Vertical stratification patterns of tropical forest vertebrates: a meta-analysis
ABSTRACT
Tropical forests harbour the highest levels of terrestrial biodiversity and represent some of the most complex ecosystems on Earth, with a significant portion of this diversity above ground. Although the vertical dimension is a central aspect of the ecology of forest communities, there is little consensus as to prominence, evenness, and consistency of community-level stratification from ground to canopy. Here, we gather the results of 62 studies across the tropics to synthesise and assess broad patterns of vertical stratification of abundance and richness in vertebrates, the best studied taxonomic group for which results have not been collated previously. Our review of the literature yielded sufficient data for bats, small mammals, birds and amphibians. We show that variation in the stratification of abundance and richness exists within and among all taxa considered. Bat richness stratification was variable among studies, although bat abundance was weighted towards the canopy. Both bird richness and abundance stratification were variable, with no overriding pattern. On the contrary, both amphibians and small mammals showed consistent patterns of decline in abundance and richness towards the canopy. We descriptively characterise research trends in drivers of stratification cited or investigated within studies, finding local habitat structure and food distribution/foraging to be the most commonly attributed drivers. Further, we analyse the influence of macroecological variables on stratification patterns, finding latitude and elevation to be key predictors of bird stratification in particular. Prominent differences among taxa are likely due to taxon-specific interactions with local drivers such as vertical habitat structure, food distribution, and vertical climate gradients, which may vary considerably across macroecological gradients such as elevation and biogeographic realm. Our study showcases the complexity with which animal communities organise within tropical forest ecosystems, while demonstrating the canopy as a critical niche space for tropical vertebrates, thereby highlighting the inherent vulnerability of tropical vertebrate communities to forest loss and canopy disturbance. We recognise that analyses were constrained due to variation in study designs and methods which produced a variety of abundance and richness metrics recorded across different arrangements of vertical strata. We therefore suggest the application of best practices for data reporting and highlight the significant effort required to fill research gaps in terms of under-sampled regions, taxa, and environments.
I. INTRODUCTION
The distribution of biodiversity across the Earth's surface is a prominent motivation for the research of evolutionary ecologists and biogeographers alike. Eco-evolutionary mechanisms for the production and coexistence of diversity include speciation, dispersal, and extinction pathways, all of which occur across multiple spatial and temporal scales (Wiens et al., 2006; Harvey et al., 2020). One exciting aspect of species coexistence is the fine-scale vertical structuring of forests, with structural complexity working to expand the potential niche space within which species can partition and/or fill (MacArthur, 1958; MacArthur, Recher & Cody, 1966; Gouveia et al., 2014). Tropical forests in particular demonstrate the greatest diversity and vertical complexity of all terrestrial systems (Terborgh, 1985; Denslow, 1987; Johnson, 1998), and they have formed the basis for much vertical stratification research (Kays & Allison, 2001; Ozanne et al., 2003).
The complex three-dimensional structure of a forest creates vertical gradients in abiotic conditions such as wind, light, humidity, and temperature (Allee et al., 1949; Terborgh, 1985; Campbell & Norman, 2012; Jucker et al., 2020), which have a strong influence on patterns of diversity. For example, in a mesic Panamanian forest, thermal variance over 24 h was on average 5 °C in the canopy, 3 °C in the understorey, and 1 °C in the soil (Basham & Scheffers, 2020). These steep abiotic gradients correlate with unique assemblages of species that are layered from ground to canopy, often referred to as ‘vertical stratification’. Since its inception in the early and mid-20th century with principal articles by Allee (1926), MacArthur (1958), MacArthur et al. (1966), and Pearson (1971), vertical stratification has become a dominant theme in research related to species co-existence and niche theory, with numerous studies conducted at the levels of populations, species, and communities (Orians, 1969; Bernard, 2001; Basham et al., 2019; Basham & Scheffers, 2020; Thiel et al., 2021). For example, birds from New Guinea were seen to have a range of species-specific stratification patterns, but as a community there was consistently higher abundance and richness in the canopy (Bell, 1982b). Conversely, a study of birds from the Ghats in India showcased strong patterns of stratification with community-level abundances and richness greater towards the ground (Jayson & Mathew, 2003). Although a general consensus has formed in ecology of vertical stratification being a widespread phenomenon in the tropics (Parker & Brown, 2000; Nakamura et al., 2017; Oliveira & Scheffers, 2019), the generality of trends across biogeographic scales has yet to be synthesised and evaluated.
Variation in the vertical patterns of diversity are due to differences in morphology, ecology, physiology, and behaviour within and across taxa, all of which dictate how species interact with the local-scale vertical dimension of forest habitat structure, resources (e.g. food, light, water, and roosting sites), and climate (see online supporting information, Table S1; Terborgh, 1985; Cascante-Marín et al., 2006, Roll, Geffen & Yom-Tov, 2015; Acharya & Vijayan, 2017). For example, physical habitat structure changes from ground to canopy, and dense understorey vegetation may prevent the flight of some bat species (Hodgkison et al., 2004) but provide structure for locomotion for small rodents (Abreu & De Oliveira, 2014). Food requirements also vary considerably by species, and the distribution of food resources can differ from ground to canopy within and among forests of different compositions (Rader & Krockenberger, 2006; De Moraes Weber et al., 2011). These drivers lead to considerable variation in the patterns of stratification of species and communities across the tropics.
Furthermore, biotic interactions, both intra- and interspecific, can play a significant role in shaping vertical stratification. Bats often travel closer to the ground as a form of canopy predator avoidance (Zubaid, 1994; Rex et al., 2011), and competition for space and resources is frequently discussed as a driver of vertical stratification across all taxa (Terborgh, 1980; Rader & Krockenberger, 2006; Abreu & De Oliveira, 2014; Chmel et al., 2016). Mutualisms can also shape vertical distributions such as the relationship between plants and their pollinators, which can be highly mutualistic (e.g. fig trees and obligate fig-wasps) (Kato et al., 1995; Nefdt & Compton, 1996). However, stratification drivers may also vary significantly across macro-scale gradients such as elevation or latitude, often caused by shifts in plant composition or climate (Scheffers et al., 2013; Ashton et al., 2016; Acharya & Vijayan, 2017). Thus, a synthesis is required to understand how macro-scale gradients and drivers influence stratification across vertical space.
Here, we provide a comprehensive analysis of global vertical stratification patterns across tropical forest vertebrates. We use the PRISMA Ecology and Evolutionary Biology checklist (O'Dea et al., 2021) as a guide to facilitate best reporting practices where applicable. In our review of empirical studies, we collate abundance and richness data for all vertebrates sampled across the vertical axis of tropical forests – the most vertically complex and diverse terrestrial ecosystems (Allee et al., 1949; Smith, 1973; Terborgh, 1985). With our collated database, we assess general patterns of stratification using standard geographic [latitude, biogeographic realm (hereafter: biorealm), elevation], habitat (forest type, canopy height) and climate (season) variables (Table 1). Second, we review local-scale drivers of stratification such as habitat structure (e.g. foliage density), nesting behaviour (e.g. roosting height), age (e.g. differences in distribution and behaviour between adults and juveniles), foraging/food, species interactions (e.g. competition), and species morphology (e.g. body size) (Table S1). Thus, we attempt to assess the generality of macro-scale patterns of stratification trends within and among vertebrate taxa using an empirical meta-analysis, and place these findings in context to reviewed local-scale drivers.
| Variable | Covariate description | Unit |
|---|---|---|
| Response | ||
| Biodiversity metric | Abundance or richness metric | Continuous – proportion of maximum abundance or richness in each study unit |
| Predictors – random effects | ||
| Strata (slope) | Mean vertical height of sampled strata | Continuous – proportion of maximum vertical height of forest in each study unit |
| Study unit (intercept) | The study from which the data were taken; studies were separated into separate units if they provided data from multiple locations or seasons (see Table S1 for study units) | Categorical – e.g., Pearson (1977a), Pearson (1977b) (2) |
| Predictors – fixed effects | ||
| Strata | Mean vertical height of sampled strata | Continuous – proportion of maximum vertical height of forest in each study unit |
| Taxa | Taxonomic grouping | Categorical – bats, birds, small mammals, amphibians |
| Study scale | An index of study scale derived from rankings of spatial breadth (1–5), temporal resolution (1–5), and temporal breadth (1–5); see Appendix S2 for details | Continuous – numeric index |
| Latitude | Latitude of the study unit | Continuous – decimal degrees |
| Elevation | Elevation (altitude) of the study unit | Continuous – metres above sea level |
| Canopy height | The maximum forest canopy height of the study unit. | Continuous – meters |
| Forest type | IUCN-defined forest type of the study unit | Categorical – lowland moist forest, montane moist forest, dry forest (single records of mangrove forest and swamp forest were assigned to lowland moist forest) |
| Biorealm | A coarse categorisation of the biogeographic realm of the study unit | Categorical – Americas, Africa, Asia and Oceania |
| Sampling season | The sampling season from which data were collected at the study unit | Categorical – dry, wet, sampling combined across the year |
| Model weights | ||
| Interval weights | The weighting of individual model response variables according to the number of intervals that the strata was divided into, as described in the Section II.1 | Continuous – bounded between 0 and 1 |
II. MATERIALS AND METHODS
(1) Literature review/data collection
We conducted a comprehensive, structured literature search through Web of Science. We used key search terms in the following combination: (canopy OR arboreal OR vertical OR stratify OR stratification) AND (amphibian OR reptile OR vertebrate OR mammal OR bird OR bat OR rodent) AND (tropical OR tropics). From our first-stage initial search for published studies and theses on Web of Science (date of search: 22nd February 2020) we obtained 1106 results (see Appendix S1 for PRISMA flow chart). We also searched through specific combinations of search terms on Google Scholar and reviewed the first 100 results for each taxon. Titles and abstracts were searched to remove non-eligible studies (i.e. those that did not relate to the review topic). The remaining list of studies was investigated, along with references within, and this resulted in 350 potential studies/theses at the second stage. At the third stage we reduced this to 62 studies based on five selection criteria: (1) studies were conducted at latitudes between 30° and −30° (we use the subtropical latitudes of 30° which include the Atlantic rainforests of Brazil); (2) studies recorded abundance and/or richness across the vertical gradient (minimum of two separate strata), from the ground or understorey to a stratum defined as subcanopy or canopy; (3) studies were conducted at the community level; excluding single-species studies or those focused on specific groups of species (a sub-set of the community, e.g. hummingbirds); (4) studies recorded sampling effort across vertical strata, to ensure comparability across strata or allow data correction to account for uneven sampling; (5) studies were of primary forest or old-secondary forest, to eliminate studies of heavily degraded or early successional forests which have distinctly different vertical structure and complexity.
We collected data and statistics from tables, main text, and/or figures (extracted using the online ‘Plot Digitizer’ tool; https://plotdigitizer.com), with 100% of studies processed by E.W.B. and 50% of studies checked by a second author (D.H.K.) for accuracy. For every study, we recorded reported abundance or richness values and calculated the mean height of the sampled strata from the minimum and maximum strata height (e.g. a stratum spanning 2–4 m would be assigned a mean height of 3 m). Abundance data were standardised for sampling effort within studies where effort varied across strata by dividing abundance by the sampling effort in that strata (e.g. number of trapping events). It was not possible to standardise richness using this method because sampling effort and richness saturation do not follow a regular pattern; instead, we only include richness data from studies that applied the same effort across strata, reported a richness estimator metric per strata which accounts for varying sampling effort (e.g. the Chao metric), or where strata had been sampled sufficiently to saturate species richness, thereby negating any discrepancy in sampling effort. We delineated studies into unique study units (Fig. 1A, Table S2) if they encompassed multiple spatial or if possible, seasonal units, which constitute potential changes in conditions that influence stratification. For example, Pearson (1977a) sampled stratification in birds in Indonesia, Papua New Guinea, Ecuador, Peru, Bolivia, and Gabon; in our database Pearson (1977a) represents six study units. Basham & Scheffers (2020) recorded amphibian stratification in the dry and wet seasons in Panama, therefore representing two seasonal study units in our database.
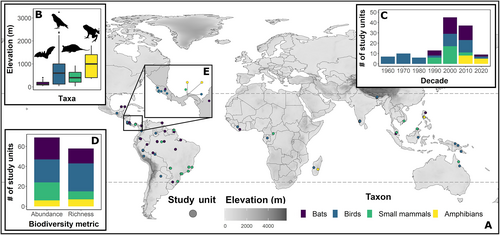
Some studies recorded abundance within strata of varying widths (birds; N = 12 study units; amphibians, N = 1 study unit). For example, in one study unit we may see five birds recorded between 1 and 2 m in height (width = 1 m) and 10 birds recorded between 2 and 4 m in height (width = 2 m). Study units that recorded abundance with varying strata widths were corrected for strata size by dividing abundance into 1 m height intervals. From this example, the 1 m wide stratum would be assigned five birds at 1.5 m (mean height), whereas the 2 m wide stratum with 10 birds would be assigned five birds to a mean height of 2.5 m and another five birds to a mean height of 3.5 m. All observations that were divided into intervals were then assigned a model weight to account for possible bias caused by creating multiple intervals. These model weights were created by dividing the default model weight applied to each row in a data frame (weight = 1) by the number of 1 m intervals produced by that stratum (see Appendix S2 for details). While abundance recorded in strata of varying widths was divided into intervals, this could not be done for richness because a given species can occur across a range of heights. We attempted to correct for any differences in strata width in the construction of our models (see Section II.3). Lastly, 13 studies reported counts, means, and standard deviations of individual species' vertical habitat use, in which case we summed the species (richness) and individuals (abundance) present at 1 m vertical height intervals.
There was considerable variation in the use of abundance (including abundance proxies), and richness metrics, for example, bat studies often report captures per net hour (Bernard, 2001), a small mammal study reported density per hectare (Zubaid & Ariffin, 1997), and some bird studies recorded counts of foraging observations (Frith, 1984). We recognise the use of this broad range of metrics as a limitation to our study, and therefore applied a range of criteria to select justifiably comparable studies of vertical habitat use/stratification and transformed data to account for variation. Chiefly, for each study we converted richness/abundance into a proportion of the maximum richness/abundance value reported within that study unit. We applied the same process to vertical height within each study. Thus, all values of height and richness and abundance were standardised between 0 and 1.
We collected geographical, biological, and methodological metadata from each study and location for incorporation into the analysis, which included mean elevation, latitude, biorealm, canopy height, forest type, and sampling season (see Table 1 for description of variables collected). We used the IUCN habitat classification scheme (https://www.iucnredlist.org/resources/habitat-classification-scheme) to standardise forest type definitions for all studies. Some studies did not report canopy height and/or sampling season data, therefore, for these missing data we used a global canopy database (Simard et al., 2011) for missing canopy heights, and researched regional climate data to estimate seasonal coverage of sampling. Furthermore, we sought to incorporate an index of the temporal and spatial robustness of each study unit (hereafter referred to as ‘study scale index’) to control for high variation in sampling effort and spatial and temporal coverage among studies. For example, one study may have sampled heavily (high temporal resolution) over a period of a month (low temporal breadth) at a single observation tower (small spatial breadth), whereas another may have sampled lightly 1 day per month (low temporal resolution) over 4 years (very high temporal breadth) in three different national parks (high spatial breadth). Due to the variation in sampling methods and reporting, this index must be seen as an estimate of study extent only. Particularly difficult to define is the difference in scale and effort between studies of different taxa due to the orders of magnitude that exist between collecting data for different taxa or in different systems. For example, one study may conduct a 1 h transect survey on birds resulting in 100 bird sightings, whereas another study may set up numerous time-consuming trap installations for small mammals and only record 10 captures. Thus, we attempted to rank studies relative to their taxa, which generally relied upon similar methodologies. We calculated quantitative indices of spatial and temporal breadth, and a qualitative index of temporal resolution (the intensity of sampling across the sampling period), which were ranked from 1 to 5 (see Appendix S3 for details). This index of study scale was incorporated into the analysis as a standard fixed-effect model covariate.
(2) Local vertical stratification drivers
To understand general research themes pertaining to the local drivers of vertical stratification (Table S1), we documented the referenced and investigated factors thought to influence stratification patterns in each study. We considered ‘referenced’ factors as those briefly cited within the text, and ‘investigated’ factors as those that were incorporated into statistical analyses or expanded upon in detail. We exclude macro-scale drivers here because individual studies often did not feature sufficient spatial or temporal coverage to test for macro-scale patterns, but rather considered the specific drivers at that location. Here, drivers were recorded at the study level, not the study unit, because discussions of local stratification drivers in these papers were not separated into study units as defined herein.
(3) Data analysis
To understand pantropical patterns in the vertical stratification of biodiversity, we conducted a two-tiered analysis. In our first-tier analysis, we directly modelled observations of species richness and abundance as a function of vertical height using data sets collected from 62 studies, comprising 86 independent study units (Fig. 1). From this analysis, we obtained estimates of the directionality of vertical biodiversity stratification (i.e. the per study change in species richness and abundance corresponding to a 1 unit increase in vertical stratum). Our second-tier analysis focused on modelling the directionality of vertical stratification, as estimated from the first-tier analysis, as a function of macro-scale variables (e.g. elevation, latitude, and biorealm). This analysis estimates the degree of change in stratification directionality per unit change in macro-scale variables (i.e. the per taxon change in stratification direction per unit increase in elevation and latitude or among biorealms).
In our first-tier analysis, we used data derived from studies that recorded observations of species richness and abundance across vertical gradients throughout the tropics. As mentioned above (see Section II.1), these observations were converted into proportions of maximum abundance or species richness observed within each study. These data are necessarily bounded between 0 and 1 and violate assumptions of standard linear models. To address this, we implemented beta-distributed generalised linear mixed models (GLMMs), with candidate sets based on a priori hypotheses. To construct these candidate sets, we first created a base model (hereafter referred to as a ‘study model’) featuring only terms related to the studies from which the data originated (e.g., study unit and study scale). This study model was fitted both with fixed and random effects, as well as weighted using model weights to account for possible bias caused by creating multiple intervals for abundance data (see Table 1). We used a single fixed effect, ‘study scale’, to account for variation introduced from methodological differences across studies (Table 1). We then used ‘study unit’ as a random effect which allowed the intercept (interpreted as the magnitude of each biodiversity metric at the lowest vertical stratum) and slope (interpreted as the change in biodiversity metrics per unit change in vertical stratum) to vary by study unit (Table 1). We then created sequential sets of candidate models by modifying this study model with additional fixed effects such as ‘taxa’ and ‘strata’ as a first, second, and third-order polynomial term (Table 1). Next, we iteratively added variables as fixed effects for geographic factors (‘latitude’, ‘elevation’, and ‘biorealm’), forest ecosystems (‘forest type’ and ‘canopy height’), and ‘sampling season’ to our models (Table 1). For richness data specifically, we incorporated a variable in our models to account for uneven strata widths within studies. However, the addition of this variable did not affect our model's performance or predictions. Therefore, to reduce model complexity and prevent overfitting, this variable was not included in the final candidate set from which results were drawn. All continuous covariates were scaled to a mean of 0 and unit variance to promote model convergence. Models that did not converge due to over-parameterisation were removed. The resulting candidate model set contained 36 models representing ecological processes underlying our biodiversity metrics.
We evaluated the performance of each of these models using an information theoretic approach, with Akaike's information criterion corrected for small sample size (AICc) as our order-determining criterion (Burnham & Anderson, 1998). The relative importance of each model is determined by comparing each model's AICc values using ΔAICc. These ΔAICc values can be used to calculate model weights (ω), which represent the probability of each model being the best approximating model in the candidate set. Hence, a candidate set's model weights must sum to a cumulative weight of 1; a subset of the models summing to a cumulative weight of 0.95 are considered the confidence set. We considered models in the confidence set, as well as covariates featured therein, to be the best models for explaining the data, given the evidence. However, accepting that no single model, or covariate, contains sole explanatory power of the data, we leverage inference across our entire model set to ensure maximum confidence in explanatory power and to minimise loss of information. We used model averaging to obtain model predictions and parameter estimates weighted by their relative likelihood. We also compared the model-averaged stratification coefficients from study units which recorded both metrics to assess the variation in directionality of abundance and richness stratification responses. Lastly, we conducted a sensitivity analysis by re-running the first-tier analysis with z-transformed response variables. The resulting coefficients of stratification did not alter the conclusions of the analysis and we therefore elected to keep the proportional response variables.
To conduct our second-tier analysis, we obtained model-averaged estimates of random effects (the per study change in biodiversity metrics corresponding to 1 unit increase in vertical stratum) obtained from the first-tier analysis. As previously described, this secondary analysis allowed us to evaluate how the directionality of vertical stratification varied across geographic, temporal, and methodological factors. Unlike the first-tier analysis, our response variables in the second-tier analysis are independent and normally distributed and therefore can be modelled using simple linear fixed effects models. In this analysis, we created taxon-specific candidate model sets to model the directionality of species richness and abundance stratification as a function of six a priori hypothesised landscape/climate variables: elevation, latitude, forest type, canopy height, biorealm, and sampling season (Table 1).
Data management, statistical analyses, and data visualisations were all conducted in the R programming environment (R version 4.0.3; R Core Team, 2022), using the following packages in order of workflow: tidyverse (data curating, manipulating, and visualising data), scales (scaling covariates before fitting models), glmmTMB (fitting beta-distributed GLMMs via a Template Model Builder framework), sjPlot [obtaining model estimates using function ‘get_model_data()’], AICcmodavg [creating AICc tables using function ‘aictab()’ and multimodel inference using function ‘modavgCustom()’], emmeans (obtaining estimated marginal means and post-hoc contrasts), and ggpubr (creating publication-ready figures).
III. RESULTS
(1) Taxonomic patterns
Our analysis showed clear differences in vertical stratification patterns of abundance and species richness among taxa. Study units of bat richness and abundance showed a wide variation in patterns, with no significant overall direction (Fig. 2); however, there was a greater frequency of the most significant study units for abundance that were stratified towards the canopy (Fig. S2B). Bird studies showed strong variation in both abundance and richness stratification, with approximately half of study units demonstrating increasing richness and abundance towards the canopy, and half a decrease towards the canopy (Figs 2 and S2). Unlike the variation and upward stratification trends found among bats or birds, both amphibians and small mammals were unidirectional in their patterns of stratification, with the greatest abundance and richness towards the ground (Figs 2 and S2).
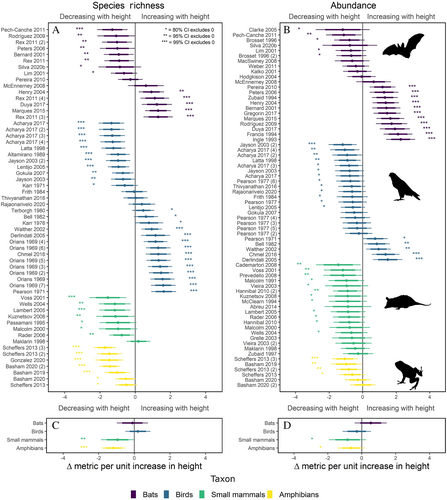
We also observed that abundance and richness stratification patterns from the same study units were strongly correlated (Fig. S3A). Correlations were most closely aligned in birds (Fig. S3B), which had greater data coverage and variation between negative and positive stratification patterns across studies. Of all study units that recorded both abundance and richness (N = 41), only six showed a different stratification direction for abundance and richness (bats N = 4; birds N = 1; small mammals N = 1; Fig. S3B).
(2) Environmental/geographical variables
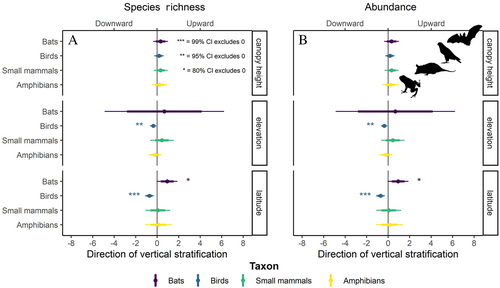
In testing the influence of environmental and geographical variables on patterns of vertical stratification, birds were the only taxon to show any significant effect. Birds showed a significant effect for all variables except canopy height, which was not significant (Fig. 3, Table 2). Elevation was the strongest predictor; bird abundance and richness were weighted towards the canopy in lowland forests, and towards the understorey in montane forests (Fig. 3, Table 2). Latitude showed a similar pattern to elevation, with bird communities weighted towards the understorey in forests further from the equator (Fig. 3). Interestingly, communities sampled in the Americas were more strongly weighted towards the canopy than communities in Asia and Oceania (Table 2). The stratification patterns of bats, small mammals, and amphibians were not significantly influenced by environmental and geographical variables in this analysis (Fig. 3, Table 2).
| Metric | Taxa | Variable | Slope estimate | P value | Significant relationships |
|---|---|---|---|---|---|
| Richness | Small mammals | Biorealm | −0.245 | 0.476 | NA |
| Richness | Small mammals | Season | 0.216 | 0.864 | NA |
| Richness | Small mammals | Forest type | −0.167 | 0.719 | NA |
| Richness | Bats | Biorealm | −1.197 | 0.234 | NA |
| Richness | Bats | Season | −0.760 | 0.375 | NA |
| Richness | Bats | Forest type | 1.246 | 0.116 | NA |
| Richness | Birds | Biorealm | 0.090 | 0.013 | Americas > Asia and Oceania |
| Richness | Birds | Season | −1.268 | <0.001 | Dry season > sampling combined, wet season > sampling combined |
| Richness | Birds | Forest type | 0.595 | 0.007 | Moist lowland > moist montane |
| Richness | Amphibians | Biorealm | 0.030 | 0.771 | NA |
| Richness | Amphibians | Season | −0.045 | 0.738 | NA |
| Richness | Amphibians | Forest type | −0.054 | 0.899 | NA |
| Abundance | Small mammals | Biorealm | −0.245 | 0.476 | NA |
| Abundance | Small mammals | Season | 0.216 | 0.864 | NA |
| Abundance | Small mammals | Forest type | −0.167 | 0.719 | NA |
| Abundance | Bats | Biorealm | −1.197 | 0.234 | NA |
| Abundance | Bats | Season | −0.760 | 0.375 | NA |
| Abundance | Bats | Forest type | 1.246 | 0.116 | NA |
| Abundance | Birds | Biorealm | 0.090 | 0.013 | Americas > Asia and Oceania |
| Abundance | Birds | Season | −1.268 | <0.001 | Dry season > sampling combined, wet season > sampling combined |
| Abundance | Birds | Forest type | 0.595 | 0.007 | Moist lowland > moist montane |
| Abundance | Amphibians | Biorealm | 0.030 | 0.771 | NA |
| Abundance | Amphibians | Season | −0.045 | 0.738 | NA |
| Abundance | Amphibians | Forest type | −0.054 | 0.899 | NA |
(3) Local vertical stratification drivers
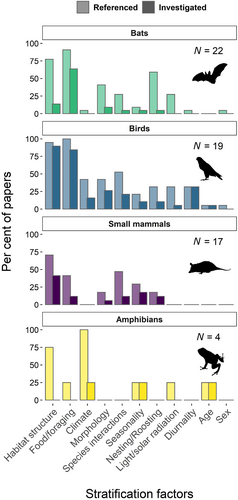
We documented 11 factors hypothesised to drive the vertical stratification of vertebrates (Table S1), of which 10 were investigated directly and one was only discussed (Fig. 4). The stratification drivers referenced most commonly were habitat structure and food/foraging. These were consistently cited and investigated across studies of different taxa, but most frequently for studies of bats and birds (Fig. 4). Climate, species interactions, and morphology were also strongly represented, although not equally across taxa. For example, the small number of amphibian studies (N = 4) frequently cited the importance of vertical climate gradients in influencing stratification patterns, yet this received no attention for bats and less so for small mammals. Equally, roosting and nesting behaviours were noted as important variables for birds, bats, and small mammals, which may utilise different strata for sheltering, but this was not mentioned for amphibians.
IV. DISCUSSION
To our knowledge, our study provides the first pantropical analysis of vertical stratification in vertebrates, and we identified variation in the stratification of abundance and richness within and among birds, bats, small mammals, and amphibians. Stratification of bats was variable but trended towards greater richness and abundance in the canopy. Stratification in bird richness and abundance also was variable but generally exhibited greater richness and abundance towards the canopy (Figs 2 and S2), whereas both amphibians and small mammals were stratified with greatest abundance and richness towards the ground (Figs 2 and S2). As a generalised pattern, we also show that vertebrate abundance is correlated with richness across vertical strata (Fig. S3).
(1) Broad-scale variation
It is a long-held belief that tropical ecosystems are highly stratified as a result of numerous ecological processes (Table S1). Variation in vertical stratification patterns among studies was best explained by taxonomic class, but variation was also partitioned through other biological and geographical factors. Latitude was the most significant macroecological explanatory variable for vertical stratification patterns, of birds in particular (Fig. 3). Across latitude there are gradients in rainfall, solar radiation, and seasonality, among numerous other factors (De Frenne et al., 2013; Oliveira & Scheffers, 2019), which combine to influence the composition and structure of ecosystems. For example, species richness declines from low- to high-latitudes (Rosenzweig, 1995; Hillebrand, 2004). Here we showed that bird abundance and richness were stratified towards the canopy in forests closer to the equator (Fig. 3). This may reflect differences in vertical forest structure across latitude, e.g. greater vertical complexity, plant species richness, tree density, canopy height, and abundance and richness of canopy epiphytes (Gouveia et al., 2014; Ashton et al., 2016; Taylor et al., 2022). Nonetheless, inference on the effect of latitude on vertical stratification may be limited because we did not include temperate regions which vary significantly in vertical structure relative to low-latitude forests (Terborgh, 1985).
Beyond latitude, elevation also plays an important role in shaping vertical stratification of vertebrates through local modification of climate and shifts in forest composition and vertical structure (Asner et al., 2014; Acharya & Vijayan, 2017). Specifically, we showed that bird communities shifted from richness and abundance weighted towards the canopy in lowland forests to the understorey in montane forests (Fig. 3). These shifts match increases in relative vegetation density in the understorey compared to the canopy with increasing elevation (Asner et al., 2014; Acharya & Vijayan, 2017). Variation in stratification patterns were not explained by elevation, or other macroecological variables, for bats, small mammals, or amphibians (Fig. 3). However, bats were sampled across an elevation gradient limited to 50–500 m and small mammals from 50 to 1000 m (Fig. 1B), while amphibians lacked data overall (N study units = 7). We suggest the study of vertical stratification across elevation gradients as an important area for future research, particularly for communities at elevations above 1000 m.
Of all sampled taxa, we found a significant effect of biogeographic realm on vertical stratification only for birds, with more upwardly weighted communities in the Americas compared to communities in Asia and Oceania. However, more data are needed to confirm this result due to high variation in stratification patterns among bird studies. Comparisons to the Afrotropics were limited due to data scarcity. One might expect differences in stratification among biorealms if the unique evolutionary history of resident biota leads to alternate sets of traits and/or ecological strategies. For example, the preferred reproductive habitat of amphibians (i.e. the ratio of terrestrial to aquatic breeders) is not homogeneously distributed across the tropics due to long-term isolation and speciation (Holt et al., 2013; Lion et al., 2019), which could fundamentally alter the vertical stratification of a community. Observing similar patterns of stratification across biogeographic realms with vastly different phylogenetic or evolutionary histories, may be an indication of convergent eco-evolutionary strategies of vertical habitat use.
Temporally, we did not identify a clear effect of sampling season on stratification: studies sampled in either dry or wet seasons showed abundance and richness weighted more towards the canopy than studies which sampled across both seasons (Table 2). This pattern might reflect insufficient data with which to assess a relationship, given there is an extensive literature showing clear shifts in vertical stratification between wet and dry seasons. For example, in our review of the literature, studies in the lowland Amazon rainforest demonstrated that seasonal inundation and flooding play critical roles in vertical resource distribution and physical structure which impacted the stratification of bats (Pereira, Marques & Palmeirim, 2010). Moreover, arboreal amphibians are known to descend to the ground in Panama during dry seasons (Basham & Scheffers, 2020), and birds shift in vertical height in numerous tropical forests across seasons (Bell, 1982a; Frith, 1984). There is ample evidence from the literature that the direction of vertical stratification oscillates across seasonal and daily timescales, with individuals moving across the vertical axis to track shifts in structure, food resources, and microclimate. Of the 62 studies included here, many were sampled only in a single season and inter-seasonal sampling data were absent from most studies which sampled across seasons. Thus, we advocate greater temporal coverage and reporting in future research in order to characterise seasonal changes in stratification accurately.
(2) Local-scale drivers
Broad-scale factors such as elevation, latitude or biorealm could provide valuable explanations of biogeographic patterns of vertical stratification. However, many local-scale mechanisms also are thought to drive vertical stratification. Below, we summarise and discuss the evidence for local drivers of patterns in stratification in vertebrates, primarily those that were investigated or referenced by studies in our database but were too nuanced or site-specific to include in our empirical analysis.
(a) Habitat structure and morphology
Across all vertebrate classes, a majority of studies posited habitat structure as a key factor driving stratification patterns (Fig. 4). Previous studies have found stratification of birds to be positively correlated with foliage density (Orians, 1969; Pearson, 1977a; Acharya & Vijayan, 2017), of bats to be positively influenced by the degree of canopy openness (Bonaccorso, 1976; Hodgkison et al., 2004; Marques, Ramos Pereira & Palmeirim, 2015), and of small mammals to be influenced both by canopy cover and microhabitat distribution (Malcolm & Ray, 2000; Wells et al., 2004; Abreu & De Oliveira, 2014). We identified herein a delineation between volant (birds and bats) and non-volant (small mammals and amphibians) taxa, with significant variation and a general upward stratification trend for volant taxonomic classes contrasting with a unidirectional downward stratification trend for non-volant taxonomic classes (Figs 2 and S2). Thus, it is possible that an interaction exists between locomotive morphology and physical habitat structure in determining vertical stratification patterns across taxa.
(b) Food and foraging
Food and foraging was another commonly proposed driver, especially for bats and birds where over 95% of studies argued that it may be important in determining vertical stratification (Table S1, Fig. 4). The distribution of food resources may not be a mutually exclusive driver as it will be closely tied to structural characteristics such as foliage density (Shanahan & Compton, 2001; Thiel et al., 2021). However, there may be significant variation in food requirements among species within a vertebrate class [e.g., between frugivores and insectivores (Bell, 1982b; Denzinger & Schnitzler, 2013)]. Thus, in addition to the quantity and distribution of food sources, variable guild structure among communities is likely an important consideration in the variation of stratification patterns, as can be seen in birds and bats which have diversified to fill specialised foraging niches (Pearson, 1977a; Bell, 1982b; Bernard, 2001).
In contrast to bats and birds, most small mammals are foraging generalists, feeding on arthropods, nuts, flowers, seeds, and fruit, which can accumulate and be easily accessed on the ground (August, 1983; Wells et al., 2004). Considering the ground-skewed stratification patterns we found for small mammals, we suggest that generalist ground-foraging behaviour is likely a key driver of their stratification patterns. Amphibian richness and abundance was also heavily ground-skewed, yet, amphibians mostly consume arthropods, which are generally abundant across vertical strata (Dial et al., 2006; Ashton et al., 2016). We thus surmise that foraging may not be a key determinant of vertical stratification in amphibians, but this awaits more comprehensive examination.
(c) Climate and environmental gradients
Of the vertebrate taxa investigated herein, amphibians are ectothermic, and are thus more reliant on environmental moisture and heat. Desiccation tolerance of amphibians has been shown to vary between ground- and canopy-dwelling species (Tracy, Christian & Tracy, 2010). Therefore, the steep vertical climate gradients present in forests, which are often closely associated with habitat complexity, may be key drivers of vertical stratification in amphibians (Scheffers et al., 2013; Oliveira & Scheffers, 2019; Basham & Scheffers, 2020) (Fig. 4). However, harsh climates can be mitigated through avoidance behaviours or access to climatically buffered microhabitats such as within epiphytes (González Del Pliego et al., 2016; Seidl et al., 2019).
While small mammals, bats, and birds are endotherms, and thus may be less affected by climate than amphibians, vertical climate gradients may still play a key role in shaping their vertical stratification. Daily vertical movements of birds coincide with changes in both light and heat, with birds descending to the ground during the hottest periods of the day (Bell, 1982b; Frith, 1984; Rajaonarivelo et al., 2020). Although small mammal studies did not suggest that vertical climate gradients were a potential explanatory variable (Fig. 4), research has shown that thermoregulatory processes (which are related to body size) strongly influence their period of activity (i.e., diurnal versus nocturnal) (Bonebrake, Rezende & Bozinovic, 2020). Further research likely will uncover previously undocumented interactions between vertical stratification, vertical climate gradients, species morphology, and species activity period.
(d) Species interactions
Intra- and interspecific interactions influence species distributions in many ways. Reduced competition is often cited as a force driving species to exploit novel vertical niches (Terborgh, 1980; Rader & Krockenberger, 2006; Abreu & De Oliveira, 2014; Chmel et al., 2016). Specifically, this hypothesis suggests that the stratification of habitats and resources allows for stable coexistence of multiple species through reduced competition (Koen, 1988; Chmel et al., 2016). Predator–prey relationships may also influence vertical stratification, with numerous bird and bat studies suggesting that the canopy may be avoided during movement due to the presence of canopy predators such as raptors and owls (Zubaid, 1994; Rex et al., 2011; Acharya & Vijayan, 2017). While predator avoidance may cause downward shifts in bats and birds, there is evidence that a number of amphibian lineages have evolved to utilise arboreal phytotelmata (plant-held water bodies) in order to avoid terrestrial predators (Bickford, 2004; McKeon & Summers, 2013). These observations highlight the variable, multidirectional responses of different taxa to a common driving force. Furthermore, interactive reproductive behaviours involving advertisement and territoriality may impact stratification. For example, birds were seen to access higher strata when singing in New Guinea (Bell, 1982b) and Madagascar (Rajaonarivelo et al., 2020). It is clear that the complexity and specificity of species interactions may drive complex patterns of vertical stratification, but research on interactions across vertical height has only just begun to consider the potential depth of this field.
V. FUTURE DIRECTIONS
A complicating factor in understanding vertical stratification patterns occurring across a wide variety of scales, habitats, and climates is the interactions that may occur between these factors. Some of the first explorations of vertical stratification by MacArthur (1958), MacArthur et al. (1966), and Pearson (1971), among others, highlighted the importance of vertical habitat structure, vertical and seasonal climate, and species interactions, and outlined the expected variation in stratification patterns that could occur within and among communities and taxa. In this analysis, we necessarily applied numerous corrections and standardisation to data from a wide range of study designs, biodiversity metrics, and levels of data reporting, which naturally limited the statistical strength of our results. Furthermore, a number of studies were excluded due to poor reporting of sampling effort or the absence of necessary metadata. We advocate detailed reporting of metadata and the placing of data in the public domain by future authors, both for repeatability and reproducibility (Cassey & Blackburn, 2006), and for use in syntheses (Feng et al., 2019).
In our study, we did not wish to confound the analysis of basic biogeographic patterns by incorporating sites that were degraded or otherwise non-primary forest. It is clear from the data presented herein that many tropical vertebrate communities, particularly bats and birds, reside above ground in habitats provided by forest vegetation. Tropical forests are globally threatened by human activity and there is strong evidence of disruption of community stratification caused by logging and habitat degradation (Malcolm & Ray, 2000; Dinanti, Winarni & Supriatna, 2018), in addition to forest clearing. Furthermore, climate change is likely to impact vertical structure and forest composition across the tropics (Nakamura et al., 2017). Stronger seasonal changes in temperature, precipitation, and humidity may increase extreme conditions across the vertical gradient, but particularly in forest canopies, which are more exposed than lower strata to thermal and hydric changes (Chen et al., 1999; De Frenne et al., 2021). Climate change may thus expose canopy flora and fauna to increasingly inhospitable conditions, causing extirpations that could reduce or collapse vertical complexity and niche space (Oliveira & Scheffers, 2019; Basham & Scheffers, 2020). Further work specifically examining the threats of global change to biodiversity across vertical strata could reveal the extent to which conservation initiatives targeting canopy systems are needed (e.g. the restoration of canopy environments within secondary forests via the transplantation of epiphytes).
We also recognise the incredible diversity and importance of other tropical forest organisms such as plants, invertebrates and microbiota, which are likewise stratified in vertical space. Studies have shown strong but varied patterns of vertical stratification across many invertebrate groups such as ants (Bruhl, Gunik & Linsenmair, 1998; Hashimoto et al., 2006; Basset et al., 2015), butterflies (DeVries, Murray & Lande, 1997; Nice et al., 2019; Mena et al., 2020), beetles (Charles & Bassett, 2005; Grimbacher & Stork, 2007), and mites (Karasawa & Hijii, 2008; Beaulieu et al., 2010), among others. Plant stratification underlies the biotic and abiotic stratification seen in forests (Smith, 1973; Parker, Lowman & Nadkarni, 1995). For example, tropical forest epiphytes such as orchids and bromeliads are abundant throughout the canopy and provide habitat for unique and diverse assemblages of invertebrates above ground (Gonçalves-Souza, Brescovit & Romero, 2010; Rogy, Hammill & Srivastava, 2019; Phillips et al., 2020), indeed many amphibian species are reliant on phytotelmata and epiphytes (Seidl et al., 2019; Basham et al., 2022). Lastly, microbiota such as nematodes, bacteria, and fungi are extremely diverse in forests and are strongly stratified in vertical space (Lodge & Cantrell, 1995; Powers et al., 2009; Beaulieu et al., 2010; López-Mondéjar et al., 2015; Zotz & Traunspurger, 2016), and there appears to be general similarity in drivers of stratification between invertebrates and vertebrates. As for vertebrates, variance in vertical stratification patterns within invertebrate taxa has been attributed to food resources (e.g., dung versus nectar; Grimbacher & Stork 2007), life-cycle processes (e.g., larval host-plant dependency; Nice et al., 2019), morphology (e.g., wing shape or flight performance; Schulze, Linsenmair & Fiedler, 2001) and climate (e.g., thermal tolerance; Leahy et al., 2022), all of which vary within and across taxa. The growing wealth of data that now exist on stratification in non-vertebrate forest biodiversity lies ready for similar but uniquely tailored analyses such as that presented here.
In Table 3 we provide a list of specific recommendations to advance our understanding of vertical stratification in forest communities.
| Topic | Recommendation |
|---|---|
| Where | Africa – All taxa were poorly represented from the Afrotropics, despite its importance as a tropical forest biodiversity hotspot. |
| High elevation sites (especially for bats) – Understanding how communities stratify across elevational gradients could improve our understanding of how assemblages are shaped, as well as aiding the prediction of localised responses to climate change. | |
| How | Sampling effort – In analyses, authors should account for as well as report sampling effort in different strata, in particular for species richness. Reporting observed richness is not sufficient when sampling effort differs between strata. At the most basic level, species rarefaction analysis should be performed in conjunction with species richness estimation, to allow for comparisons of richness levels while controlling for different sample sizes (Colwell et al., 2004). |
| Upper canopy access – Many studies only sample into the midstorey of forests (often due to logistical difficulties), precluding the collection of data from the upper canopy which may differ significantly in structure, resources, and species composition. | |
| Community-based data – Although single-species studies are vitally important for understanding their natural history and ecology, community-level studies are needed to gather sufficient data and track patterns across larger scales. | |
| Multiple sampling methods – Multiple methods should be used if possible to increase accuracy and reduce methodological bias, e.g., acoustic sampling could be combined with mist netting to sample the full community of bats. | |
| What | Reptiles – Globally, we did not find any studies that fully sampled a reptile community across the vertical gradient. |
| Amphibians – There were few amphibian studies across the vertical gradient, and none in the lowland tropical forests of the Amazon, Congo Basin, or Papua New Guinea, the largest remaining intact rainforests. | |
| Primates – Although there is a sizeable literature on arboreal primates (Kays & Allison, 2001), nearly all were studies of specific species rather than full communities. |
VI. CONCLUSIONS
(1) From this synthesis of empirical vertical stratification data on tropical vertebrates, we compiled a set of factors that may drive vertical structuring of communities, as well as recommendations for future research.
(2) We found large variation in the patterns of vertical stratification of abundance and richness within and among vertebrate taxa. Stratification in bat richness and abundance was variable, but abundance trended towards the canopy. Bird richness and abundance stratification was also variable, with no overriding pattern in stratification direction. On the contrary, both amphibians and small mammals showed consistently higher abundance and richness towards the ground and understorey.
(3) Significant efforts are still required to fill research gaps in terms of adequate sampling across spatial and temporal scales, and for under-sampled regions, taxa, and environments. There remains huge potential for vertical stratification research, both in tropical forests and beyond.
ACKNOWLEDGEMENTS
E.W.B. was supported through the University of Florida's (UF) School of Natural Resources and Environment (SNRE) Graduate School Fellowship Award. D.H.K. acknowledges support from the National Science Foundation Graduate Research Fellowship Program (DGE-1842473). J.A.B. was supported through a Graduate Preeminence Doctoral Fellowship from the UF, awarded through the SNRE, as well as additional financial support provided by the UF SNRE Robin Nadeau Ecology Graduate Research Award. B.R.S. was supported by an Alfred P. Sloan Fellowship.
AUTHOR CONTRIBUTIONS
E.W.B. and B.R.S. conceptualised the study and wrote the manuscript. E.W.B. and D.H.K. conducted the literature search and extracted data. J.A.B. and E.W.B. conducted data analyses. All authors assisted in writing and approved the final manuscript.
Open Research
DATA AVAILABILITY STATEMENT
Data and code are provided on Github at: https://github.com/schefferslab/Vert-Strata-Meta.




