further supporting mRNA stabilizing roles for MSY2 (Medvedev, Pan & Schultz, 2011). MSY2 phosphorylation likely contributes to the transition from mRNA stability to instability during oocyte maturation. MSY2 is phosphorylated by active CDK1 with the onset of GVBD (Medvedev et al., 2008). Blocking CDK1 after GVBD not only leads to an increased ratio of non-phosphorylated MSY2 but also obstructs mRNA degradation. Overexpression of a non-phosphorylatable form of MSY2 prevents mRNA degradation during oocyte maturation. MSY2 phosphorylation could lead to the dissociation of MSY2 from mRNAs due to charge repulsion or cause a switch in its function from protecting the mRNA to promoting the recruitment of RNA degradation machinery to the mRNA. Whether this is the case remains to be investigated.
(c) ZAR1/2 proteins are involved in mRNA stability regulation
RBPs zygote arrest protein 1 (ZAR1) and ZAR2 are also involved in maternal mRNA stability regulation (Table 1). Rong et al. (2019) reported that the double deletion of Zar1/2 leads to a delay in meiotic resumption, and these oocytes not only contain reduced levels of many maternal mRNAs but also exhibit impaired synthesis of some key proteins, such as cyclin B1 and WEE1B (WEE2), as well as BTG anti-proliferation factor 4 [BTG4, an adaptor of carbon catabolite repression 4 (CCR4)–negative on TATA-less (NOT) deadenylase complex]. These results indicate that ZAR1 and ZAR2 are likely required for the stabilization and/or translational activation of some maternal mRNAs. However, Yamamoto et al. (2013) and Charlesworth et al. (2012) demonstrated that ZAR1 and ZAR2 repress translation in immature Xenopus oocytes (Yamamoto et al., 2013; Charlesworth et al., 2012). They replaced the C-terminal domain (RNA binding domain) of ZAR1 and ZAR2 with MS2 coat protein (MCP) and tagged a reporter mRNA with the MS2 stem-loop. Co-injection of these two mRNAs into oocytes leads to binding of N-ZAR–MCP fusion protein to the MS2-tagged reporter mRNA. Through this system, N-ZAR1/2–MCP fusion was found to repress reporter translation in immature oocytes. Translational repression was relieved in maturing oocytes, possibly due to ZAR1/2 degradation. These data suggest that ZAR1 and ZAR2 may play dual roles: repressing translation and stabilizing mRNAs in GV oocytes. The decreased level of protein synthesis observed in the Zar1/2 null mouse oocyte could solely be caused by reduced mRNA levels (caused by reduced stability) rather than decreased translational activity. A recent study showed that the maternal mRNAs in mammalian oocytes are stored around the mitochondria in hydrogel-like membraneless compartments (Cheng et al., 2022). This process is facilitated by ZAR1, which helps the assembly of such compartments and protects the mRNA from degradation. In mature eggs, degradation of ZAR1 drives dissolution of such compartments to ensure the timely degradation of maternal mRNAs. In addition to ZAR1 and ZAR2, a third member of the ZAR protein family has been identified in Xenopus, which is termed ZAR1L (Yamamoto et al., 2013). Very recently, ZAR1L has been demonstrated as a translational repressor in Xenopus prophase I oocytes (Heim et al., 2022). Loss of Zar1l leads to premature activation of the M-phase-promoting kinase CDK1 and Mos, which, in turn, accelerate the hormone-induced meiotic resumption of Xenopus oocytes. Further investigation showed that ZAR1L binds to RNP granules containing the translation repressor 4E-T and the polyadenylation regulator CPEB1. However, it remains unclear whether ZAR1L is also involved in mRNA stability regulation.
(2) Deadenylation initiates maternal mRNA degradation
(a) mRNA degradation machinery CCR4–NOT complex
Deadenylation- and exoribonuclease-mediated degradation are the major patterns by which most mRNAs undergo degradation. Deadenylation is the first and often a rate-limiting step for mRNA turnover (Garneau, Wilusz & Wilusz, 2007). Translationally inactivated mRNAs are deadenylated by deadenylases. The poly(A) tail is first shortened to about 80 nt by the PAN2/PAN3 dimer (Table 2), then the remaining tail is removed by the CCR4–NOT (CNOT) deadenylase complex or PARN (Fig. 4A, Table 2) (Wiederhold & Passmore, 2010; Schoenberg & Maquat, 2012). CCR4–NOT subunits are conserved from yeast to humans, including two subunits that have ribonuclease activity (i.e. CCR4 with an endonuclease–exonuclease–phosphatase (EEP) domain and chromatin assembly factor 1 (CAF1) with a DEDD domain) and six non-catalytic subunits (CNOT1–4, CNOT9, and CNOT10). In vertebrates, two CCR4 paralogs (CNOT6 and CNOT6L) and two CAF1 paralogs (CNOT7 and CNOT8) have been identified (Winkler & Balacco, 2013). It was reported that CNOT6L, instead of CNOT6, is preferentially expressed in mouse oocytes (Sha et al., 2018; Horvat et al., 2018). Deletion of Cnot6l in mice disturbs the deadenylation and degradation of a subset of maternal mRNAs during oocyte maturation, with 90% of these oocytes having distorted meiotic spindles and failing to extrude the first polar body and reach metaphase II, although they can undergo GVBD (Sha et al., 2018; Horvat et al., 2018). Therefore, CNOT6L-dependent degradation of a specific subset of maternal mRNAs is critical for oocyte meiotic maturation. However, Cnot6 inactivation does not affect the maternal mRNA degradation detected at the end of MI in mouse oocytes (Luong et al., 2020). These data support the concept that CNOT6L, instead of CNOT6, plays a prominent role in maternal mRNA degradation. In addition, CNOT7 and CNOT8 were demonstrated to function redundantly in mouse oocytes (Aslam et al., 2009).
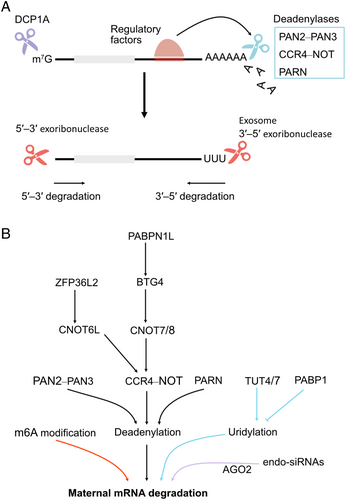
(b) ZFP36L2 recruits the CCR4–NOT complex to target mRNAs
Notably, none of the CCR4–NOT subunits contain an RNA-binding motif, leading to the speculation that the CCR4–NOT complex requires RBP(s) to mediate its loading to target mRNAs. Not surprisingly, recent studies in mice revealed that Zinc finger protein 36-like 2 (ZFP36L2; Xenopus C3H4 homolog), binding the AU-rich element (ARE), acts as a CNOT6L adaptor to recruit CCR4–NOT to ARE-containing maternal mRNAs and triggering their deadenylation and degradation during oocyte maturation (Table 1) (Ball et al., 2014; Dumdie et al., 2018; Sha et al., 2018). A mutation of Zfp36l2 in which the N-terminal 29 amino acid residues were deleted leads to 40% fewer oocytes released and early developmental arrest at the two-cell stage (Ball et al., 2014). These results indicate that ZFP36L2 is essential for ovulation and/or oocyte maturation. A later study reported that oocyte-specific depletion of Zfp36l2 resulted in defects in oocyte maturation and fertilization by preventing global transcriptional silencing (Dumdie et al., 2018). Sha et al. (2018) demonstrated that ZFP36L2 directly binds to CNOT6L and recruits CNOT6L to target ARE-containing maternal mRNAs during oocyte maturation in mice. Thus, ZFP36L2 is likely a key adaptor of CNOT6L in mediating ARE-containing mRNA degradation during oocyte maturation (Fig. 4B).
(c) BTG4 recruits the CCR4–NOT complex to target mRNAs
BTG4 (a member of the BTG/TOB family) was reported to be another CCR4–NOT adaptor that recruits CCR4–NOT to target mRNAs by interacting with its subunits CNOT7 or CNOT8 (Table 1) (Yu et al., 2016; Liu et al., 2016; Pasternak et al., 2016). The direct interactions of BTG4 with CNOT7 and CNOT8 have been well documented (Yu et al., 2016; Liu et al., 2016; Pasternak et al., 2016). This interaction is likely mediated by the N-terminus of BTG4 because a mutation at residue W95 abolished the interaction with CNOT7 and CNOT8 (Pasternak et al., 2016; Yu et al., 2016). Yu et al. (2016) reported that Btg4 knockout caused infertility in female mice due to early developmental arrest at the two-cell stage, while Btg4−/− females displayed normal oogenesis and ovulated MII oocytes. Truncation of the C-terminal 156 amino acids mimicked this phenotype. The same phenotype was also observed in a different Btg4-knockout mouse line by Liu et al. (2016). Additionally, using siRNA-induced Btg4 knockdown, Pasternak et al. (2016) reported that 63% of Btg4-depleted oocytes failed to arrest at MII and spontaneously progressed into anaphase II. This extra phenotype, which was not observed in the other two relevant studies, was not likely caused by off-target effects of the siRNA because the phenotype could be rescued by full-length BTG4; and co-depletion of Cnot7 and Cnot8 mirrored this phenotype of Btg4 depletion. The observation of this phenotype required high-resolution imaging of live oocytes, which might be a possible explanation for the omission of this phenotype in the other two studies.
The transcriptome profile analysis demonstrated that 46–65% mRNAs (among about 13,000 mRNAs detected) showed higher abundance of Btg4−/− MII oocytes, while Btg4−/− and wild-type oocytes exhibited similar transcriptome profiles at the GV stage (Yu et al., 2016; Liu et al., 2016). As further evidence, a significant overlap was found between mRNAs destabilized in meiosis I and mRNAs stabilized in Btg4−/− oocytes (Luong et al., 2020). These results suggest that the mRNA deadenylation and subsequent degradation in maturing oocytes are likely insufficient in the absence of BTG4. This was further supported by results showing that a subset of mRNAs that were reduced in wild-type oocytes failed to be deadenylated and were thus less accessible to degradation in Btg4−/− oocytes (Liu et al., 2016; Pasternak et al., 2016). Notably, the defective degradation of certain mRNAs in Btg4−/− oocytes could be rescued by full-length BTG4 (Liu et al., 2016), indicating that the phenotype is caused by Btg4 loss-of-function rather than off-target effects and that the N-terminus appears to be essential for BTG4 function. Btg4−/− mice mimicked the phenotypes of Btg4W95A/− mice, in which the BTG4-CNOT7/8 interaction was abolished (Yu et al., 2016). Taken together, BTG4 is required for the deadenylation of maternal mRNAs through interaction with CNOT7 and CNOT8, two subunits of the CCR4–NOT deadenylase complex. Of note, not all mRNA degradation events in oocytes rely on BTG4 because only a subset of mRNAs were stabilized in Btg4 null oocytes. Other mechanisms may also be involved in maternal mRNA elimination. Of note, Liu et al. (2016) reported that CNOT7 protein levels were lower in Btg4−/− MII oocytes, however, Yu et al. (2016) reported that CNOT7 protein levels was comparable in Btg4−/− and WT MII oocytes. This inconsistency might be caused by the different antibodies used in the two studies.
Interestingly, Pasternak et al. (2016) also demonstrated that the failure in MII arrest of Btg4-depleted oocytes was caused by the insufficient synthesis of Emi2, which is required for MII arrest by inhibiting APC activity (Ohe et al., 2010; Tang et al., 2010). Emi2 synthesis is impaired in the absence of BTG4 and thus the APC was not efficiently inhibited in MII oocytes (Pasternak et al., 2016). The shortage of Emi2 synthesis was not likely caused by a decrease in polyadenylated Emi2 mRNA levels. This leads to the speculation that the mRNAs escaping from deadenylation may compete for the translation machinery, for example by sequestering PABPs, thus obstructing fresh protein synthesis required for MII arrest such as Emi2. This was supported by evidence that the expression of a microinjected EGFP reporter mRNA decreased by 79% in Btg4-depleted MII oocytes (Pasternak et al., 2016). Additionally, microinjection of polyadenylated EGFP mRNA into MII arrested oocytes released the oocyte from MII arrest, however, nude EGFP mRNA without a poly(A) tail showed no effect. Collectively, these data suggest that Btg4 ablation leads to defects in mRNA deadenylation and degradation, and ultimately that mRNAs escaping from deadenylation impair protein synthesis required for MII arrest. This finding also highlights that researchers should avoid delivering high concentrations of exogenous polyadenylated mRNAs into oocytes, which can perturb the translation of endogenous proteins.
Endogenous BTG4 protein in mouse oocytes is detectable only after meiotic resumption and reaches a maximal level at the MII stage (Yu et al., 2016). Not surprisingly, its translation is activated by CPE-mediated cytoplasmic polyadenylation. The timely synthesis of BTG4 might provide an insight into long-standing questions regarding which factors trigger mRNA degradation in maturing oocytes. BTG4 was also demonstrated to bind eIF4E directly on the C-terminus, a domain required for binding to eIF4G and the 5′-cap of mRNA (Yu et al., 2016). This observation suggests that BTG4 might prevent eIF4E binding to the 5′-cap of mRNA and/or eIF4G and therefore cause translational repression. This finding also connects translational repression and deadenylation-mediated mRNA degradation.
Of note, BTG4 does not contain an RNA-binding domain itself, but a very recent study demonstrated that nuclear poly(A)-binding protein 1 like (PABPN1L) acts as an mRNA-binding adaptor of BTG4 by promoting the accumulation of BTG4 and recruiting BTG4 to the poly(A) tails of maternal mRNAs in maturing oocytes (Table 1) (Zhao et al., 2020). PABPN1 was demonstrated to bind directly to poly(A) tails and facilitate mRNA deadenylation (Benoit et al., 2005). Like phenotypes of Btg4 null mice, Pabpn1l null mice exhibit normal oogenesis but are infertile due to early developmental arrest at the two-cell stage (Zhao et al., 2020). Loss of PABPN1L leads to defects in the deadenylation and degradation of a subset of maternal mRNAs in maturing oocytes. It was reported that BTG4 also interacts with PABPC1L (also known as ePAB) (Liu et al., 2016), indicating that PABPC1L might work as another BTG4 adaptor. Other members of the BTG/TOB family have also been reported to interact with poly(A)-binding proteins (Okochi et al., 2005). BTG2 was reported to recruit CCR4–NOT to PABPC1 (Stupfler et al., 2016) but this needs to be further confirmed in oocytes.
Therefore, ZFP36L2 and PABPs-modulated BTG4 work cooperatively to load the CCR4–NOT complex to maternal mRNAs to trigger deadenylation and subsequent degradation in maturing oocytes (Fig. 4B). Following the loss of the poly(A) tail, the 5′ cap is removed by decapping enzymes. In mouse oocytes, the level of DCP1A (a component of the decapping complex) rises during meiotic maturation and then dramatically increases at metaphase II (Flemr et al., 2010). This finding indicates that DCP1A-mediated decapping may promote maternal mRNA degradation during oocyte maturation. The naked transcripts are susceptible to be degraded by 3′–5′ exoribonuclease and 5′–3′ exoribonuclease. EXOSC10, an exosome-associated RNase, was recently reported to be involved in mRNA degradation during mouse oocyte maturation (Wu & Dean, 2020). Oocyte-specific loss of Exosc10 leads to dysregulation of a subset of maternal mRNAs. Although EXOSC10 is localized to the nucleus in both GV oocytes and embryos, it could pass into the cytoplasm after GVBD.
All these factors are deposited in oocytes and are responsible for stage-specific degradation of maternal mRNAs during M-decay. Maternally expressed BTG4 and the CCR4-NOT deadenylase complex continue to serve for Z-decay, but likely require reinforcement from zygotic factors for timely removal of maternal mRNAs (Sha et al., 2020b; Zhao et al., 2022).
(3) Terminal uridylation facilitates maternal mRNA degradation
Compared with mRNAs in the somatic cells, a proportion of maternal mRNAs in oocytes contain shorter poly(A) tails and a larger proportion of 3′ terminal oligouridylation. Uridylated poly(A) tails are uncommon in newly fertilized eggs but increase dramatically from the zygote to the two-cell stage in both zebrafish, frogs, and mice (Chang et al., 2018). Further analysis in Xenopus oocytes found that uridylation increases during oocyte maturation but decreases upon egg activation. Thus, there may be at least two waves of uridylation: first from GV to metaphase II and second from the zygote to the two-cell stage. Terminal uridylyl transferases (TUTases) TUT4 (also known as ZCCHC11) and TUT7 (also known as ZCCHC6) have been shown to catalyse the 3′ terminal uridylation of maternal mRNAs (Table 2) (Chang et al., 2018; Morgan et al., 2017). They are highly conserved ‘writers’ of U tails in vertebrates. TUT4 and TUT7 uridylate mRNAs with short poly(A) tails at their 3′ end (Morgan et al., 2017; Chang et al., 2018; Sha et al., 2020b). In mouse oocytes, Tut4 and Tut7 transcripts are detectable at the GV stage, but they are almost completely eliminated during oocyte maturation. Zygotic Tut4/7 mRNAs were re-transcribed as early as the two-cell stage (Sha et al., 2020b). This is consistent with the notion that there may be at least two rounds of uridylation surge during the oocyte-to-embryo transition. In both zebrafish and Xenopus, injecting morpholinos complementary to the start codon of Tut4/7 mRNAs into fertilized eggs abolished the increase in uridylation during the oocyte-to-embryo transition (Chang et al., 2018). Knockdown of Tut4/7 also prevents the uridylation increase during oocyte maturation. By contrast, splicing-blocking morpholinos did not show detectable effects. Based on these results, Chang et al. (2018) proposed that the TUT4/7 proteins translated from maternal mRNAs, rather than those from zygotically transcribed mRNAs (which require splicing), are responsible for uridylation during the oocyte-to-embryo transition. This conclusion is not convincing because it is not clear if the splicing-blocking morpholinos efficiently knocked down zygotically expressed Tut4/7. As mentioned above, maternal Tut4/7 mRNAs are almost completely eliminated during oocyte maturation, and therefore zygotically transcribed Tut4/7 likely account for the second wave of TUT4/7 protein synthesis. Moreover, in mice, while Tut4 mRNA is more abundant than Tut7 mRNA at the GV stage, the mRNA level of Tut7 was twofold higher than that of Tut4 at the two-cell stage (Sha et al., 2020b). Consistently, TUT7 is one of the most efficiently translated proteins among those detected at 4 h after fertilization in zebrafish (Chang et al., 2018). It is plausible that TUT7 might account for the majority of maternal mRNA uridylation from the zygote to the two-cell stage, as further evidenced by the observation that knockdown of Tut4 did not show a significant effect on mRNA uridylation.
Mice with the conditional double-knockout of Tut4/7 in oocytes have a different repertoire of maternal mRNAs than wild-type mice and meiotic maturation is disrupted, although ovulation is not affected (Morgan et al., 2017; Chang et al., 2018; Sha et al., 2020b). These TUT4/7-deficient oocytes are rarely fertilized and fail to progress to the two-cell stage, indicating they are incompetent for fertilization and subsequent embryonic development. TUT4/7-catalysed uridylation is required for both oocyte maturation and fertility. Of note, PABP1 (also known as PABPC1; Table 1) antagonizes uridylation of polyadenylated mRNAs, and this has been proposed to contribute to the specificity of TUTases for uridylation. Upon deadenylation, mRNAs with short poly(A) tails (less than 25 nt) lose PABP and instead are recognized by TUT4 and/or TUT7, which mediates tagging the oligo(U) tail (Lim et al., 2014). The oligo(U) tail acts as a degradation mark and is recognized by downstream mRNA degradation factors to trigger mRNA degradation. Oligouridylated mRNAs (with more than two uridines) are more sensitive than monouridylated mRNAs to degradation factors and are less stable (Lim et al., 2014; Chang et al., 2018). Further evidence showed that depletion of TUT4 and TUT7 in zebrafish and Xenopus oocytes delays maternal mRNA clearance, leading to defects in early embryogenesis (Chang et al., 2018). At 2–4 h after fertilization, 25.3% of maternal transcripts decreased significantly in a TUT4/7-dependent manner and these transcripts decreased at a reduced rate (47% of the original rate) in TUT4/7-knockdown embryos compared to control embryos in zebrafish. The zygotic transcript levels were not affected by TUT4/7 knockdown at this time. These results suggest that the failure in maternal RNA decay is due directly to defects in RNA degradation in Tut4/7-knockdown oocytes. The loss of TUT4/7 results in the global accumulation of mRNAs with a short poly(A) tail. In all species examined (fish, frog, and mouse), uridylation is consistently observed almost exclusively among deadenylated mRNAs. These data indicate that, in vertebrate oocytes, TUT4/7 selectively target deadenylated RNAs and facilitate their degradation. Furthermore, inhibition of mRNA degradation factors, such as 5′-3′ exoribonuclease XRN1 and 3′-5′ exoribonucleases RRP41 and DIS3-like exonuclease 2 (DIS3L2), leads to an increase in the proportion of oligouridylated mRNAs in cultured mammalian cells (Lim et al., 2014). This implies that blocking downstream degradation factors causes the accumulation of oligouridylated mRNAs which should be eliminated by mRNA degradation factors. Another study showed that, among the 4329 Z-decay transcripts detected, 2984 (68.93%) were stabilized in Tut4/7-depleted embryos (Sha et al., 2020b). Taken together, uridylation-induced maternal mRNAs degradation is not only involved in M-decay but is also a key mechanism of Z-decay.
It is imperative to characterize the downstream factor(s) and mechanisms that recognize the oligo(U) tails during maternal mRNA degradation in oocytes. One such example is PABPN1. Zhao et al. (2022) demonstrated that cytoplasmic PABPN1 is an early expressed zygotic factor and that it docks on uridylated transcripts and recruits 3′-5′ exoribonuclease DIS3L2 to its targets, facilitating maternal mRNA decay (Zhao et al., 2022). Pabpn1 mRNA levels were significantly increased after fertilization and remained at a relatively high level until the eight-cell stage. Pabpn1 depletion in oocytes blocked both the degradation of maternal mRNAs and the activation of the zygotic genome. Upon Pabpn1 knockdown, the median levels of mRNAs that should be degraded during the oocyte-to-embryo transition were 2.32-fold higher at the late two-cell stage. While Dis3l2 was proposed to be actively translated from maternally deposited mRNAs (Zhao et al., 2022; Chang et al., 2018), it likely preferentially interacts with PABPN1 to allow its recruitment onto mRNAs. DIS3L2 RNA immunoprecipitation (RIP) assays show that cytoplasmic PABPN1 effectively promoted DIS3L2 binding to representative Z-decay mRNAs (Zhao et al., 2022). Thus, both the ‘writer’ and a ‘reader’ of the uridylation pathway are activated during the oocyte-to-embryo transition in vertebrates. Conclusively, the terminal uridylation pathway is a novel mechanism modulating maternal mRNA degradation (Fig. 4B).
(4) N6-methyladenosine (m6A) modification destabilizes maternal mRNAs
mRNA epigenetics (also known as epitranscriptomics) has recently become the focus of an increasing number of studies (Meyer & Jaffrey, 2014; Fu et al., 2014; Saletore et al., 2012). Recent findings indicate that N6-methyladenosine (m6A) of RNA is a new mechanism that regulates mRNA splicing, alternative polyadenylation, and mRNA stability. The consensus m6A sites have been identified as RRACU (R = G/A). In mammals, the m6A modification is catalysed by a methyltransferase complex (writer) comprising METTL3 and METTL14, and adapter Wilms' tumor 1-associating protein (WTAP) (Liu et al., 2014; Ping et al., 2014). The m6A modifications can be removed by the demethylases (erasers) fat mass and obesity-associated protein (FTO, also known as alpha-ketoglutarate-dependent dioxygenase) and AlkB Homolog 5 (ALKBH5) (Bartosovic et al., 2017; Zheng et al., 2013). The YTH-domain family (YTHDF) members YTHDF2 and YTHDF3 specifically bind the m6A-modified mRNAs and act as m6A readers and destabilize m6A-modified mRNAs (Wang et al., 2014). A study in zebrafish revealed that over 30% of the maternal mRNAs can be m6A modified, and clearance of these mRNAs is accelerated by YTHDF. Further, YTHDF depletion delays the degradation of these m6A-modified maternal mRNAs and prevents zygotic genome activation (Zhao et al., 2017). Similarly, conditional Ythdf2 deletion in mouse oocytes causes the deregulation of maternal mRNA dosage during oocyte maturation. Analysis of MII oocytes revealed an increased dosage of transcripts of 201 genes and decreased expression of 68 genes upon conditional Ythdf2 deletion (Ivanova et al., 2017). Although YTHDF is not likely required for the process of fertilization per se as indicated by the successful extrusion of the second polar body, YTHDF2 is required to produce metaphase II oocytes competent to sustain early embryonic development (Table 2). Upon conditional Ythdf2 deletion, fewer zygotes made two-cell stage embryos of normal morphology and two-cell embryos presented various cytokinesis defects such as micronuclei and enucleated cells (Ivanova et al., 2017). The number of dysregulated transcripts caused by Ythdf2 loss-of-function is surprisingly small, yet with a clear phenotype. These findings suggest that the m6A-reader YTHDF2 is crucial for the regulation of proper degradation of a small subset of maternal mRNAs during oocyte maturation, which is necessary for the maternal-to-zygotic transition (Fig. 4B). However, Luong et al. (2020) reported that there was minimal overlap between mRNAs destabilized in meiosis I and those stabilized by Ythdf2 loss-of-function (Luong et al., 2020). This implies that YTHDF2 is not the only mechanism that accounts for maternal mRNA degradation, or it works redundantly with other factors. Overall, the current data are not sufficient to reveal the precise mechanisms by which m6A modification regulates maternal mRNA stability and it is unclear whether m6A regulates maternal mRNA degradation in a stage-dependent manner during oocyte meiotic maturation.
(5) Small RNAs mediate maternal mRNA degradation
Three main classes of small RNAs [miRNAs, siRNA, and PIWI-interacting RNAs (piRNAs)] are known to regulate gene expression through interactions with AGO proteins at the posttranscriptional level. Numerous studies have demonstrated that small RNAs, particularly siRNAs, play a critical role during maternal mRNA clearance. The biogenesis of small RNAs in oocytes is illustrated in Fig. 5. The function of small RNAs during oocyte maturation has been extensively reviewed previously (Schultz, Stein & Svoboda, 2018; Suh & Blelloch, 2011; Svoboda, 2017; Svoboda, Franke & Schultz, 2015; DeVeale, Swindlehurst-Chan & Blelloch, 2021; Li, Zhang & Liu, 2021) and will be discussed only briefly herein to provide recent updates.
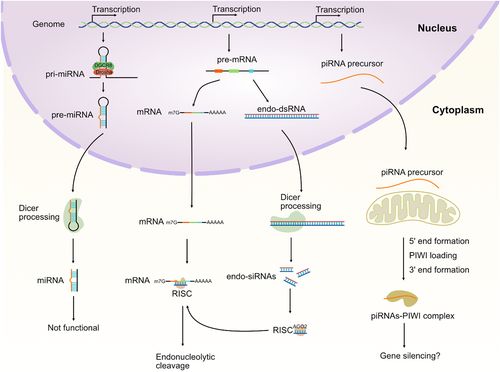
Deep sequencing of small RNAs has identified miRNAs, piRNAs, and a large number of siRNAs in mammalian oocytes (Tam et al., 2008; Watanabe et al., 2006, 2008). Studies in mammals indicate that siRNA function is essential during oocyte meiosis (Liu et al., 2017a; Stein et al., 2015). RNase III Dicer is required for both miRNA and siRNA biogenesis. Dicer depletion in oocytes causes misregulated transcripts and meiotic defects such as abnormal spindle formation and chromosomal organization (Liu et al., 2010; Liu et al., 2017a; Suh et al., 2010; Murchison et al., 2007). However, miRNA function appears to be dispensable for oocyte maturation, at least in mammals (Suh et al., 2010; Ma et al., 2010; Liu et al., 2017a). In mice and pigs, depletion of DiGeorge syndrome critical region 8 (DGCR8, a key component of miRNA biogenesis) in oocytes did not change the oocyte transcriptome and these oocytes undergo normal meiotic maturation, fertilization, and embryonic development (Suh et al., 2010; Liu et al., 2017a). Consistently, through testing the ability of endogenous miRNAs to repress target gene expression, it was demonstrated that miRNA function is suppressed during mouse oocyte meiotic maturation (Ma et al., 2010; Freimer et al., 2018). Thus, the effect caused by Dicer depletion is due to the loss of siRNA function rather than miRNA function. To determine if the function of siRNAs in oocytes is mediated by endonucleolytic cleavage, Stein et al. (2015) generated mice expressing an inactive form of Ago2 (Ago2ADH) specifically in oocytes and thus disrupted the function of siRNAs and blocked RNA interference (RNAi)-induced endonucleolytic cleavage. They demonstrated that meiotic maturation is impaired in Ago2ADH oocytes and most oocytes fail to progress to anaphase I, with severe defects in spindle formation and chromosome alignment, while oogenesis is normal (Stein et al., 2015). Moreover, the transcriptome of oocytes from these mice is widely misregulated. Gene ontology (GO) analysis of misregulated transcripts shows that up-regulated genes are enriched in the cell cycle, cell division, translational regulation, and ribosomes as well as microtubules (Stein et al., 2015). These results suggest a crucial role for siRNAs through AGO2-mediated endonucleolytic cleavage in mammalian oocytes. In addition, it has been demonstrated that a population of siRNAs in oocytes are derived from mRNAs and are speculated to regulate the stability of their precursor mRNAs (Tam et al., 2008; Watanabe et al., 2008). RNA-seq analysis showed that the levels of mRNAs that produce siRNAs in oocytes are increased in AGO2- or Dicer-null oocytes (Stein et al., 2015; Tam et al., 2008). These results suggest that the action of siRNAs in oocytes is mediated by AGO2 endonucleolytic cleavage of target mRNAs (Fig. 5). Of note, siRNA function has been widely described in mouse oocytes (Tam et al., 2008; Watanabe et al., 2008; Liu et al., 2010) but rarely reported in other species; thus, siRNA function in oocytes appears to be mouse specific. The siRNA might be derived from antisense pseudogenes, however, the mechanisms of the production (source) and timing of activation of siRNAs remain unclear.
piRNAs may control maternal mRNAs but this is much less clear than for siRNAs. piRNAs form effector complexes with PIWI proteins (a subset of Argonaute proteins) and suppress transposable elements through piRNA-induced silencing complex-mediated cleavage of target transcripts, as well as transcriptional gene silencing through DNA methylation or histone modification (Ozata et al., 2019). In mice, three PIWI proteins MIWI (PIWIL1), MILI (PIWIL2), and MIWI2 (PIWIL4) have been discovered (Li et al., 2021; Li et al., 2013; Vourekas et al., 2012). piRNAs are expressed predominantly in the germline in mammals and interact with PIWI proteins. piRNAs are essential for male gametogenesis, playing a role in the repression of transposable elements (Malone & Hannon, 2009). PIWI family deletion in mice leads to sterility in males but shows no discernible phenotype in female mice; oocytes from PIWI protein-deficient mice have no discernible phenotype (Cook & Blelloch, 2013). These data indicate that the piRNA pathway does not seem to be essential for female fertility. However, recent hamster knockout studies showed that a piRNA factor is necessary for oocytes, completely in contrast to the findings in mice (Ishino et al., 2021; Zhang et al., 2021; Hasuwa et al., 2021). Most mammals have four PIWI genes, including PIWIL3, which is not expressed in mice. PIWIL3 binds to a subset of piRNAs in hamster and human oocytes (Ishino et al., 2021; Yang et al., 2019). PIWIL3-depleted female hamsters display reduced fertility (Hasuwa et al., 2021). Deficiency of piRNA factors PIWIL1, phospholipase D family member 6 (PLD6), and Mov10 like RISC complex RNA helicase 1 (MOV10L1) in hamsters led to female infertility, with embryos arrested at the two-cell stage (Hasuwa et al., 2021; Ishino et al., 2021; Zhang et al., 2021). Therefore, piRNA might play an essential role in oocytes but this may differ among mammalian species. RNA-seq analyses in metaphase II oocytes show that the lack of PIWIL1 significantly altered the oocyte transcriptome (Hasuwa et al., 2021). Among 1612 differentially expressed genes, 66.13% showed increased mRNA abundance in PIWIL1-deficient hamster oocytes, while none of the upregulated genes were mapped to be the PIWIL1–piRNA targets. Thus, hamster PIWIL1 may regulate mRNAs through a piRNA-independent mechanism. siRNAs may also be functionally redundant with piRNAs for silencing a class of mRNAs in oocytes (Fig. 5) (Taborska et al., 2019).
Long non-coding RNAs (lncRNAs) have also been discovered in oocytes of different species (Cao et al., 2019; Fan et al., 2015; Dang et al., 2016; Karlic et al., 2017). RNA-seq profiling of lncRNA shows 1600 annotated lncRNAs expressed during the oocyte-to-zygote transition and a large population of these are removed before zygotic genome activation (Karlic et al., 2017). However, their function in oocytes remains largely unknown. Very recently, two studies demonstrated that lncRNA2193 and lncRNA Sirena1 appear to play a role in oocyte maturation (Ganesh et al., 2020; Yang et al., 2020b). lncRNA2193 knockdown by injecting siRNAs into GV porcine oocytes decreases GVBD and causes meiotic defects (Yang et al., 2020b). Furthermore, Bubr1, Mad2, c-mos, Cdk1, and cyclin B1 (Ccnb1), which are known to be crucial for oocyte meiosis, are predicted targets of lncRNA2193 (Yang et al., 2020b). Therefore, lncRNA2193 likely globally coordinates oocyte meiotic maturation through regulating maternal mRNA expression. In addition, Ganesh et al. (2020) reported that lncRNA Sirena1 is involved in the cytoplasmic organization of mitochondria in the mouse fully grown oocyte, however, it appears non-essential for female fertility. Knockout of Sirena1, lncRNA-OET-06-154, or lncRNAOET-19-199 in mice did not affect fertility (Ganesh et al., 2020; Karlic et al., 2017). Overall, most lncRNAs seem to have no function in mouse oocytes (Goudarzi et al., 2019).
VII. CONCLUSIONS
- (1)
Various events of oocyte meiotic maturation depend on timely synthesis and regulated destruction of protein. Because the transcription machinery is dormant during this period, the proteins are translated solely from the pre-existing maternal mRNAs that are transcribed and stored during oocyte growth. However, the precise mechanisms that orchestrate the timing of protein translation required for meiotic progression and developmental competence acquisition remain incompletely understood.
- (2)
A large proportion of mRNA transcripts in oocytes are stored in a dormant state, selectively recruited for translation at the appropriate time to coordinate oocyte maturation events. Consensus has been reached that the poly(A) tail length of mRNAs determines the translational activity in oocytes. In addition, cis-acting elements within the 3′ UTR of an mRNA, such as CPEs, play a key role in the control of the shortening and elongation of the poly(A) tail. These elements and their binding factors interact with each other and coordinate to control the fate of an mRNA. Although some regulators and elements that regulate mRNA translation and degradation have been discovered, the mRNA-specific mechanisms are still not fully understood. How the subcellular distribution and RNP organization regulate mRNA translational status and stability also require further study.
- (3)
Subcellular localization of mRNAs into granules has been proved to play fundamental roles not only in the storage of dormant mRNAs but also in the control of spatiotemporal translation in maturing oocytes. Increasing evidence has indicated that cytoskeleton reorganization functions in maternal mRNA localization and local translation. The interaction networks among mRNAs, RBPs, cytoskeleton, and motor proteins are still not fully understood. The development of sensitive and accurate methods for mRNA detection in live and fixed cells at single molecule resolution will allow the tracking and quantification of a single mRNA or protein in a 3D manner to decipher how they interact and regulate the translational status of mRNAs. Elucidating the molecular details of oocyte meiotic maturation will help to identify new biological features to predict and select high-quality oocytes better for fertility treatments.
- (4)
Maternal mRNAs are gradually and selectively degraded from the onset of oocyte maturation, and most maternal mRNAs, if not all, are eliminated by the early embryo stage. The concept has been developed that translational repression, poly(A) tail shortening, decapping, and slicing of mRNAs by the degradation machinery occur sequentially. In these processes, RBPs and small RNAs play a key role in specifying which mRNAs are degraded when this takes place. In this scenario, the timing of synthesis of certain RBPs and components involved in the small RNA pathway are the key mystery, which remains to be further deciphered.
ACKNOWLEDGEMENTS
The authors are indebted to Dr John Carroll for advice and comments on this review. This work was supported by the National Natural Science Foundation of China (No. 31772596 to X. Z. and No. 31672417 to C. L.). The research of D. A. is supported by The National Health and Medical Research Council, Australia.




