Broad-scale climate variation drives the dynamics of animal populations: a global multi-taxa analysis
ABSTRACT
Climate is a major extrinsic factor affecting the population dynamics of many organisms. The Broad-Scale Climate Hypothesis (BSCH) was proposed by Elton to explain the large-scale synchronous population cycles of animals, but the extent of support and whether it differs among taxa and geographical regions is unclear. We reviewed publications examining the relationship between the population dynamics of multiple taxa worldwide and the two most commonly used broad-scale climate indices, El Niño-Southern Oscillation (ENSO) and North Atlantic Oscillation (NAO). Our review and synthesis (based on 561 species from 221 papers) reveals that population changes of mammals, birds and insects are strongly affected by major oceanic shifts or irregular oceanic changes, particularly in ENSO- and NAO-influenced regions (Pacific and Atlantic, respectively), providing clear evidence supporting Elton's BSCH. Mammal and insect populations tended to increase during positive ENSO phases. Bird populations tended to increase in positive NAO phases. Some species showed dual associations with both positive and negative phases of the same climate index (ENSO or NAO). These findings indicate that some taxa or regions are more or less vulnerable to climate fluctuations and that some geographical areas show multiple weather effects related to ENSO or NAO phases. Beyond confirming that animal populations are influenced by broad-scale climate variation, we document extensive patterns of variation among taxa and observe that the direct biotic and abiotic mechanisms for these broad-scale climate factors affecting animal populations are very poorly understood. A practical implication of our research is that changes in ENSO or NAO can be used as early signals for pest management and wildlife conservation. We advocate integrative studies at both broad and local scales to unravel the omnipresent effects of climate on animal populations to help address the challenge of conserving biodiversity in this era of accelerated climate change.
I. INTRODUCTION
Population fluctuations of animals are driven by changes in extrinsic and intrinsic factors (Barraquand et al., 2017; Wan et al., 2019). The legendary ecologist Charles Elton (1924) suggested that climate is a major extrinsic factor determining large-scale geographic distributions and a driver of local animal population dynamics. He famously argued that broad-scale (geographic range) climate variation played a key role in explaining large-scale synchrony in the multiannual periodic fluctuations of Norwegian lemmings (Lemmus lemmus) and Canadian snowshoe hares (Lepus americanus) (reviewed by Lindstrom et al., 2001). Testing this Broad-Scale Climate Hypothesis (BSCH) has stimulated the development of local-scale climate and population dynamics hypotheses in ecology. Many local climate factors (snow, rainfall, temperature) have indeed been shown to be closely associated with fluctuations of animal populations (e.g. Lima, Keymer & Jaksic, 1999; Pech et al., 1999; Brown & Ernest, 2002). For example, recent research shows that high local precipitation increases primary production, which increases rodent abundance (Krebs et al., 2019), in turn leading to the prevalence of plague (Parmenter et al., 1999). Similarly, local temperature has been identified as a key factor triggering population outbreaks of several rodents (Stenseth et al., 2006; Kelly et al., 2013; Holland et al., 2015). However, general support for Elton's claim of a BSCH has been lacking until now.
During the past two decades, researchers have realized that global climate changes, and climate fluctuations, indexed for example by the El Niño-Southern Oscillation (ENSO) and the North Atlantic Oscillation (NAO), cause climatic anomalies around the world, and trigger extreme weather events that then become major natural disasters (Zhang & Wang, 1998; Post & Stenseth, 1999; Zhang, 2001; Stenseth et al., 2002a; Forcada et al., 2006; Stige et al., 2007; Tian et al., 2011; Heffernan et al., 2014; Butler, Metzger & Harris, 2017). Studies addressing these relationships were rare before the 1990s (but see Mysak, 1986; Zabel & Taggart, 1989). Indeed, only 3.2% (160 out of 4956) of all ENSO- or NAO-related papers located in an ISI Web of Science search were published before 1990 (see online Supporting Information, Fig. S1A), and the earliest papers with long-term data and quantitative analysis were published in 1992 (Mickelson, Dann & Cullen, 1992; Morishita, 1992).
From the 1990s onwards, the increasing availability of precise measurements of changes in global climate has stimulated relevant research on broad-scale climatic impacts on animal population dynamics. For example, there are at least two review papers on the association of the dynamics of animal populations with ENSO and NAO (Stenseth et al., 2002a; Forchhammer & Post, 2004), or climate dipoles (Zuckerberg et al., 2020). The integration of broad-scale (i.e. regional or continental) patterns and local-scale mechanisms has been attempted to provide a better understanding of the effects of climate change on broad-scale ecological patterns and processes (Heffernan et al., 2014; Scheffers et al., 2016). However, a comprehensive assessment of the taxa- or region-specific responses of animals to broad-scale climate remains a major knowledge gap, and this stimulated the present review.
In this study, we analysed recent advances in understanding the effects of broad-scale climate indices on the population dynamics of animals from multiple taxa. Our aims are: (i) to test the generality of Elton's (1924) BSCH – that climate explains large-scale synchrony of population fluctuations; (ii) to examine the extent to which the effects of broad-scale climate drivers are taxon or region specific; (iii) given that broad-scale climate is expected to influence local populations through local climate, to review conceptually the kinds of mechanisms that could modify the effect of local climate on populations; (iv) to explore the potential to use broad-scale climate drivers to provide early-warning signals for pest outbreaks and population crashes that might be of conservation concern.
Although there are many broad-scale climate indices (Stenseth et al., 2003), we focus on two prevalent ones, ENSO and NAO, and their effects on animal population dynamics as reflected by changes in population abundance, growth rate, reproduction rate, or survival rate.
II. METHODS
We used the ISI Web of Science to search for relevant publications. ISI covers more than 34,600 journals and allows easy location, saving, sorting and exporting the results of literature searches. We systematically searched the literature published during 1900–2021 with a combination of the following key words and topics, ‘El Niño-Southern Oscillation, or ENSO, or Northern Atlantic Oscillation, or NAO, or El Niño, or La Niña, or Southern Oscillation’ and ‘population dynamics, or population abundance, or animals, or birds, or mammals, or insects’. A total of 4956 published papers were found, of which 2717 were potentially relevant to our topic. We determined suitability for inclusion in our study by using the following screening criteria: a study must contain ≥10 years of data for population abundance and growth, survival or reproduction rates, and must provide the results of a quantitative statistical analysis. After screening abstracts, we found 933 papers that potentially contained ≥10 years of data and these were included for full-text screening. During full-text screening, we excluded 336 papers that did not report at least 10 years of data, 154 papers that lacked evidence of the effects of ENSO or NAO on animal population abundance, 105 papers that contained only phenological shifts (not as a proxy of population abundance), 37 papers that addressed marine species, 20 epidemic studies without population abundance data for vector organisms, 19 papers for which we could not access their full text, 15 papers reporting qualitative studies, 13 papers reporting community studies, 11 papers without clear positive, negative, or non-significant associations, and two papers containing only seasonality changes (Fig. S1B). Finally, 221 papers (131 for ENSO, 103 for NAO, 13 for both) covering 561 species were used in this study (Table S1). The timescale we concentrated on was dictated by the period of the climate indices we used. The typical period of ENSO and NAO is 2–7 years (Climate Prediction Center, 2005; Seip, Gron & Wang, 2019), thus a series of ≥10 years would typically cover about two ENSO or NAO cycles. The studies we located covered a period of 370 years (mostly during the past three decades) and encompassed all continents of the world (Fig. 1; Fig. S2, Table S1). To ensure reproducible screening, systematic reviews were formatted to be compatible with the metagear package of R (Lajeunesse, 2016), which is a comprehensive research synthesis tool for systematic reviews and meta-analysis.
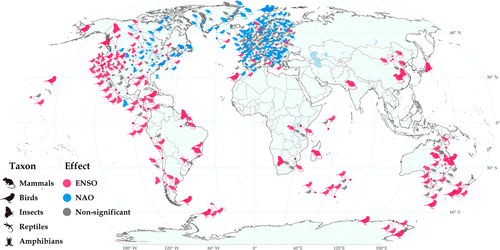
There were considerable differences in the variables used in the analysed studies. Eighty-seven of 221 studies used population abundance; 137 out of 221 studies used population growth rate (N = 31), reproduction (N = 64) or survival rate (N = 50). Note that because some studies used multiple kinds of variables, the total number of studies summed across different variables can differ from the total number of studies. We did not include studies on associations between broad climate and other proxies of population dynamics such as phenology, body growth, and range shifts. Some studies included the effects of density dependence (79 out of 221), autocorrelation (18 for spatial, 118 for temporal) or detrending (46). Nine kinds of ENSO indices were used for 131 studies: Southern Oscillation Index (SOI) (N = 65), sea surface temperature (SST) (N = 32), multivariate ENSO index (MEI) (N = 11), ENSO phases (N = 9), ENSO index (N = 7), Oceanic Niño Index (ONI) (N = 5), ENSO precipitation index (ESPI) (N = 2), annual precipitation (N = 2), and wet season precipitation (N = 1). Three studies included two kinds of ENSO index. Four kinds of NAO indices were used in 103 studies: North Atlantic Oscillation index (NAOI; N = 44), winter NAOI (N = 58), NAO phases (N = 1), and Atlantic SST (N = 1). One study included two kinds of NAO index, and 13 studies reported the effects of both ENSO and NAO (Table S2). The heterogeneity of these data should be taken into account when conducting meta-analysis, a consideration that has been ignored in previous analyses (although see Spake et al., 2021; Zhang et al., 2021).
Both vote-counting and meta-analysis were used to assess taxon-specific responses of animals to broad-scale climate change based on the results of the literature search. We scored each population in the literature survey as one of four categories of relationship with ENSO or NAO phases: a significant association with positive, negative or dual (i.e. both positive and negative) ENSO/NAO phases, and non-significant associations with ENSO or NAO phases. A significant association with the positive ENSO phase (ENSO+) was defined as a significant and positive association of animal populations with SST or El Niño events, or significant negative association with SOI. A significant association with the negative ENSO phase (ENSO−) was defined as significant positive association of animal populations with SOI or La Niña events, or significant negative association with SST. A significant association with both ENSO phases (ENSO+/−) was defined as significant associations with both ENSO+ and ENSO− for populations of the same species, due to the opposing effects of ENSO in different regions or with different time lags, or in different time periods. Similarly, significant associations with positive (NAO+), negative (NAO−) or both (NAO+/−) NAO phases were defined as significant positive, negative or dual association of animal populations with the NAO index.
We tested the null hypotheses that the probability of association of animal populations with either the positive ENSO/NAO phase (p) or negative ENSO/NAO phase (q) was 0.5 by using chi-squared contingency tests (Likelihood Ratio Test, or G test) (Sokal & Rohlf, 2012). The expected probability of dual association () of animal populations with both positive and negative ENSO (ENSO+/−) or NAO (NAO+/−) should be: . For ENSO and NAO, we tested the null hypotheses that the expected and observed frequency of dual association should be equal by using chi-squared contingency tests. An overall significant association with ENSO or NAO phases was assessed using the subtotal of associations with ENSO+, ENSO− and ENSO+/−, or with NAO+, NAO− and NAO+/−. We summarize the frequency (number) in each category for different taxa in Tables 1 and 2. Although studies with non-significant associations might be reported less frequently in the literature, we found 78 out of 221 studies (35.3%) reporting non-significant associations. We tested for a significant difference between significant and non-significant associations of animal populations with ENSO/NAO phases. A significant difference from the null hypothesis indicates that more or less species than the random expectation in a taxon responded to ENSO or NAO positive or negative phases or showed a dual response to both phases. G tests and chi-squared tests were carried out using the DescTools (v. 0.99.44) libraries in R (v.4.1.3) (Signorell et al., 2021; R Core Team, 2022).
| Taxon | ENSO associations | |||||
|---|---|---|---|---|---|---|
| Significant association with | Non-significant association | Total† | ||||
| ENSO+ | ENSO− | ENSO+/− | Subtotal† | |||
| Mammals | 58 (10) * | 34 (16) * | 9 (5) | 97 (27) *** | 31 (13) | 121 (34) |
| Birds | 28 (23) | 38 (36) | 25 (15) * | 81 (64) *** | 24 (24) | 100 (81) |
| Insects | 92 (8) *** | 8 (5) *** | 3 (1) | 103 (12) *** | 41 (4) | 131 (13) |
| Reptiles | 1 (1) | 2 (2) | 0 (0) | 3 (3) * | 0 (0) | 3 (3) |
| Total | 179 (42) | 82 (59) | 37 (21) | 284 (106) | 96 (41) | 355 (131) |
- Results of chi-squared contingency tests: *p < 0.05, **p < 0.01, ***p < 0.001.
- † Some species or studies had multiple kinds of associations; Subtotal ≤ sum of the number of ENSO+, ENSO− and ENSO+/− associations, and Total ≤ sum of the number of significant (subtotal) and non-significant associations.
| Taxon | NAO associations | |||||
|---|---|---|---|---|---|---|
| Significant association with | Non-significant | Total† | ||||
| NAO+ | NAO− | NAO+/− | Subtotal† | |||
| Mammals | 8 (7) | 8 (7) | 7 (3) | 15 (15) | 8 (7) | 18 (20) |
| Birds | 52 (24) *** | 23 (21) *** | 50 (9) *** | 118 (49) *** | 34 (30) | 139 (68) |
| Insects | 13 (6) | 24 (5) | 0 (0) * | 36 (10) | 37 (4) | 73 (13) |
| Amphibians | 4 (1) | 1 (1) | 0 (0) | 5 (2) ** | 0 (0) | 5 (2) |
| Total | 77 (38) | 56 (34) | 57 (12) | 174 (76) | 79 (41) | 235 (103) |
- Results of chi-squared contingency tests: *p < 0.05, **p < 0.01, ***p < 0.001.
- † Some species or studies had multiple kinds of associations; Subtotal ≤ sum of the number of NAO+, NAO− and NAO+/− associations, and Total ≤ sum of the number of significant (subtotal) and non-significant associations.
Because vote-counting provides only limited information for detecting effects (Bushman & Wang, 2009; Gurevitch et al., 2018), we also calculated effect sizes based on model coefficients of 381 observations for 318 species taken from tables or plots digitized by WebPlotDigitizer (https://apps.automeris.io/wpd/) from 128 studies (Table S1); effect sizes were not available for the remaining 93 studies. We used these effect sizes in a meta-analysis, following Stewart (2010) and Gurevitch et al. (2018). Effect size was measured using the magnitude of climate effects on population dynamics of animals from the model coefficients in the original studies, including correlation, regression, generalized additive models (GAM), generalized linear models (GLM), and other kinds of quantitative analysis. Due to data heterogeneity for response variables of both species and ENSO or NAO indices (see above), we corrected the model coefficients when calculating effect size [both dependent variables (y) and independent variables (x) of the models often varied among different studies]. As is typical for experimental studies with treatment and control groups, we corrected the effect size (c) using Hedges' g or the log-transformed response ratio: or in which and are the response of the treatment and control groups, and is the pooled standard deviation of the treatment and control groups (e.g. Davidson et al., 2018; Zhou et al., 2019; Chen et al., 2020; Spake et al., 2021; Zhang et al., 2021). In modelling studies, regression coefficients of response variables (y) (e.g. abundance, trait) against independent variables (x) (e.g. climate) were used to estimate the effect size of x on y (Radchuk et al., 2019; van Klink et al., 2020). In theory, effect size could be corrected using , but this requires extraction of the x and y values from published papers, which is often impossible, therefore, we calculated the effect size based on model coefficients and corrected by taking data heterogeneity into account.
We used linear mixed-effects models to test the difference of effect size of positive ENSO/NAO phase, negative ENSO/NAO phase, dual ENSO/NAO phase, and non-significant association on animal populations of different taxa by taking climate indicators and population variables as random factors. This helped to remove any bias due to effects of data heterogeneity. Unlike vote-counting methods, the effect size of a non-significant association is not expected to be affected by biased reporting in the literature. Therefore, the magnitude of effect sizes () of the same climate and population variable for each taxon were normalized by the minimum value () and range () of all values: to remove data heterogeneity among and between different climate or population variables for different taxa. Studies were excluded if the sample size of the climate or population variables of the same group and same taxon was <3. We also excluded studies if the sample size of positive, negative, dual and non-significant associations was <3. Based on these two rules, we excluded analysis of dual associations for both ENSO and NAO, effect of ENSO on reptiles, and effect of NAO on amphibians. In our final data set, there were 349 model coefficients for mammals (N = 69), birds (N = 153), and insects (N = 127) which were used for testing the difference in effect size of ENSO and NAO on animal populations. These model coefficients included two population variable groups: abundance (N = 50 for mammals; N = 59 for birds; N = 124 for insects) and rates (i.e. population growth rate, reproduction rate, survival rate) (N = 19 for mammals; N = 94 for birds; N = 3 for insects), and seven climate variable groups: SOI (N = 13 for mammals; N = 39 for birds; N = 3 for insects); SST (N = 17 for birds; N = 35 for insects); MEI (N = 14 for mammals; N = 8 for birds; N = 47 for insects); wet season precipitation (N = 11 for mammals); annual precipitation (N = 3 for mammals); NAOI (N = 21 for mammals; N = 35 for birds; N = 4 for insects); and winter NAO index (N = 7 for mammals; N = 54 for birds; N = 38 for insects).
We tested for significant differences in magnitude of effect size (measured by the absolute value of model coefficients) of positive, negative, and non-significant ENSO/NAO associations on mammals, birds, and insects using linear mixed-effects models. Linear mixed-effects models and least-square means for multiple comparisons were carried out using lme4 (v.1.1–29) and lsmeans (v.2.3.0) libraries in R (Bates et al., 2015; Lenth, 2016; R Core Team, 2022).
III. IMPACT OF ENSO
ENSO, the irregular change of SST over the Niño 3.4 region around the equatorial Pacific, has been recognized as an important factor for global climate anomalies such as floods and droughts (Bjerknes, 1969; Loon & Rogers, 1981; NOAA, 1994; Diaz, Hoerling & Eischeid, 2001). Similarly, El Niño and La Niña events could cause a short-term increase and decrease, respectively, of global average surface temperature (Brown, Li & Xie, 2015), while climate warming has increased the amplitude of SOI fluctuations during the last 50 years (Zhang, Guan & Yang, 2008). ENSO could affect the population dynamics of organisms via its influence on local weather (Zhang, 2001; Zhang & Wang, 1998).
Out of 131 papers we reviewed, 106 studies reported significant associations of animals with ENSO events (Fig. 1, Table 1, Table S1), encompassing 284 (out of 355) species. These included 97 mammal species from 27 studies, 81 birds from 64 studies, 103 insects from 12 studies, and three reptiles from three studies (Table 1). Of the 284 species, 179 species showed significant associations with ENSO+, 82 species showed significant associations with ENSO−, 37 species showed significant associations with ENSO+/−, and 96 species showed no significant associations with ENSO phases (Table 1). Note that some species had multiple kinds of associations, and thus, the totals in Table 1 may differ from the sum of the number of all associations.
(1) Mammal responses
Ninety-seven of 121 mammal species showed a significant association with ENSO phases. More mammal species than expected by chance (ENSO+ versus ENSO− = 58 versus 34, G = 6.33, df = 1, p = 0.01) showed a significant association with ENSO+ (Table 1). These included mammal species in Canada, the USA, Chile, Tanzania, India, and China (Table S1; Lima et al., 1999; Lima, Stenseth & Jaksic, 2002b; Murua, Gonzalez & Lima, 2003; Waite et al., 2007; Zhang, Tao & Li, 2007; Jiang et al., 2011; Sinclair et al., 2013; Byrom et al., 2014; Srivathsa et al., 2019; Chaudhary et al., 2021). Commonly represented taxa included rodents, wild boars, and carnivores (Table S1). Thirty-four mammal species showed significant associations with ENSO− (Table 1). These studies were conducted in Canada, Costa Rica, Chile, Brazil, Norway, Finland, France, Poland, Tanzania, South Africa, Madagascar, India, China, and Australia (Lima et al., 2001a; Zhang, 2001; Ogutu & Owen-Smith, 2003; Zhang et al., 2003; Letnic & Dickman, 2006; Waite et al., 2007; Wiederholt & Post, 2010; Dunham, Erhart & Wright, 2011; Jiang et al., 2011; Sinclair et al., 2013; Yan et al., 2013; Boyle & Hone, 2014; Campos, Jack & Fedigan, 2015; Shuai et al., 2020; Magnusson et al., 2021; McLeod et al., 2021). Studies reporting associations with ENSO− included numerous rodent species, and small numbers of kangaroos, ungulates, primates, and carnivores.
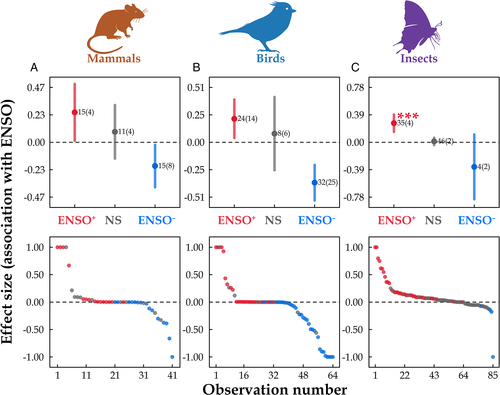
Nine species showed significant ENSO+/− associations. These included foxes, rodents, and deer in the USA, Brazil, and China (Bakker et al., 2009; Magnusson, Layme & Lima, 2010; Zhang et al., 2010; Jiang et al., 2011; Marshal & Bleich 2011; Table S1). This frequency was not significantly different from that expected by chance, indicating that dual associations appeared to occur randomly across populations, taxa and geographic regions (G = 2.14, df = 1, p = 0.14). The mechanisms of these dual associations (ENSO+/−) were complex, including different lags in time-delayed effects, geographic differences, or seasonal differences. The contrasting effects of ENSO (SST)-driven precipitation for Yangtze vole (Microtus fortis) in China exemplifies this situation: ENSO (SST)-driven precipitation had positive effects on vole abundance in the current year but negative effects for the previous year. Thirty-one mammal species including rodents, primates, and ungulates showed non-significant associations with ENSO in Canada, the USA, Chile, Brazil, Kenya, Tanzania, Mozambique, Zimbabwe, South Africa, China, and Australia (Lima et al., 2001b; Ogutu & Owen-Smith 2003; Krebs et al., 2004; Zhang et al., 2007; Ogutu et al., 2008; Jiang et al., 2011; Marshal et al., 2011; Sinclair et al., 2013; Boyle & Hone 2014; Ferreira et al., 2016; Campos et al., 2017; McLeod et al., 2021; Polyakov et al., 2021; Table 1). Of 121 mammal species, 105 showed a <1 year time-lag in response to ENSO events, while 18 species had a time-lag ≥1 year (Table S3). There was no difference in magnitude of effect size for ENSO+, ENSO−, and non-significant association with ENSO on mammal populations (F = 1.06 df = 2, p = 0.36, Fig. 2A).
The most frequently studied mammal species were rodents, ungulates, primates, and carnivores (felids), from studies carried out in America, Europe, Africa, East Asia, and Australia.
(2) Bird responses
Eighty-one of 100 bird species showed a significant association with ENSO phases; there was no significant difference in the frequency of association between positive and negative ENSO phases (G = 1.52, df = 1, p = 0.22) (Table 1). Out of 81 bird species, 28 had a significant association with ENSO+ (Table 1), including studies in Canada, the USA, Mexico, Ecuador, Brazil, South Georgia, Denmark, Spain, Italy, French Sub-Antarctic Islands, Australia, and Antarctica (Kingsford et al., 1999; Brichetti, Foschi & Boano, 2000; Grant et al., 2000; Almaraz & Amat, 2004; Anders & Post, 2006; Chambers & Loyn, 2006; Forcada et al., 2006; Jenouvrier, Barbraud & Weimerskirch, 2006; Kim, Slack & Chavez-Ramirez, 2008; Balbontin et al., 2009; Norman & Chambers, 2010; Rolland, Weimerskirch & Barbraud, 2010; Cubaynes et al., 2011; Lamanna et al., 2012; Garcia-Perez et al., 2014; Dugger et al., 2016; McArthur et al., 2017; Gianuca et al., 2019; Hill et al., 2019; Wren, Shaffer & Polovina, 2019; Boano et al., 2020; Tavares et al., 2020; Tompkins & Anderson, 2021; Table S1). A wide diversity of bird taxa was represented including land birds (e.g. swallows, swans), raptors, and numerous seabirds (e.g. penguins, albatrosses, boobies; Table S1).
Thirty-eight bird species showed a significant association with ENSO− (Table 1), including studies from Canada, the USA, Mexico, Jamaica, Costa Rica, Panama, Ecuador, Peru, Brazil, South Georgia, the UK, Spain, Seychelles, French Sub-Antarctic Islands, Australia, New Zealand, and Antarctica (Chastel, Weimerskirch & Jouventin, 1993; Lyver, Moller & Thompson, 1999; Sillett, Holmes & Sherry, 2000; Gaston & Smith, 2001; Ramos et al., 2002; Barbraud & Weimerskirch, 2003; Jenouvrier et al., 2005; Mazerolle et al., 2005; Chambers & Loyn, 2006; Forcada et al., 2006; Sedinger et al., 2006; Vargas et al., 2006, 2007; Lee, Nur & Sydeman, 2007; Balbontin et al., 2009; Devney, Short & Congdon, 2009; Norman & Chambers, 2010; Rolland et al., 2010; Wolf et al., 2010; Ancona et al., 2011; Baylis et al., 2012; Schmidt et al., 2014; Woehler et al., 2014; Wolfe, Ralph & Elizondo, 2015; Horswill et al., 2016; Townsend et al., 2016; Anderson et al., 2017; Sandvig et al., 2017; Barbraud et al., 2018; Velarde & Ezcurra, 2018; Woodworth et al., 2018; Jones & DuVal, 2019; McKechnie et al., 2020; Tavares et al., 2020; Cleeland et al., 2021; Smart, Smith & Riehl, 2021). These 38 bird species spanned a broad range of taxa, with seabirds (penguins, petrels, albatrosses, auks, gulls) and New World warblers well represented.
Twenty-five bird species including seabirds (e.g. albatross, penguins, shearwaters), New World warblers, a raptor and an owl showed associations with ENSO+/− in Canada, the USA, Mexico, North Atlantic, Selvagem Grande Island, French Sub-Antarctic Islands, Australia, New Zealand, and Antarctica (Peacock, Paulin & Darby, 2000; Wilson et al., 2001; Jenouvrier et al., 2005; Olivier et al., 2005; Seamans & Gutierrez, 2007; Kim et al., 2008; Sandvik, Coulson & Saether, 2008; Rolland et al., 2010; Boyle & Hone, 2012; Ramos et al., 2012; Pardo et al., 2013; McKellar et al., 2015; Humphries & Moller, 2017; Ancona et al., 2018; Cleeland et al., 2021). The frequency of dual associations was significantly different from that expected by chance alone, indicating that dual associations appeared to occur non-randomly across species, taxonomic groups and geographical areas (G = 5.95, df = 1, p = 0.01).
Twenty-four bird species showed non-significant associations with ENSO (Table 1). These studies were conducted in Canada, the USA, Ecuador, Peru, the UK, France, Hungary, Spain, Seychelles, French Sub-Antarctic Islands, Australia, and Antarctica (Mickelson et al., 1992; Kingsford et al., 1999; Beadell et al., 2003; Wilson & Arcese 2003; Chase, Nur & Geupel, 2005; Grosbois et al., 2006; Kim et al., 2008; Vargas et al., 2008; Ballerini et al., 2009; Catry et al., 2009; Devney et al., 2009; Pearce-Higgins et al., 2009; Rolland, Barbraud & Weimerskirch, 2009; Stutchbury et al., 2009; Rolland et al., 2010; Glenn et al., 2011; McClure et al., 2012; Garcia-Perez et al., 2014; Woehler et al., 2014; Ross et al., 2016; Hernandez, Oro & Sanz-Aguilar, 2017; Huang et al., 2017; Barbraud et al., 2018; Laczi et al., 2019) and included seabirds (penguins, albatrosses), sparrows, swallows, owls, kestrels, and flamingos.
Eighty-seven of 100 bird species had a <1 year time-lag in response to ENSO events (Table S3). The difference in magnitude of effect sizes for ENSO+, ENSO−, and non-significant associations was not significant (F = 0.56, df = 2, p = 0.58, Fig. 2B). For ENSO–population associations, the most frequently studied bird species were seabirds such as albatrosses, penguins, and shearwaters, and studies were from the Americas, Europe, sub-Antarctic islands, Australia, and Antarctica.
(3) Insect responses
Significant associations with ENSO were found for 103 of 131 insect species. More insect species than expected by chance alone (ENSO+ versus ENSO− = 92 versus 8, G = 82.88, df = 1, p < 0.001) showed a significant association with ENSO+ (Table 1). These included 19 butterfly species in Canada and the USA (Vandenbosch, 2003; Harrison et al., 2015; Pardikes et al., 2015), two fruit fly species in Mexico (Aluja et al., 2012), 67 social wasp species in French Guiana (Dejean et al., 2011), one moth species in Argentina (Paritsis & Veblen, 2011), locusts in China (Zhang & Li, 1999), and two planthoppers in Japan (Morishita, 1992) (Table S1). Only eight species showed a significant association with ENSO− (Table 1): hessian fly and three butterfly species in the USA (Woli et al., 2014; Harrison et al., 2015), social wasps in Chile (Estay & Lima, 2010), locusts in South Africa, Botswana and Namibia (Todd et al., 2002), and two moth species in Australia (Maelzer & Zalucki, 2000) (Table S1). Dual associations with ENSO+/− were found only for three butterfly species in the USA (Harrison et al., 2015) (Table 1, Table S1), which was not significantly different from that expected by chance (G = 0.81, df = 1, p = 0.37). Forty-one insect species including butterflies and spruce beetles showed no significant associations with ENSO in the USA (Vandenbosch, 2003; Hart et al., 2014; Harrison et al., 2015; Pardikes et al., 2015) (Table 1).
For insects, 97.7% of species (128/131) showed a <1 year time-lag in their response to ENSO events (Table S3). The difference in magnitude of effect size for ENSO+, ENSO−, and non-significant association was significant (F = 12.76, df = 2, p < 0.001, Fig. 2C). The magnitude of ENSO effects on insect populations was significantly or marginally higher than that of the non-significant associations (F = 0.21, p < 0.001 for ENSO+; F = 0.29, p = 0.06 for ENSO−). The most frequently studied insect species were butterflies, wasps, locusts, and flies and studies were from the Americas, Africa, and East Asia.
(4) Reptile responses
One reptile species (green turtle Chelonia mydas in Australia) showed a significant association with ENSO+ (Limpus & Nicholls, 1997) (Table 1), and two reptile species [a lizard (Anolis apletophallus) in Panama, broad-snouted caiman (Caiman latirostris) in Argentina] had significant associations with ENSO− (Herrera, Solari & Lucifora, 2015; Stapley, Garcia & Andrews, 2015) (Table 1).
(5) Summary
In summary, a majority of studies of mammal, bird, and insect populations reported significant associations with ENSO; mammal and insect populations tended to increase during ENSO+ (Table 1); the magnitude of the effect of either ENSO+ or ENSO− on insect populations was higher than reported effect sizes for non-significant associations (Fig. 2C). The probability that mammal and insect populations were associated with both ENSO phases was not significantly different from that expected based on random occurrence across species, taxonomic groups and geographic areas, but was significantly different for birds. The proportion of species showing a time-lag >1 year relative to ENSO was only 2.3% (3/131) for insect species, but 14.9% (18/121) for mammal species and 25.0% (25/100) for bird species (Table S3), suggesting that ENSO may exert longer indirect effects (e.g. through food, habitat or predators) on mammal and bird species than on insects. ENSO was associated with the population dynamics of mammals, birds and insects in most parts of the world (Fig. 1), indicating a global influence on animals. The geographical range over which animals are influenced by ENSO is generally consistent with ENSO's core geographical range of influence (i.e. Pacific region and the tropics) as reported by climate scientists (Bjerknes, 1969; Loon & Rogers, 1981; NOAA, 1994; Diaz et al., 2001). Recent studies indicate that ENSO can also affect the climate of the North Atlantic–European area (e.g. Brönnimann et al., 2007; Ding et al., 2017) which is consistent with our compilation of ENSO's impact on animals in this region (Fig. 1), for example, in reports on lemmings and voles in Europe (Zhang, 2001), and lynx (Lynx canadensis) in Canada (Zhang et al., 2007, Yan et al., 2013). These studies provide support for Elton's hypothesis that broad-scale climate can affect the geographic synchrony of population cycles of animals. Evidence of such associations remains rare in North Africa, Central-Western Asia, and Southeast Asia, and future work should focus more on these regions to widen our understanding of this phenomenon.
IV. IMPACT OF NAO
The NAO is defined as a difference in atmospheric pressure at sea level between the low-pressure centre of Iceland and the high-pressure centre of the Azores, which controls the direction and strength of westerly winds across the North Atlantic (Hurrell, 1995; Hurrell et al., 2003). The NAO can significantly affect the climate in central and northern Europe, leading to more storms around the Mediterranean Sea and eastern North America, and potentially affecting animals throughout Europe and North America (Post & Stenseth, 1999).
Of 103 papers that we reviewed, 76 studies reported significant associations of animals with NAO events (Fig. 1, Table 2, Table S1) encompassing 174 (out of 235) species, including 15 mammals (15 studies), 118 birds (49 studies), 36 insects (10 studies), and 5 amphibians (2 studies) (Table 2, Table S1). Of these 174 species, 77 (38 studies) showed significant associations with NAO+, 56 (34 studies) with NAO−, 57 (12 studies) with NAO+/−, and 79 (41 studies) showed non-significant associations with NAO phases (Table 2).
(1) Mammal responses
Fifteen of 18 mammal species showed a significant association with NAO phases (Table 2). Of these 15 species, eight had a significant association with NAO+ (Table 2), spanning Canada, the USA, Greenland, Ireland, Norway, Sweden, Germany, Estonia, Latvia, Lithuania, and the Czech Republic, and including voles, hares, deer and moose (Alces alces) (Post & Stenseth, 1998; Forchhammer et al., 2002; Tkadlec et al., 2006; Bastille-Rousseau et al., 2013; Hagen et al., 2017; Cepelka et al., 2020; Reid et al., 2021; Table S1). Eight species had a significant association with NAO− (Table 2), including rodents, carnivores, and ungulates [deer, musk ox (Ovibos moschatus), sheep] that were studied in Canada, Greenland, the UK, Sweden, Finland, and the Czech Republic (Forchhammer et al., 2002; Hone & Clutton-Brock, 2007; Helle & Kojola 2008; Palo, 2009; Simard et al., 2010; Yan et al., 2013; Cepelka et al., 2020; Table S1). There was no significant difference in the frequency of associations between positive and negative NAO phases (G = 0, df = 1, p = 1) (Table 2).
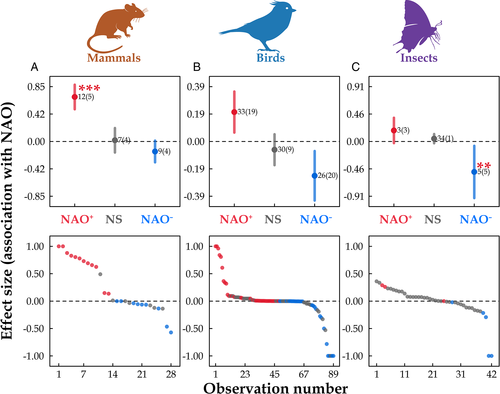
Seven mammal species showed significant associations with NAO+/−, including six ungulates and a carnivore that were studied in Canada, the UK, and Norway (Post & Stenseth, 1999; Stenseth et al., 1999; Forchhammer et al., 2001; Table S1). The frequency of associations with NAO+/− was not significantly different from random (G = 1.15, df = 1, p = 0.28). Eight mammal species including rodents, ungulates (deer, musk ox, porcupine), and a fox showed non-significant associations with NAO in Canada, the USA, Greenland, Iceland, Norway, Poland, Belarus, and the Czech Republic (Forchhammer et al., 2002; Lima, Merritt & Bozinovic, 2002a; Stenseth et al., 2002b; Klvana, Berteaux & Cazelles, 2004; Mysterud & Ostbye 2006; Palsson et al., 2016; Cepelka et al., 2020; Table 2). Of 18 mammal species, 17 showed a <1 year time-lag in response to NAO events, while 10 species had a time-lag ≥1 year (Table S3). The difference in magnitude of effect size for NAO+, NAO−, and non-significant associations on mammal populations was significant (F = 18.31 df = 2, p < 0.001, Fig. 3A). The effect size magnitude was significantly higher for NAO+ than that of non-significant associations (F = 0.56, p < 0.001), but the difference was non-significant for NAO− (F = 0.03, p = 0.97, Fig. 3A). The most frequently studied mammal species were rodents and ungulates in North America and Europe.
(2) Bird responses
Significant associations with NAO phase were found for 118 of 139 bird species. More bird species than expected (NAO+ versus NAO− = 52 versus 23, G = 11.51, df = 1, p < 0.001) showed a significant association with NAO+ (Table 2), in studies from the USA, Greenland, Iceland, the UK, Norway, Sweden, Finland, Denmark, Germany, Poland, the Czech Republic, France, Switzerland, and Spain (Sætre, Post & Král, 1999; Jonzen et al., 2002; Moller, 2002; Jones, Doran & Holmes, 2003; Olsen & Schmidt, 2004; Engen et al., 2005; Hemery et al., 2008; Nevoux, Barbraud & Barbraud, 2008; Solonen & Ursin, 2008; Balbontin et al., 2009; Bustnes, Anker-Nilssen & Lorentsen, 2010; White et al., 2011; Boyle & Hone, 2012; Naef-Daenzer et al., 2012; Jonsson et al., 2013; Garcia-Perez et al., 2014; Kvasnes et al., 2014; Grimm et al., 2015; Veit & Manne, 2015; Fox et al., 2016; Gladalski et al., 2016; Guery et al., 2017; Pavon-Jordan, Santangeli & Lehikoinen, 2017; Tjornlov et al., 2020; Table S1). Diverse bird taxa were represented, including auks, eiders, cormorants, gulls, swallows, tits, raptors and Old World flycatchers.
Twenty-three bird species showed a significant association with NAO− (Table 2), in studies from Canada, the USA, the UK, Norway, Sweden, Denmark, Romania, and Spain (Jonzen et al., 2002; Almaraz & Amat, 2004; Olsen & Schmidt 2004; Grosbois & Thompson, 2005; Votier et al., 2005; Weatherhead, 2005; Anders & Post, 2006; Zydelis et al., 2006; Figuerola, 2007; Balbontin et al., 2009; Goodenough, Elliot & Hart, 2009; Lewis et al., 2009; Pearce-Higgins et al., 2009; Gaston & Robertson, 2010; Bustnes et al., 2013; Jensen et al., 2014; Veit & Manne, 2015; Guery et al., 2017; Schimpf et al., 2020; Baltag, Kovacs & Sfica, 2021; Saunders et al., 2021; Table S1). They spanned diverse taxa from loons, cormorants, and eiders to New World blackbirds and Old World finches. Seabirds were relatively poorly represented.
There were 50 bird species that showed significant dual associations with NAO+/−, which was significantly different from a random occurrence (G = 32.35, df = 1, p < 0.001). These studies were from Canada, North Atlantic, Ireland, Germany, France, Italy, and Spain (Saether et al., 2003; Sandvik et al., 2008; Costantini, Carello & Dell'Omo, 2010; Barnagaud et al., 2011; Franke et al., 2011; Sandvik, Erikstad & Saether, 2012; Jonker, Chakarov & Kruger, 2014; Zuberogoitia et al., 2016; Cleasby et al., 2017). Taxonomic coverage was similar to that for other NAO studies cited above, except that there were more seabird species.
Thirty-four bird species including diverse taxa from seabirds (cormorants, shearwaters, eiders) to tits, geese, owls, falcons, blackbirds, and flycatchers showed non-significant associations from studies in Canada, the USA, Mexico, Iceland, the UK, the Norwegian Sea, Norway, Sweden, the Baltic Sea, Finland, Denmark, the Netherlands, Germany, Poland, the Czech Republic, France, Switzerland, Spain, and Italy (Sætre et al., 1999; Smedshaug, 2001; Blomqvist et al., 2002; Jonzen et al., 2002; Sanz, 2002; Wang et al., 2002; Olsen & Schmidt, 2004; Engen et al., 2005; Grosbois et al., 2006; Zydelis et al., 2006; Rivalan et al., 2007; Hemery et al., 2008; Ambrosini et al., 2011; Boyle & Hone, 2012; Ekroos et al., 2012; Lamanna et al., 2012; McClure et al., 2012; Bustnes et al., 2013; Jonsson et al., 2013; Garcia-Perez et al., 2014; Poysa & Vaananen, 2014; Lorentsen et al., 2015; Mesquita et al., 2015; Veit & Manne, 2015; Masoero et al., 2016; Golawski et al., 2017; Gonzalez-Braojos, Sanz & Moreno, 2017; Hernandez et al., 2017; Martin, Onrubia & Ferrer, 2019; Morganti, Ambrosini & Sara, 2019; Table 2). Of 139 bird species, 121 had <1 year time-lag in their responses to NAO events (Table S3). The difference in effect size magnitude for NAO+, NAO−, and non-significant associations was not significant (F = 1.69 df = 2, p = 0.19, Fig. 3B). The most frequently studied avian species were sea birds (cormorants and ducks), swallows, and tits, in studies from North America and Europe.
(3) Insect responses
Thirty-six out of 73 insect species showed significant associations with NAO phases (Table 2); there was no significant difference in the frequency of association of insect populations with positive or negative NAO phases (G = 3.32, df = 1, p = 0.07). Thirteen of these insect species showed significant associations with NAO+ (Table 2), in studies from the USA, Mexico, and the UK (Jonas & Joern, 2007; Saldaña, Lima & Estay, 2007; Westgarth-Smith et al., 2007; Lima et al., 2008; Aluja et al., 2012; Westgarth-Smith et al., 2012; Table S1). The taxa studied included butterflies, aphids, grasshoppers, and fruit flies. Associations with NAO− were found for 21 grasshopper species in the USA (Jonas & Joern, 2007; Jonas, Wolesensky & Joern, 2015), and true bugs, moths, and Lepidoptera in the UK, Finland, and Serbia (Halkka et al., 2006; Dennis & Sparks 2007; Ducic et al., 2012; Table 2, Table S1). No insect species showed a dual association with NAO+/−, which was significantly lower than the random expectation (G = 5.93, df = 1, p = 0.01, Table 2). Thirty-seven insect species including 34 butterfly species and 3 moth species showed non-significant associations with NAO in the UK, Sweden, and Finland (Conrad, Woiwod & Perry, 2003; Klemola et al., 2003; Westgarth-Smith et al., 2012; Young et al., 2014; Table 2). All 73 insect species had a <1 year time-lag in response to NAO events (Table S3). Effect size magnitude of NAO− on insect populations was significantly higher than that of non-significant associations (F = 0.51, p < 0.01, Fig. 3C), but the difference was non-significant for NAO+ (F = 0.13, p = 0.48, Fig. 3C). The most frequently studied insect species were butterflies, flies, grasshoppers, and moths in North America and Europe.
(4) Amphibian responses
Four salamander species in the USA (Warren & Bradford 2010) showed a significant association with NAO+ and one plethodontid salamander in Italy (Salvidio et al., 2016) had significant association with NAO− (Table 2).
(5) Summary
In summary, a majority of bird and amphibian populations were reported to have significant associations with NAO phase. Bird populations tended to increase in NAO+ and more birds tended to have dual associations with NAO+/−, whereas no insects were reported to have dual associations. Associations between NAO and population dynamics of animals were reported only from North America and Europe west of Romania and Lithuania, suggesting that the geographical area of influence of NAO on animals is smaller than that of ENSO. This is consistent with the range of NAO effects on weather in Europe and North American reported by climatologists (Hurrell, 1995; Hurrell et al., 2003). The proportion of species showing a time-lag >1 year relative to NAO was 0% for insects, 55.6% (10/18) for mammals and 18.0% (25/139) for birds (Table S3), suggesting that NAO may exert longer indirect effects (e.g. through food, habitat or predators) on mammal and bird species than on insects.
V. PERSPECTIVES
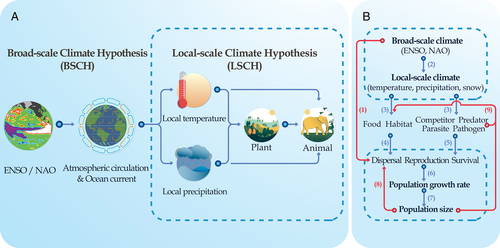
Our review provides support for the BSCH from a wide range of studies during the past two decades. We have documented extensive and far-reaching evidence of effects of ENSO and NAO on animal populations. After Elton (1924) formulated the BSCH, he speculated that sunspots or volcanic eruptions could be the drivers of large-scale climate effects on populations (Elton, 1942; see Lindstrom et al., 2001). In contrast to the Local-Scale Climate Hypothesis (LSCH), which focuses on the effects of local climate, the BSCH emphasizes the effects of large-scale phenomena such as ENSO and NAO on population dynamics. In Fig. 4A, we show both the differences between the LSCH and BSCH and their close links. ENSO and NAO alter atmospheric circulation and ocean currents, thereby affecting local climate and hence local populations of animals. Broad-scale climate can thus impact local climate through altering atmospheric circulation, monsoon strength, precipitation and temperature patterns (Wu et al., 2009), which can affect animal populations either directly or indirectly through effects on food resources, habitat, or predation (Tian et al., 2011, 2017; Trenberth, 2011; Heffernan et al., 2014). Advances in the study of associations between population dynamics and ENSO and/or NAO have expanded our understanding of the effects of climate from local-scale to broad-scale regimes.
A variety of studies have shown that broad-scale climate factors can affect animals via changes to phenology (Both et al., 2006; Saino et al., 2011; Saracco et al., 2019), habitat (Forcada et al., 2006; Stige et al., 2006; Kausrud et al., 2008; Butler et al., 2017; Flesch, Rosen & Holm, 2017; Lister & Garcia, 2018), or vegetation (Zhang et al., 2003; Jiang et al., 2011). However, for most of the studies reviewed herein, exactly how broad-scale climate factors affect animal population dynamics via local climate remains unclear. There is an urgent need to integrate the two scales of study to unravel the effects and mechanisms of climate change on the population dynamics of animals (Heffernan et al., 2014).
If broad-scale climate affects population dynamics via local-scale climate, what is the contribution of broad-scale climate to population ecology? In Fig. 4B we illustrate known relationships between broad-scale and local-scale climate and their potential impacts on animal population dynamics. Effects of broad-scale climate act through changes to local climate to affect animals, potentially via a bottom-up chain effect. Broad-scale effects of climate could explain the broad-scale geographic synchrony of population variations or cycles (i.e. act as a synchronizer of cycles), while intra- and inter-specific interactions could dampen the influence of broad-scale climate on population variation (i.e. act as an ecological buffer). Broad-scale climate could affect animal populations via multiple pathways, which may have non-monotonic effects (see Section V.4). We also discuss whether broad-scale climate could serve as an early-warning signal for important population changes such as pest outbreaks or population crashes.
(1) Bottom-up chain effects
A biotic interaction chain occurs when one species indirectly affects another via intermediary species (Wootton, 1994). Here, we define the bottom-up chain effect as the indirect effects of broad-scale climate on population abundance via local climate through the biotic interaction chain (Fig. 4B). Broad-scale climate is likely to influence animals indirectly by affecting the local climate, and consequently food resources, habitat, species interactions, and population dynamics (Fig. 4B). Movements of organisms such as seasonal bird migrations create teleconnection (strong links) among distant regions, and these processes at the broad scale can interact with processes at local scales, resulting in non-linear dynamics (Heffernan et al., 2014). Migratory birds are highly susceptible to shifts of broad-scale climate; there is evidence for changes to breeding [fecundity, clutch size, breeding probability, provision of food for offspring, fledging success, and nesting success (Ancona et al., 2011; Tompkins & Anderson 2021)], food sources or prey abundance (Pardo et al., 2013; Saunders et al., 2021; Tompkins & Anderson 2021), survival [differential survival according to age, changes in migratory tactics and foraging costs (Dugger et al., 2016; Guery et al., 2017; Tjornlov et al., 2020)], and phenology [hatching date and laying dates of birds, or plant and insect phenology (Lorentsen et al., 2015; Gonzalez-Braojos et al., 2017; Morganti et al., 2019)]. Thus, it is important to investigate how bottom-up chain effects link broad-scale and local-scale climate effects, and to disentangle the independent effects of broad- and local-scale climate. Only a few studies have addressed this issue to date (Castilla & Camus, 1992; Jaksic, 2001; Ottersen et al., 2001; Forchhammer & Post, 2004; Jiang et al., 2011; Sinclair et al., 2013; Yan et al., 2013; Tian et al., 2017). Hierarchical climate analyses, by integrating broad-scale and local-scale data, perform better than non-hierarchical analyses in determining how broad-scale climate causes population changes through local abiotic and biotic factors. They can also include within- and across-generation responses that encompass the ecological repercussions of climatic change, thereby helping to disentangle various ecological responses. Additionally, such studies can help to identify key pathways of the effects of climate on the population dynamics of animals.
(2) Ecological buffers
We define an ecological buffer as a factor weakening the synchrony of population variation or cycles across a large geographic range (Fig. 4B). Biological buffers at the level of individuals, such as genetic, physiological, behavioural, or morphological plasticity, are important in adapting to climate change (Hoffmann & Sgro, 2018; Huey, Buckley & Du, 2018; Llewelyn et al., 2018; Meester, Stoks & Brans, 2018; Sinervo et al., 2018). Similarly, ecological buffers may play a significant role in determining the impacts of ongoing climate changes at the population and community levels.
Previous studies indicate that the population-dynamic effects of climate variation could be facilitated by biotic interactions (Post, 2013). Intra- or inter-specific interactions may weaken (buffer) the effects of global climatic changes and contribute to spatial or temporal dynamics in population responses (Wang et al., 2006; Sandvik et al., 2008). The population-dynamic effects of climate variation could be weakened by predation (Forchhammer & Asferg, 2000; Schmitz et al., 2003; Wilmers et al., 2006; Wilmers, Post & Hastings, 2007; Post et al., 2009), intra-specific interactions (Gamelon et al., 2017), inter-specific interactions or, more generally, ecosystem functioning (Young et al., 2016). Biodiversity loss therefore is likely to reduce the capacity of ecosystems to resist accelerated climate change, but further investigations are needed.
(3) Synchronizers of cycles
The drivers of the population cycles of animals (e.g. voles and lemmings, Canadian snowshoe hare and lynx) have long puzzled ecologists. Predator–prey interactions have been proposed as the main driving force behind the periodic population oscillations of hare and lynx (Krebs, Boonstra & Boutin, 2018), but empirical modelling failed to support this (Zhang et al., 2007; Yan et al., 2013). Elton (1924) believed that broad-scale climate factors must be the driving force behind the geographic synchrony of population cycles, but the underlying mechanisms were unclear. Moran (1953) suggested that stochastic density-independent but correlated processes could synchronize two (or more) independent populations across large geographic ranges (defined as the Moran effect) (Lindstrom et al., 2001). Yan et al. (2013) showed that broad-scale climate drivers (i.e. ENSO, NAO) were essential external factors in timing and producing such cycles, together with delayed density dependence and predation, and this was supported by recent studies in North America (Pokallus & Pauli, 2015; Pomara & Zuckerberg, 2017). It thus appears that predation and density dependence alone are not able to produce sustained predator–prey population cycles, and that inclusion of broad-scale climate in the models is essential in producing, timing, and maintaining the cycles in this predator–prey system.
(4) Non-monotonic effects
Recently, non-monotonic effects of climate on population dynamics at different spatial or temporal scales have been recognized (Zhang et al., 2015). Non-monotonic effects of climate mean that signs of the association of climate with population variation may switch between positive and negative when examined at different spatial scales, temporal scales, or along environmental gradients.
At the physiological level, the fitness of an individual is likely to be highest at intermediate (optimal) values of an environmental gradient (e.g. temperature, humidity), while more extreme environments are more harmful, producing a dome-shaped non-monotonic response. For example, a warming climate has depressed the population cycling of many insect pests by altering their thermal optima (Johnson et al., 2010; Lehmann et al., 2020). At the ecological level, environmental variables (particularly broad-scale climate) can act via multiple pathways to affect population dynamics, directly or indirectly. Temperature or precipitation may affect the development, reproduction, or survival of animals directly at the physiological level, but also indirectly by altering the productivity of plant food resources or habitats at the ecological level. For example, as compared to the positive effects of increased temperature over short periods, long-term climate warming decreased the frequency of locust outbreaks (Tian et al., 2011) and the prevalence of human disease (Tian et al., 2017) in ancient China. Precipitation showed opposite associations with human plague occurrence in north China with a dry climate, and in south China with a wet climate (Xu et al., 2011). Such studies suggest that climate could have non-monotonic effects on animal populations.
Out of the 561 species we reviewed herein, the population dynamics of 81 species showed a dual association (a non-monotonic association) with broad-scale climate factors (37 for ENSO, 57 for NAO, and 13 for both; Tables 1 and 2). This non-monotonicity was caused by opposite responses of the same species to ENSO or NAO in different regions (N = 48), different temporal periods or with different frequencies (N = 42), via different pathways (N = 16), via a dome-shaped or a quadratic response (N = 4), or by age-dependent responses (N = 2) (non-monotonicity likely due to more than one cause for 30 species, Table S1). Overall, the observed dual associations of animals with ENSO were not significantly different from random. However, the probability of dual association of birds was higher than expected based on random occurrence across populations, and that of insects was lower (Tables 1 and 2). There are various possible explanations: broad-scale climate may have complicated effects on (or relationships with) local climate (Hurrell, 1995; Hurrell et al., 2003), and could have opposing effects on populations of animals in different seasons or with different time lags or in different areas (Table S1).
Our analysis thus suggests that non-monotonic effects of climate may be common, but it remains necessary to break down these data into different scales and conduct separate analyses to identify the mechanisms by which climate affects population variation.
(5) Early warning signals and wildlife management
Predictions of population cycles can be useful for pest management, biodiversity conservation, and resource management. A large climatic signal often appears much earlier than subsequent animal population changes. For example, ENSO can be detected several months before local climates are altered (NOAA, 1994; Diaz et al., 2001).
Our analysis suggests that ENSO or NAO can have a time-lag (mostly less than 1 or 2 years) for mammals and birds but not for insects (Table S3). Thus, it may be possible to enable long-term forecasting of rodent outbreaks, production of animals for human use, or climatic threats to endangered species. The use of ENSO signals has been suggested for early pest management in China (Zhang & Wang, 1998; Jiang et al., 2011) and Australia (Letnic & Dickman, 2006), for fisheries and aquaculture management in the Pacific (Bertrand et al., 2020; Yu et al., 2021), and for early warnings for conservation of primates in Brazil (Wiederholt & Post, 2010). Our ability to use ENSO or NAO cycles to predict animal population trajectories strongly depends on understanding the relationship of these broad-scale climate factors to animal populations via their effects on local-scale climate factors, and the links between these remain worthy of further investigation.
VI. CONCLUSIONS
(1) During the past two decades, it has become clear that global climate changes, and climate fluctuations, indexed for example by the El Niño-Southern Oscillation (ENSO) and the North Atlantic Oscillation (NAO), cause climatic anomalies around the world, and trigger extreme weather events that increase the frequency of natural disasters. Increasingly precise measurements of changes in global climate in the 1990s stimulated many studies of broad-scale climatic impacts on animal population dynamics, expanding the examination of climate-related population dynamics from the local to the global scale.
(2) Syntheses of these findings will be useful for long-term forecasting of population changes and may find applications in pest control, resource management, and biodiversity conservation. Until now, the taxa- and region-specific responses of animals to broad-scale climate changes have not been reviewed, and the underlying mechanisms by which broad-scale climate translates into population dynamics via local climate are not fully understood.
(3) Our review and synthesis based on 561 species reveals that population changes of mammals, birds and insects are strongly influenced by ENSO and NAO, providing clear evidence in support of Elton's BSCH. Mammal and insect populations tended to increase during positive ENSO phases, while bird populations tended to increase in positive NAO phases. Some taxa or regions may be more or less vulnerable to climate fluctuations and some geographical areas show multiple weather effects related to ENSO or NAO phases. Changes in ENSO or NAO could be used as early signals for pest management or wildlife conservation.
(4) Beyond confirming that animal populations are influenced by broad-scale climate variation, we uncovered extensive variation among taxa. The direct biotic and abiotic mechanisms by which broad-scale climate factors affect animal populations remain poorly understood. We therefore emphasize the need for integration of both broad-scale and local studies to clarify the effects of climate on population dynamics under accelerated climate change. Continued, accurate, long-term monitoring is necessary for a better understanding of both the broad- and local-scale effects of climate on animal populations. Future studies could be directed towards studying and utilizing bottom-up chain effects, ecological buffering effects, synchronizers of cycles, non-monotonic effects, and early warning signals for wildlife management.
ACKNOWLEDGEMENTS
We are grateful to Dr Leif Christian Stige, University of Oslo for his valuable comments and suggestions. R. D.’s visit to work on this paper at the Zhang Laboratory was supported by the Chinese Academy of Sciences. M. H. was supported by Hatch Act funding to the University of California, Davis Agricultural Experiment Station. This study was partially supported by the following grants: Major Program of National Natural Science Foundation of China (Grant 32090021), ANSO Project of Chinese Academy of Science projects (ANSO-CR- KP-2020-08), the Strategic Priority Research Program of Chinese Academy of Sciences (Grant XDB11050300), and the ISZS/IUBS Program of Biological Consequence of Global Change (BCGC). The authors declare no competing interests.
AUTHOR CONTRIBUTIONS
Z. Z. designed the study; X. W., M. H., R. D., and Z. Z. contributed to data compilation, data editing, and analysis; all coauthors contributed to paper writing.
Open Research
DATA AVAILABILITY STATEMENT
All data needed to evaluate the conclusions in this paper are available in the Supporting Information; the database for reproducible screening can be accessed at https://github.com/wanxinru/BSCH. Any additional data related to this paper of interest to readers may be provided by the authors upon request.




