Aliens in caves: the global dimension of biological invasions in subterranean ecosystems
ABSTRACT
Alien species are a significant threat to natural ecosystems and human economies. Despite global efforts to address this challenge, the documented number of alien species is rapidly increasing worldwide. However, the magnitude of the impact of alien species may vary significantly across habitats. For example, some habitats are naturally less prone to biological invasions due to stringent abiotic and biotic characteristics, selecting for a limited number of introduced species possessing traits closely related to the native organisms. Subterranean ecosystems are quintessential examples of habitats with strong environmental filters (e.g. lack of light and scarcity of food), driving convergent adaptations in species that have successfully adapted to life in darkness. Despite these stringent environmental constraints, the number of records of alien species in subterranean ecosystems has increased in recent decades, but the relevant literature remains largely fragmented and mostly anecdotal. Therefore, even though caves are generally considered very fragile ecosystems, their susceptibility to impacts by alien species remains untested other than for some very specific cases. We provide the first systematic literature survey to synthesise available knowledge on alien species in subterranean ecosystems globally. This review is supported by a database summarising the available literature, aiming to identify gaps in the distribution and spread of alien invertebrate species in subterranean habitats, and laying the foundations for future management practices and interventions. First, we quantitatively assessed the current knowledge of alien species in subterranean ecosystems to shed light on broader questions about taxonomic biases, geographical patterns, modes of dispersal, pathways for introductions and potential impacts. Secondly, we collected species-specific traits for each recorded alien species and tested whether subterranean habitats act as ecological filters for their establishment, favouring organisms with pre-adaptive traits suitable for subterranean life. We found information on the presence of 246 subterranean alien species belonging to 18 different classes. The dominant alien species were invertebrates, especially insects and arachnids. Most species were reported in terrestrial subterranean habitats from all continents except Antarctica. Palaearctic and Nearctic biogeographic regions represented the main source of alien species. The main routes of introductions into the recipient country are linked to commercial activities (84.3% of cases for which there was information available). Negative impacts have been documented for a small number of case studies (22.7%), mostly related to increased competition with native species. For a limited number of case studies (6.1%), management strategies were reported but the effectiveness of these interventions has rarely been quantified. Accordingly, information on costs is very limited. Approximately half of the species in our database can be considered established in subterranean habitats. According to our results, the presence of suitable traits grants access to the stringent environmental filter posed by subterranean environments, facilitating establishment in the new habitat. We recommend that future studies deepen the understanding of invasiveness into subterranean habitats, raising public and scientific community awareness of preserving these fragile ecosystems.
I. INTRODUCTION
In a globalised planet, there has been an increase in human-mediated relocations of species beyond their natural ranges (Meyerson & Mooney, 2007; Hulme et al., 2008; Liebhold & Tobin, 2008). Alien species are defined as organisms introduced accidentally or deliberately into a habitat where they are not normally found, often representing a serious threat to biodiversity and the functioning of ecosystems (Pyšek et al., 2020; Clavero & García-Berthou, 2005; Simberloff et al., 2013). In recent years, the number of successful biological invasions has continued to rise, despite increasing global conservation efforts to address this challenge (Pagad et al., 2015), often resulting in substantial impacts to ecosystems (Vilà et al., 2010, 2011) and economies (the global cost of invasive alien species is estimated to be a minimum of $26.8 billion annually; Diagne et al., 2021).
With increasing study of the potential impacts of alien species across taxonomic groups and habitat types (e.g. Courchamp et al., 2017; Cuthbert et al., 2019; Haubrock et al., 2019; Mofu et al., 2019), there is a growing awareness that not all natural environments are equally likely to be invaded (Pyšek, Chytrý & Jarošík, 2009; Pyšek et al., 2010). Due to their abiotic and biotic characteristics, some habitats may be less prone to biological invasions than others. As foreseen by Charles Darwin, preadaptation and competition are the two key opposing forces behind the success or failure of an invasion (Cadotte et al., 2018). In other words, when a habitat exerts a strong environmental filter, colonisers showing traits that are closely related to local native organisms may be more successful than others. Conversely, when competition is the most important factor shaping a community, selection will act against trait similarity and colonisers with comparable traits are generally excluded – the so-called ‘Darwin's naturalisation hypothesis’. As a consequence, an enhanced understanding of community assembly rules in a functional perspective is crucial to assessing invasion risks (Hamilton et al., 2005; Statzner, Bonada & Dolédec, 2008; Cadotte et al., 2018).
Caves and other subterranean systems are quintessential examples of habitats with strong environmental filters, selecting for convergent adaptations in species that have successfully adapted to life in darkness (Pipan & Culver, 2012; Trontelj, Blejec & Fišer, 2012). Eye reduction, depigmentation and enhanced development of tactile and olfactory organs are among the most conspicuous features possessed by subterranean species; these shared features have evolved in response to selective environmental pressures imposed by subterranean environments. As a result, one can predict that the conditions in deep subterranean habitats should act as effective ecological filters for the establishment of alien species, favouring only those organisms with suitable pre-adaptive traits (Reeves, 1999; Mammola, 2017). Alien species can successfully establish in surface/subterranean ecotones such as cave entrances and other shallow subterranean spaces due to their higher availability of resources and greater richness and diversity of native species (Lloyd et al., 2000; Prous, Ferreira & Martins, 2004; Prous, Ferreira & Jacobi, 2015).
Despite an increased number of records of alien species in subterranean ecosystems during recent decades, relevant literature remains scarce and fragmented. Moreover, occurrences mostly refer to caves or to artificial hypogean habitats (i.e. bunkers and abandoned mines), with few studies on other kinds of – still largely unexplored – subterranean habitats (e.g. the Milieu Souterrain Superficiel; see Mammola et al., 2016). For these reasons, the true extent of alien species invasions in the subterranean realm is largely unknown and in-depth studies are needed to clarify the importance of this threat in terms of biological conservation and how best to address any related environmental issues. Consequently, assessing the effects of alien species on subterranean ecosystems is perceived as an important and urgent question in cave-based science (Mammola et al., 2020).
To facilitate this goal, we here provide a first synthesis of the available literature on alien species in subterranean ecosystems. We asked three general questions: (i) hat are the most frequent alien taxa present in subterranean habitats? (ii) What are the origins, the recipient countries, and the pathways of alien species introductions in subterranean ecosystems? (iii) What are the environmental and socio-economic impacts of these species? We then extracted information on species-specific traits for each alien species documented in subterranean ecosystems across the sampled literature, aiming to answer a further question: (iv) do successful colonisers of subterranean environments display pre-adaptive traits? Specifically, we tested the relationship between the presence/absence of adaptive traits facilitating the colonisation of subterranean ecosystems and the probability of establishment in a subterranean environment. Considering that the strength of competition in caves is often lower than that of environmental filtering (Mammola, 2019), we predict that successful colonisers should possess traits that are similar to those of local native organisms (Cadotte et al., 2019).
II. MATERIALS AND METHODS
(1) Scope of the analysis
We focused on terrestrial and freshwater subterranean habitats globally. Following the function-based classification of Earth's ecosystems (Keith et al., 2022), the habitats we considered were ‘Subterranean’ (S) [including ‘Subterranean lithic’ (S1) and ‘Anthropogenic subterranean voids’ (S2) biomes] and ‘Subterranean-freshwater’ (SF) [including ‘Subterranean freshwater’ (SF1) and ‘Anthropogenic subterranean freshwater’ (SF2) biomes]. We excluded marine caves and anchialine systems, i.e. the ‘Subterranean tidal’ (SM1) biome sensu Keith et al. (2022). The diversity of alien species, pathways of introduction, and management in marine systems seems to be much lower than in terrestrial ecosystems [see Gerovasileiou et al. (2016, 2022) for extensive coverage of alien species in marine caves].
Furthermore, we did not consider studies focusing on alien photosynthetic organisms (lampenflora) in caves opened to tourism (i.e. illuminated by artificial lights; see e.g. Cigna, 2011; Falasco et al., 2014; Mulec, 2019; Piano et al., 2015; Piano, Nicolosi & Isaia, 2021). We excluded studies on lampenflora because the species pool of photosynthetic organisms colonising a cave usually originates from the surface habitat in the proximity of the cave rather than a different biogeographic region. In addition, this topic has been the subject of other reviews (Baquedano Estévez et al., 2019; Falasco et al., 2014; Piano et al., 2022).
We considered alien subterranean species to be alien species moved by human activities beyond the limits of their natural geographic range into a new area (sensu Richardson, Pyšek & Carlton, 2011) and invading any of the subterranean systems considered herein (i.e. S1, S2, SF1 and SF2, see above). We acknowledge that this is a broad generalisation: nativeness is a nuanced and highly dynamic concept (Lemoine & Svenning, 2022; Verbrugge, Leuven & Zwart, 2016) whose assessment necessarily entails a certain degree of interpretation and subjectivity. However, given the scarce information available, we found this simplification to be appropriate for our analysis.
(2) Standardised literature search
We conducted a systematic literature review focused on the occurrence of subterranean alien species. In August 2021, we performed standardised literature searches in the Clarivate Analytics Web of Science. For the literature search, we followed the PRISMA reporting standard (Moher et al., 2009; O'Dea et al., 2021).
We initially trialled several combinations of words for our Web of Science query string, aiming to improve the search specificity. During this exploratory trial, we found that the use of generic terms such as ‘Subterranean’ and ‘cave’ resulted in an excess of irrelevant articles often referring to archaeological, anthropological, or mineralogical aspects. To minimise this number of irrelevant references, we added a ‘NOT’ criterion while also restricting our search to Web of Science Categories referring to natural science and biology. The final search string was: TS = (“cave*” OR “hypoge*” OR “subterranean” OR “lava tube*”) AND TS = (“alien*” OR “invasive” OR “introduced” OR “exotic” OR “non-native” OR “non native” OR “non-indigenous” OR “non indigenous”) NOT TS = (“termite*” OR “fungi” OR “marine” OR “architecture” OR “Archaeol*” OR “microbial” OR “medicine” OR “speleogenesis” OR “art” OR “histor*” OR “agricult*”) AND WC = (Ecology, Zoology OR Entomology OR Geosciences Multidisciplinary OR Biodiversity Conservation OR Multidisciplinary Sciences OR Agriculture Multidisciplinary OR Environmental Sciences OR Plant Sciences OR Geology OR Agronomy OR Marine Freshwater Biology OR Biology OR Genetics Heredity OR Soil Science OR Biotechnology Applied Microbiology OR Forestry OR Evolutionary Biology OR Education Educational Research OR Fisheries OR Horticulture OR Microbiology OR Veterinary Sciences OR Agriculture Dairy Animal Science OR Oceanography OR Toxicology OR Anatomy Morphology OR Mycology OR Education Scientific Disciplines OR Infectious Diseases OR Ornithology).
This initial search yielded 2781 papers. We screened the titles and abstracts of all papers obtained from this search for eligibility to be included in the review, selecting N = 448 for potential inclusion. We then read the full text of each of these papers to select relevant studies based on a set of inclusion/exclusion criteria. We included studies if they: (i) investigated the state of subterranean ecosystem components potentially impacted by alien species; (ii) provided subterranean fauna inventories including the presence of subterranean alien species; and (iii) investigated the effect of management practices in subterranean ecosystems to control or eradicate subterranean alien species. We excluded studies that: (iv) focused on subterranean alien species in non-subterranean habitats; (v) focused on ‘Subterranean tidal’ ecosystems (SM1, see Section II.1). A total of 43 papers met our criteria (Fig. S1).
We cross-checked the resulting list of subterranean alien species with the Global Biodiversity Information Facility (GBIF; www.gbif.org; accessed December 2021) and International Union for Conservation of Nature (IUCN) ISSG Global Invasive Species Database (www.iucngisd.org/gisd/; accessed December 2021) to verify the current status (i.e. if the species is currently considered as alien in the specific country) and level of invasiveness of the alien species present in our database.
(3) Additional literature search
Given that the literature on alien species includes grey literature not listed on the Web of Science, including technical reports and articles not in English (Haddaway et al., 2020; Chowdhury et al., 2022), we conducted parallel searches for additional papers to maximise the comprehensiveness of our database. For each paper selected above, we inspected the reference list to retrieve additional potentially relevant literature. We also performed a search in Google Scholar (Haddaway et al., 2015) using the same key words listed in Section II.2. These additional searches resulted in 61 papers added to our database (Fig. S1).
(4) Meta-data extraction
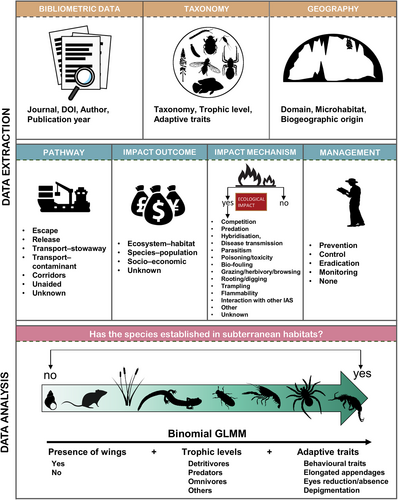
The full list of the metadata extracted and their sources is presented in Table 1. The literature database is provided as online supporting information in Table S1. For each paper, we read the full text and extracted detailed information (Fig. 1), including the year of the study and the country where the study occurred. Next, we recorded the alien species (see definition in Section II.1) mentioned in each publication, its most recent taxonomy (based on the GBIF database), the domain (terrestrial or freshwater), and the type of subterranean habitat in which the species was found using Keith et al. (2022) as: ‘Subterranean lithic’, ‘Anthropogenic subterranean voids’, ‘Subterranean freshwater’, or ‘Anthropogenic subterranean freshwater’. In addition, we subdivided the subterranean lithic habitat into ‘limestone cave’ and ‘lava tubes’.
| Metadata | Sources | Description | Levels |
|---|---|---|---|
| Species | Investigated literature | Scientific name of the subterranean alien species | – |
| Class | GBIF backbone taxonomy | Class of the subterranean alien species | – |
| Order | GBIF backbone taxonomy | Order of the subterranean alien species | – |
| Organism group | Investigated literature | Taxonomic group included in the database | Invertebrate; Vertebrate; Plant |
| Trophic level | Investigated literature | Level or position in food chain, food web, or ecological pyramid of the subterranean alien species | Detritivore; Herbivore; Omnivore; Parasite; Predator; Primary producer |
| Location | Investigated literature | Invaded country out of the native range of the subterranean alien species | – |
| Domain | Investigated literature; General literature | Type of ecosystem in which the subterranean alien species occurs | Terrestrial; Freshwater |
| Microhabitat | Investigated literature | Type of habitat in which the subterranean alien species occurs | Subterranean lithic; Anthropogenic subterranean voids; Subterranean freshwater; Anthropogenic subterranean freshwater |
| Origin continent | Investigated literature; GISD; GBIF | Continent in which the subterranean alien species originated and/or where it first arrived without human intervention. Species with a ‘Cosmopolitan’ distribution are recognised as alien although their specific geographic origin is unknown. | Asia; Africa; North America; South America; Antarctica; Europe; Oceania; Unknown; Cosmopolitan |
| Biogeographic origin | Literature; General literature; GISD; GBIF | Bioregion in which the subterranean alien species originated and/or where it first arrived without human intervention | Global; Afrotropical; Indomalayan; Nearctic; Neotropical; Oceanian; Palaearctic; Unknown |
| Established | Investigated literature | Indication of the possible naturalisation of the subterranean alien species into the new habitat/country | Naturalised; Occasional; Unknown |
| Adaptive trait | Investigated literature; General literature | Indication of the presence or absence of adaptations commonly present in the subterranean alien species | Yes; No |
| Trait | Investigated literature; General literature | Type of adaptation present in the subterranean alien species | Absence of eyes; Behavioural traits; Depigmentation; Elongated appendages; Eyes reduction; Physiological adaptations |
| Presence of wings | Investigated literature; General literature | Considered a proxy for dispersal ability | Yes; No |
| Impact | Investigated literature | General impact caused by the subterranean alien species | Positive; Negative; Neutral; Unknown |
| Mechanism | Investigated literature | Any change in ecological or ecosystem properties, excluding socio-economic effects and human values | Competition; Predation; Hybridisation; Disease transmission; Parasitism; Poisoning/toxicity; Bio-fouling; Grazing/herbivory/browsing; Rooting/digging; Trampling; Flammability; Interaction with other invasive species; Other; Unknown |
| Impact outcome | Investigated literature; InvaCost database (for socio-economic impacts only) |
Impact of subterranean alien species: changes to environmental or socio-economic parameters | Ecosystem – Habitat; Species – population; Socio-economic; Unknown |
| Pathway | Investigated literature; General literature; GISD; GBIF | Pathways of introduction: how a species is transported (intentionally or unintentionally) outside its natural geographical range | Release; Escape; Transport – contaminant; Transport – stowaway; Corridors; Unaided; Unknown |
| Management | Investigated literature | Any lethal or non-lethal action aimed at the eradication, population control or containment of a population of an invasive alien species | Prevention; Eradication; Control; Monitoring; None |
- In ‘Sources’, ‘Investigated literature’ refers to the literature extracted in our systematic survey; ‘General literature’ refers to additional literature sourced for each species using Google Scholar and by inspecting reference lists.
- GBIF, Global Biodiversity Information Facility; GISD, Global Invasive Species Database.
We included the biogeographic region of origin of the alien species (Global, Afrotropical, Indomalayan, Nearctic, Neotropical, Oceanian, Palaearctic, Unknown), based on the information reported complemented by species-specific literature searches.
We included a generic indication of the possible establishment of the subterranean alien species (Occasional, Naturalised, Unknown) based on the information provided in each paper. If not specifically stated, we considered as naturalised (i.e. established) a species forming plausible self-replacing populations (i.e. abundant, spread across multiple locations and present throughout the year) (Richardson et al., 2000). In other cases, we considered the species as ‘Occasional’. When the information was missing or insufficient to define its status, we classified it as ‘Unknown’.
For the type of impact, impact outcome, and management activities we referred to the categories/classifications present in the IUCN Global Invasive Species Database.
Based on the information reported in each publication, we registered the impact outcome of the subterranean alien species (Ecosystem/habitat, Species/population, and/or Socio-economic, or Unknown), and performed an overall assessment of the direction of this impact (Positive, Negative, Neutral or Unknown). For Socio-economic impact, we also used the InvaCost database (version 4.0) (Diagne et al., 2020a,b) to obtain an estimate of the globally reported costs of that alien species. Although the available data do not specifically refer to subterranean habitats, they provide a proxy indication of the potential socio-economic impact in subterranean habitats.
We classified the ecological impacts on the subterranean habitat caused by each species into 13 mechanisms: Competition (the alien species competes with cave-dwelling native taxa for resources); Predation (the alien species predates cave-dwelling native taxa); Hybridisation (the alien species hybridises with cave-dwelling native taxa); Disease transmission (the alien species transmits diseases to native cave-dwelling species); Parasitism (the alien taxon parasitises cave-dwelling native taxa); Poisoning/toxicity (the alien taxon is toxic or allergenic to cave-dwelling native taxa); Bio-fouling (the alien taxon deposits on surfaces or septa of cave-dwelling native taxa, compromising their functionality); Grazing/herbivory/browsing (the alien species affects the functional species composition of plant communities); Rooting/digging (the alien species alters the soil layers); Trampling (the alien taxon causes impacts on substrate properties); Flammability (the alien species modifies the fire regime by altering the inherent flammability of the ecosystem); Interaction with other invasive species (the alien species interacts with other introduced species); Other (other impacts not included above); and Unknown (no documented impact). Note that a single species may fit into multiple categories.
We noted management activities (either suggested or implemented) to prevent or limit the spread of the alien species: Prevention (any measures aimed at preventing alien species from entering a nation or habitat); Eradication (any practice that aims to eradicate the alien species completely); Control (any long-term practice for limiting abundance or density of the alien species); Monitoring (any short- or long-term monitoring program of the status of an alien species); and None (no actions in place, or none known, to prevent the presence or spread of the alien species).
We specified the pathway through which the species reached the recipient region according to the Convention on Biological Diversity (CBD) pathway categorisation (CBD, 2014). Pathways included seven categories: Release (released intentionally for the purpose of human activities, e.g. biological control, fishery, hunting activities, or others); Escape (released unintentionally from confinement, e.g. aquaria, aquaculture, or scientific research); Transport – contaminant (the alien species has a trophic or biotic relationship to organisms or items being transported and on which its survival depends); Transport – stowaway (the alien species has no trophic or biotic relationship to the organisms or items being transported or, if there is any, the alien can survive in their absence); Corridors (dispersed through the establishment of an anthropogenic dispersal corridor such as tunnels or bridges); Unaided (moved naturally across borders); and Unknown (unknown pathway). When available, we also specified the pathway by which alien species were introduced into new subterranean environments within a recipient region, following the same categorisation (see pathways in bold in Table S1).
(5) Species-level traits
We referred to specialised literature to collect species-specific traits for each subterranean species in our database. In the absence of universal criteria that could be applied to quantify the degree of subterranean adaptation, we reported the presence/absence of adaptations commonly present in subterranean species (Pipan & Culver, 2012) based on the biological information available for each species. We scored the following traits: Depigmentation, Absence of eyes, Eyes reduction, Elongated appendages, Behavioural traits, and Physiological adaptations (e.g. lower metabolic rate, reduction in the number of eggs, increased longevity). We also recorded the presence/absence of wings as a proxy for dispersal ability (presence of wings). We also collected data on the trophic level of the subterranean alien species (Detritivore, Herbivore, Omnivore, Parasite, Predator, Primary producer) based on the biological information available for each species.
(6) Data analysis
We carried out analyses in R version 4.2.0. (R Core Team, 2021). We summarised data on alien species in subterranean ecosystems using bar charts and other graphical tools from the package ggplot2 version 3.3.6. (Wickham, 2016). We visualised the geographic dimension of biological invasions in subterranean ecosystems by projecting onto a global map a network connecting the biogeographic region of origin and the recipient country for each species included in the database.
Finally, we constructed a regression model to explore the role of species traits in explaining the probability of a given alien species establishing in subterranean habitats (see bottom panel in Fig. 1). For model construction and validation, we followed Zuur & Ieno (2016). Given that the response variable is binary (species is established or not) we modelled data using a Bernoulli distribution and a cloglog link function, suitable for an unbalanced binary distribution in the response variable. We fitted the model using a generalised linear mixed model (GLMM) with the R package lme4 version 1.1–27 (Bates et al., 2015). The structure of the model, in R notation, was: y ~ Adaptive traits + Trophic level + Presence of wings + (1 | Class/Order), where: ‘Adaptive traits’ is the presence or absence of any adaptive trait related to subterranean life (see Section II.5 and Table 1 for the full list), which we interpreted as possible preadaptations aiding alien species to overcome the environmental filter posed by subterranean environments (Mammola, 2017). We only considered the explanatory variable ‘Adaptive traits’ in our model rather than each single trait given the limited number of species presenting subterranean traits, and the consequent prevalence of zeros (i.e. absence of traits).
‘Trophic level’ is a categorical variable that we included to test whether different trophic groups are more likely than others to establish in subterranean habitats. We used the trophic levels Detritivore, Predator, Omnivore, and Others; with ‘Others’ here including the least common trophic levels Herbivore, Primary producer and Parasite, which we grouped together to balance factor levels. ‘Presence of wings’ refers to the presence or absence of wings, which we interpreted as a proxy for dispersibility in a range-expanding population. The random structure of the model was used to control for data non-independence, under the assumption that taxonomically related species may express more similar traits than expected from random. We validated the model with the R package performance version 0.9.0. (Lüdecke et al., 2021).
III. RESULTS AND DISCUSSION
We included 104 publications in the final database (Table S1). Most of these papers were published after the year 2000 (Fig. S2). This body of literature encompasses 362 reports of alien species in subterranean habitats corresponding to 246 unique alien species from 18 classes invading subterranean habitats (Fig. 2).

(1) What are the most frequent alien taxa present in subterranean environments and habitats?
Most of the subterranean alien species were reported in terrestrial subterranean habitats (322/362 cases; 88.9%), rather than freshwater (40/362; 11.0%). Subterranean lithic was the most invaded terrestrial ecosystem (323 cases) of which 194 (53.6%) reports concerned limestone caves and 129 (35.6%) lava caves, followed by anthropogenic subterranean voids with 14 cases (3.87%).
From these 362 cases, we extracted information on 246 unique species invading subterranean habitats. These were mostly invertebrates (211; 85.8%), followed by vertebrates (20; 8.1%) and plants (15; 6.1%) (Fig. 3A). Among invertebrates, arthropods dominated, especially the class Insecta (59 species; 24.0%) followed by Arachnida (46; 18.7%), Entognatha (32; 13.0%), and Diplopoda (19; 7.7%). Vertebrates were represented by Actinopterygii (10 species; 4.1%), followed by Amphibia and Mammalia with five species each (2.0%). This pattern reflects the dominant groups in subterranean food webs (Deharveng & Bedos, 2018). In surface ecosystems insects are considered among the most invasive organisms (Seebens et al., 2017), although current knowledge in invasion ecology might be taxonomically and/or geographically biased (Pyšek et al., 2008). Among invertebrates, the other dominant group was the class Malacostraca (Gastropoda) (22; 8.9%) (Fig. 3A). Despite the general adverse conditions in caves for plants, the class Magnoliopsida constituted 5.3% of all species in our database, being mostly represented by species colonising the entrance zone, or penetrating the soil and reaching the cave with their roots.
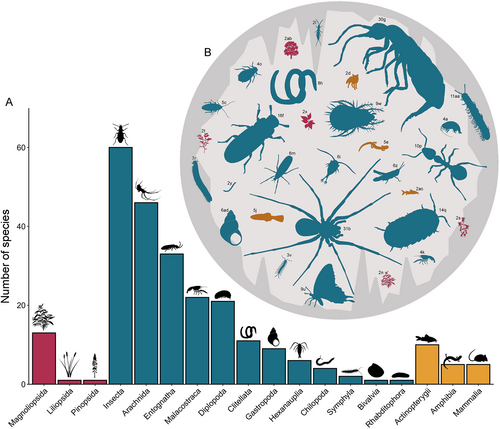
Araneae and Collembola were the most dominant orders, represented respectively by 31 species (12.6%) and 30 species (12.2%), followed by Coleoptera (16; 6.5%), Isopoda (14; 5.7%), and Hymenoptera (10; 4.1%) (Fig. 3B). Among vertebrates, Caudata and Cyprinodontiformes were the best represented orders with five species each (2.0%).
The five species identified most often in caves are the diplopod Oxidus gracilis (15 cases), followed by the fire-ant Solenopsis invicta (14), the cockroach Periplaneta americana (7), the spiders Nesticella mogera (7) and Psilochorus simoni (7) and the worm Bimastos rubidus (5).
Subterranean ecosystems are generally regarded as nutrient-poor environments that mainly depend on energy inputs from the surface (Culver & Pipan, 2019). Consequently, food webs are bottom-truncated (Gibert & Deharveng, 2002) and detritus-based; herbivores are usually absent, although cave root feeders may be present (Howarth, 1983). As expected, detritivores were the dominant feeding group among the 246 subterranean alien species detected in subterranean ecosystems, encompassing 81 species (33.0%), followed by predators (70; 28.5%), omnivores (60; 24.4%), and herbivores (18; 7.3%).
(2) What are the origins, recipient countries and pathways of alien species introductions in subterranean ecosystems?
The greatest proportion of alien species in our database has a Palaearctic origin (116; 47.2%), followed by Neotropical (26; 10.6%), Indomalayan (23; 9.3%), Afrotropical (15; 6.1%), Nearctic (18; 7.3%) and Oceanian (10; 4.1%); 15 species (6.1%) have a global distribution, with information lacking for 23 species (9.3%) (Fig. 4).
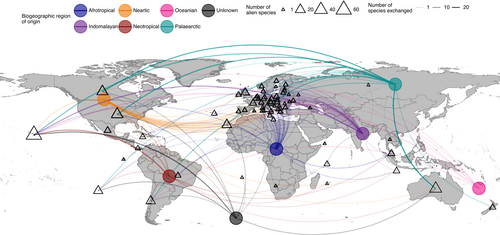
Palearctic and Nearctic biogeographic regions represent the main source of alien species, with broad bi-directional exchanges between these two regions (Fig. 4). This trend is likely due to the greater economic development of these regions and their associated international trade and globalisation networks (Turbelin, Malamud & Francis, 2017), although could be also attributed to higher research effort on alien species in these regions (Pyšek et al., 2008). In Europe, a broad contingent of species also comes from the Afrotropical and Indomalayan biogeographic regions (Fig. 4).
Records of subterranean alien species spanned 60 countries. The majority were reported from the USA, of which 56.1% are in the Hawaiian Islands (Fig. 4). This high percentage is probably due to extensive efforts by local researchers documenting the alien fauna of Hawaiian lava tubes over several decades (e.g. Howarth, 1978; Howarth et al., 2007; Howarth & Stone, 2020).
Australia, a region with a long history of biological invasions (Bradshaw et al., 2021), had the second highest number of reported subterranean alien species, followed by Spain (of which 88.5% of records were in the Canary Islands) and Italy (Fig. 4). This distribution again may reflect greater research efforts in these countries, as well as the paucity of information on the distribution of alien species in subterranean habitats in most countries. However, these data are in line with the global trend for invasive alien species observed by Turbelin et al. (2017).
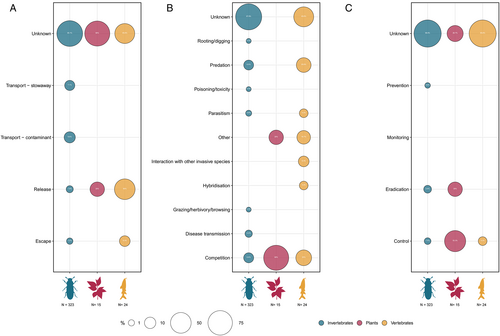
Although research efforts to understand pathways of biological invasions have increased recently (Meyerson & Mooney, 2007), information on subterranean species is scarce. We could retrieve information on the routes by which alien species were introduced into the recipient country for only 64 out of 362 cases (17.7%). Of these, only in a limited number of cases (18 out of 64, 28.1%) was information about the pathways of introduction into the subterranean habitat specified. The most widespread form of introduction into the recipient country is related to trade activities (54 out of 64 cases, 84.3%: Transport – contaminant, 31 cases, 48.4%; Transport – stowaway, 36 species, 35.9%), especially for invertebrates (Fig. 5A), and in particular for predators and omnivores (Table S1).
The trade in potted plants is possibly the main vehicle of introduction of alien species into subterranean ecosystems. Invertebrate species can be passively dispersed within the plant's pot; once the pot is placed on the ground in a garden or greenhouse, alien species may find suitable microclimatic conditions (e.g. high moisture) to thrive (Sánchez-García, 2014). Once established, they can disperse and potentially find suitable conditions in subterranean environments. This was seemingly the case for the detritivore Oxidus gracilis (CL Koch), known as the ‘greenhouse millipede’ (Iniesta et al., 2020), and the predator Caenoplana coerulea Moseley (Suárez, Martín & Naranjo, 2018), recorded in subterranean habitats globally and in the Canary Islands, respectively. The European spider Kryptonesticus eremita (Simon) plausibly might have colonised New Zealand via shipping containers, considering the proximity between the site of detection of this species and the port of Auckland (Vink & Dupérré, 2011).
The deliberate introduction of alien species represents the third most common pathway (21/64 cases; 32.8%), especially for vertebrates (Fig. 5A), and mostly among omnivores (Table S1). Animals may be deliberately released for their food value, especially in freshwater ecosystems (e.g. Hobbs, Jass & Huner, 1989). For example, the red swamp crayfish Procambarus clarkii (Girard) has spread widely throughout freshwater bodies across Europe since its first introduction in Spain (Habsburgo-Lorena, 1978; Souty-Grosset et al., 2016) now representing one of the 100 worst invasive species (DAISIE, 2008). It is increasingly being documented also in aquifers and caves (Mazza et al., 2014; Souty-Grosset et al., 2016; Di Russo et al., 2017; Cilenti et al., 2017).
On rare occasions, alien species have been introduced into subterranean environments for scientific purposes. The olm Proteus anguinus Laurenti, a specialised subterranean salamander inhabiting caves in the Dinarides, was deliberately released during the 1940s into a suitable cave in the Mendip Hills, UK (Chapman, 1993). However, there is no evidence that it became established (Lewarne & Allain, 2020). Likewise, Hydromantes salamanders have been intentionally released outside their natural range as part of scientific experiments. Evidence suggests the possible establishment of a persistent population capable of reproducing in their new subterranean habitat in the French Pyrenees (Lunghi et al., 2018). Among invertebrates, the beetle Speonomus normandi hydrophilus (Jeannel), originally distributed in the French Pyrenees, has been experimentally introduced into Dzwonnica Cave (Poland). Interestingly, there is evidence for molecular divergence between the native and introduced populations, suggesting that the local conditions might have an important influence on haplotype diversity of both populations (Kocot-Zalewska, Domagała & Lis, 2021).
Finally, escapes represent the least frequent form of introduction (6/64 cases; 9.4%), although they are more common for vertebrates (Fig. 5A). Escape is considered among the most common pathways for alien plants and vertebrates (Saul et al., 2017), especially through the horticulture trade (Turbelin et al., 2017). This route was mostly represented among omnivores in our database (Table S1).
(3) What are the impacts of alien species in subterranean habitats?
Our results reveal that in most cases the impact of alien species in subterranean ecosystems is unknown (280 out of 362 cases; 77.3%), whereas they have negative biological consequences in 82 out of 362 cases (22.7%).
The outcome was specified in our database in only 76 cases. Of these, 65 out of 76 cases (85.5%) have negative repercussions at the species/population level and 49 out of 76 (41.3%) on ecosystems/habitat.
Information on the mechanisms through which alien species impact native subterranean organisms and/or ecosystems was available for 67 cases, with the most important being competition (40/67 cases; 59.7%) and predation (26; 38.8%), followed by disease transmission (7; 10.4%) and parasitism (4; 6.0%). There were single records of negative impacts via grazing/herbivory/browsing, poisoning/toxicity, rooting/digging, interaction with other invasive species, and hybridisation. Information about mechanisms was lacking for the majority of cases included in our database (295 out of 362 cases; 81.5%) (Fig. 5B).
Competition of alien species with native organisms was most prevalent for plants and vertebrates (Fig. 5B), and mostly affects omnivores and primary producers (Table S1). Many alien species have traits that allow them to outcompete residents once they establish themselves in new areas. This is true for P. clarkii which occur at greater densities and tend to be more active in comparison with indigenous crayfish species (Reynolds, 2011). The presence of P. clarkii in subterranean ecosystems is widely reported (e.g. Mazza et al., 2014; Souty-Grosset et al., 2016; Di Russo et al., 2017; Cilenti et al., 2017), and established populations are able to thrive over a wide range of biotic and abiotic conditions from tropical to temperate zones (Gherardi & Panov, 2009; Siesa et al., 2011). Likewise, the non-subterranean spider N. mogera (Yaginuma) appears to be outcompeting local populations of the spider Erigone stygia Gertsch in the mid- to high-elevation caves on Hawai'i Island. Due to the constant supply of new individuals from surface habitats, the alien spider is replacing E. stygia, and probably exploits same prey (Howarth, 1978).
Although plants cannot colonise light-deprived underground environments, roots may penetrate the ceilings of shallow caves and other superficial subterranean habitats competing with local species and causing management issues (Howarth et al., 2007).
Predation represents the second most common impact mechanism in subterranean ecosystems (38.8% of cases), mostly among invertebrates and vertebrates (Fig. 5B), and especially for omnivores (Table S1). The red fire ant S. invicta Buren represents one of the most harmful predators in subterranean ecosystems (Elliott, 1992, 2000; Taylor, Krejca & Denight, 2005; Cokendolpher et al., 2009; Pape, 2016). S. invicta is considered one of the 14 worst invasive alien insect species worldwide (Lowe et al., 2000) and is included within the top 100 of the World's worst invasive species by the IUCN (Boudjelas et al., 2000). Although it is not strictly subterranean, it often constructs mounds near cave entrances because of suitable microclimatic conditions (Elliott, 1993). From there, individuals enter the caves and prey efficiently on numerous subterranean species, including several endangered species (Elliott, 1993; Cokendolpher et al., 2009).
Some vertebrates can be efficient predators in subterranean ecosystems and may pose a serious threat to cave-dwelling species. The presence of rats (Rattus rattus), a cosmopolitan pest widely recognised as one of the most damaging invasive species worldwide [Global Invasive Species Database (GISD), 2020], has been highlighted in numerous caves in the Hawaiian Islands. Rats enter caves in search of water and food and may prey on native species (Howarth & Stone, 2020).
Although underrepresented in the literature, subterranean alien species can also transmit disease (10.4%) or have impacts via parasitism (6.0%) (Fig. 5B). Introduction of the guppy Poecilia reticulata Peters into the subterranean karst habitat of Christmas Island (Australia) is considered a threat due to both its highly predatory activity and to its potential transmission of a parasite (Asian fish tapeworm Bothriocephalus acheilognathi Yamaguti) which could threaten eleotrid fish populations (Humphreys, 2014). The bed bug Cimex lectularius Linnaeus has been recorded to feed on bats and probably transmits Trypanosoma cruzi Chagas (Reeves, 1999). The browndog tick Rhipicephalus sanguineus Latreille introduced into North America from Europe is a vector for several diseases (Reeves, 1999).
(4) What are the socio-economic impacts of alien species in subterranean ecosystems?
Of all alien species found in subterranean ecosystems, only 2.2% have been associated with a socio-economic impact, although these costs have not been quantified in detail. Information on costs associated with alien species in subterranean ecosystems is very limited. This may not be surprising as many of these species are invertebrates, which are generally underrepresented in the literature (Cardoso et al., 2011; Titley, Snaddon & Turner, 2017). Additionally, when a species has no or little impact in a certain habitat, there will be no assessment of damage or intervention costs.
A recently developed database on the economic costs of invasive alien species globally (Diagne et al., 2020a), and associated studies using this database, provide an opportunity to look in more detail at the economic costs associated with alien species present also in caves.
Among these, only S. invicta is known to be associated with substantial costs (Angulo et al., 2022). This species is among the most notorious invasive species in subterranean ecosystems, and is considered a serious land invertebrate pest. Its invasive behaviour leads to impacts on human health, livestock, biodiversity, crops, and machinery (Wojcik et al., 2001). Elliott (1993) evaluated the efficacy and relative cost of different treatment methods in subterranean habitats, but a general estimate of the socio-economic cost of this species in such habitats is still lacking.
Some species are associated with very high economic costs in other habitats. For example, of the 100 World's worst invasive alien species, R. rattus has the second highest associated costs (Cuthbert et al., 2021), however, these reported costs resulted mainly from severe impacts on resident animal populations on islands (e.g. through predation of birds' eggs) and from efforts to eradicate them (e.g. Genovesi, 2005; Parkes, Byrom & Edge, 2017). The economic costs associated with this species in subterranean ecosystems remain largely unknown (Howarth & Stone, 2020).
Although unquantified, the introduction of alien species into subterranean ecosystems may also have social costs. These can include a decrease or loss of heritage value of cave-dwelling native species (Souty-Grosset et al., 2016). For example, the presence of alien crayfish can lead to the disappearance of festivals celebrating native crayfish (Reynolds & Souty-Grosset, 2011).
(5) What are the management interventions used to protect subterranean habitats?
Management interventions have been used in only in a limited number of cases (22/362) in subterranean ecosystems (Fig. 5C). Furthermore, the effectiveness of these interventions has seldom been tested statistically (Mammola et al., 2022), and most knowledge on eradication activities remains qualitative (Simberloff, 2002; Genovesi, 2005). Eradication actions have been undertaken to counteract the spread of the fire ant S. invicta in the southern USA. The most efficient methodology seems to be the use of boiling water to kill ants in the nest. Even though this is labour-intensive, it avoids the problem of non-target species consuming insecticidal baits (Elliott, 2000). However, it is not a cost-effective method over large areas (Elliott, 1992, 1993). The trapping and hand removal of P. clarkii from subterranean habitats has reduced populations of this species, but has not led to its eradication from these ecosystems (Mouser et al., 2018).
When eradication fails, long-term control activities can limit the impact of an alien species, reducing its density and abundance (Mooney et al., 2005). Several methods to control the dispersal of P. clarkii have been tested, with a synergistic approach using different methodologies often the most successful (Souty-Grosset et al., 2016). For rats, control activities are generally carried out by both public and private agencies, but caves usually are not included in such efforts (Howarth & Stone, 2020).
Prevention actions can stop a species from colonising new areas (Mooney et al., 2005). For example, the installation of artificial barriers can be a useful mechanism to prevent the entrance and spread of alien species in subterranean streams (Mouser et al., 2018). However, besides the cost implications of such barriers often being high, they may alter the flow regime and/or microclimatic conditions, while also preventing the movement of organisms in stream ecosystems (Ellis & Jones, 2013).
(6) Are there common traits shared by alien species that successfully establish in subterranean ecosystems?
Of the 246 alien species listed in our data set, 127 (51.6%) are considered to be successfully naturalised in subterranean habitats. Insects and arachnids make up the greatest proportion of naturalised species, with other invertebrate groups (gastropods and myriapods) underrepresented. Approximately one third of the species recorded in subterranean habitats are not considered to be established (73/246 species). No information on establishment success was available for 46 species (18.7%).
Only some of these alien species exhibit adaptations to subterranean life (90/246; 36.6%), including depigmentation, eye loss/reduction, or a preference for dark and humid habitats. This limited number of alien species strictly adapted to subterranean environments (e.g. Proteus anguinus, Parabathyscia dematteisi) mostly pertains to escapes of species introduced into subterranean habitats for scientific purposes (e.g. Chapman, 1993; Lewarne & Allain, 2020). This is probably due to the high sensitivity of such species to even small environmental variations (e.g. Barr & Kuehne, 1971; Howarth, 1980; Culver, 2005; Nicolosi et al., 2021) limiting their dispersal outside a subterranean environment.
Our modelling showed that the presence of adaptive traits is the strongest predictor of the probability that a species will become established in a subterranean habitat (binomial GLMM: estimated β ± SE: 1.44 ± 0.38, z = 3.80, P < 0.001; Fig. 6; Table 2). Additionally, the probability of establishing in a subterranean habitat was lower for species in the trophic level ‘Others’ (including herbivores, primary producers and parasites in this analysis) compared to detritivores, although this did not reach statistical significance (β ± SE: −1.08 ± 0.66, z = −1.63, P = 0.10). No other traits were found to exert a significant effect on the probability of becoming established in a subterranean habitat (Table 2). The regression model explained 47% of the variance (conditional r2: 0.47), of which over 28% was attributable to species taxonomy.
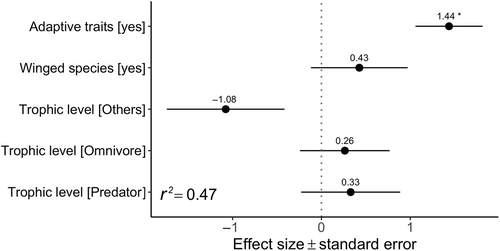
| Predictor | Estimate | S.E. | z | P |
|---|---|---|---|---|
| Intercept | −0.693 | 0.480 | −1.445 | 0.148 |
| Adaptive trait [Yes] | 1.436 | 0.378 | 3.803 | <0.001 |
| Presence of wings [Yes] | 0.427 | 0.544 | 0.784 | 0.432 |
| Trophic level [Others] | −1.078 | −0.662 | −1.628 | 0.104 |
| Trophic level [Omnivore] | 0.263 | 0.505 | 0.522 | 0.602 |
| Trophic level [Predator] | 0.329 | 0.558 | 0.590 | 0.556 |
- For predictor variables, we report in square brackets the level that is being tested. For the variables ‘Adaptive trait’ and ‘Presence of wings’, the baseline level used in the analysis is ‘No’. For the variable ‘Trophic level’, the baseline level is ‘Detritivores’.
IV. CONCLUSIONS
- (1)
Due to their simplified trophic web, low species diversity, and high spatial confinement, subterranean ecosystems are generally considered more vulnerable than surface ecosystems to anthropogenic disruption (Mammola et al., 2019). Whilst many authors have suggested that the presence of alien species may contribute significantly to the decline of subterranean species and ecosystems (e.g. Mazza et al., 2014; Suárez et al., 2018; Howarth & Stone, 2020), the true extent of their impact remains unclear (Mammola et al., 2020). Furthermore, our understanding is geographically and taxonomically biased. In-depth studies remain needed to understand the significance of alien species in subterranean ecosystems and how they affect the subterranean biota. This review provides the first comprehensive global synthesis of alien species in subterranean ecosystems. By organising the available information, it is hoped that this study will stimulate work to fill major knowledge gaps.
- (2)
From the available literature, the number of alien species observed in subterranean habitats is rather small. This is in stark contrast to surface systems, where databases on alien species are available at continental, regional, or national scales resulting from large international collaborations such as the Global Invasive Species Database (http://www.issg.org/database), the Global Register of Introduced and Invasive Alien Species (www.griis.org; Pagad et al., 2018), and alien species inventories for Europe (Roy et al., 2020). Interestingly, none of these databases report specific information on alien species in subterranean ecosystems, with caves and related environments generally not even included as a separate habitat.
- (3)
Although only limited data are available, it appears that only a few alien species represent a threat to subterranean ecosystems and to the species living therein. To colonise subterranean systems, alien species need to overcome the strong ecological filter imposed by the absence of light and the scarcity of food (Culver & Pipan, 2019). Successful invaders must therefore possess traits that enable them to cope with these environmental constraints (Reeves, 1999; Mammola, 2017). This was confirmed by our analysis, which suggested that the main predictor explaining the probability of a species becoming established in subterranean systems is the presence of pre-adaptive traits.
- (4)
Interactions between human activities and climate change might accelerate the spread of alien species into new environments, including subterranean habitats. However, investigations on the links between invasions and environmental changes in subterranean habitats are still rare (but see Mammola & Isaia, 2017). A common framework for the study of the consequences of climate changes and the routes of transport, establishment and impacts of alien species will be necessary to understand long-term consequences for subterranean ecosystems.
- (5)
Researchers in the field of subterranean biology should report the presence of alien species when preparing species inventories in addition to recording the presence of endemicity and rarity. Greater awareness of the presence and distribution of alien species will allow a greater understanding of the potential distribution and spread of alien invertebrate species in subterranean habitats, laying the foundations for future management practices and interventions. It is currently difficult to recommend management practices in the absence of well-documented relationships between native and alien species (Reeves, 1999). Adequate and rapid dissemination of information on alien species will be crucial to prevent and manage their expansion effectively (CBD, 2000), because impacts can occur in different environments through a variety of mechanisms (Ricciardi et al., 2013). We need to work towards the efficient prevention, early detection, rapid response, and management of biological invasions in these fragile habitats.
ACKNOWLEDGEMENTS
This review was prepared within the framework of the PRIN SHOWCAVE ‘A multidisciplinary research project to study, classify and mitigate the environmental impact in tourist caves’ – code 2017HTXT2R (PI: Marco Isaia), funded by the Italian Ministry of Education, University and Research. S. M. acknowledges support from the European Commission via the Marie Sklodowska-Curie Individual Fellowships project number 882221 (‘Testing macroecological theory using simplified systems’) and the Biodiversa+ project DarCo (‘The vertical dimension of conservation: A cost-effective plan to incorporate subterranean ecosystems in post-2020 biodiversity and climate change agendas’). Open Access Funding provided by Universita degli Studi di Torino within the CRUI-CARE Agreement.
AUTHOR CONTRIBUTIONS
G. N. conceived the idea, with suggestions by M. I. and S. M. G. N. collected data. G. N. and S. M. analysed the data and prepared the figs G. N. and S. M. led the writing. M. I. revised the text and provided additions to the final draft. L. V. provided arguments on alien species and estimations of costs.
Open Research
DATA AVAILABILITY STATEMENT
The literature database and R code to reproduce the analyses supporting this study are available in Figshare (https://doi.org/10.6084/m9.figshare.21779045.v2).




