The role of mate-choice copying in speciation and hybridization
ABSTRACT
Mate-choice copying, a social, non-genetic mechanism of mate choice, occurs when an individual (typically a female) copies the mate choice of other individuals via a process of social learning. Over the past 20 years, mate-choice copying has consistently been shown to affect mate choice in several species, by altering the genetically based expression of mating preferences. This behaviour has been claimed by several authors to have a significant role in evolution. Because it can cause or increase skews in male mating success, it seems to have the potential to induce a rapid change of the directionality and rate of sexual selection, possibly leading to divergent evolution and speciation. Theoretical work has, however, been challenging this view, showing that copying may decelerate sexual selection and that linkage disequilibrium cannot be established between the copied preference and the male trait, because females copy from unrelated individuals in the population, making an invasion of new and potentially fitter male traits difficult. Given this controversy, it is timely to ask about the real impact of mate-choice copying in speciation. We propose that a solution to this impasse may be the existence of some degree of habitat selection, which would create a spatial structure, causing scenarios of micro-allopatry and thus overcoming the problem of the lack of linkage disequilibrium. As far as we are aware, the potential role of mate-choice copying on fostering speciation in micro-allopatry has not been tackled. Also important is that the role of mate-choice copying has generally been discussed as being a barrier to gene flow. However, in our view, mate-choice copying may actually play a key role in facilitating gene flow, thereby fostering hybridization. Yet, the role of mate-choice copying in hybridization has so far been overlooked, although the conditions under which it might occur are more likely, or less restricted, than those favouring speciation. Hence, a conceptual framework is needed to identify the exact mechanisms and the conditions under which speciation or hybridization are expected. Here, we develop such a framework to be used as a roadmap for future research at the intersection of these research areas.
I. INTRODUCTION
Ever since Darwin's theory of sexual selection (Darwin, 1859, 1871), a key question in evolutionary biology is how sexual organisms, mostly females, choose their mates. If being choosy is more beneficial than random mating (Bateman, 1948; Williams, 1966; Majerus, 1986; Andersson, 1994), females need information about male variability in quality in order to make optimal and adaptive decisions (Valone & Templeton, 2002; Danchin et al., 2004). The question is, therefore, what information do females use before accepting or rejecting a mating invitation?
The classic view has been that females have innate, or genetic, preferences for certain male phenotypes, which remain fixed throughout their lives. Genetically inherited information is the product of selection and reflects long-term adaptations to past environments. Female genetic preferences for males with particular traits are thus considered adaptive evolutionary responses to phenotypic indicators of the benefits that a male can provide to the female as a mate. Different genetic mechanisms of female mate choice evolution include direct phenotypic benefits, sensory biases, indirect genetic benefits (good genes and sexy sons), and genetic compatibility (reviewed in Andersson, 1994; Andersson & Simmons, 2006). Although the relative importance of each mechanism may not be easy to demonstrate in all cases (Andersson & Simmons, 2006), the overall genetic mechanisms of sexual selection have been widely supported (Fisher, 1930; Kirkpatrick, 1982; Majerus, 1986; Andersson, 1994; Mead & Arnold, 2004; Andersson & Simmons, 2006). Moreover, female genetic preferences are known to have an impact on speciation (Lande, 1981; Coyne & Orr, 2004; Kraaijeveld, Kraaijeveld-Smit & Maan, 2011; Nosil, 2012).
Nonetheless, researchers are now increasingly aware that females may not exclusively select mates based on their innate preferences. On the one hand, female preferences are condition and context dependent, as the costs of choosiness – from mate assessment, female competition, male interference and predation risk – are higher to low-quality females than to high-quality ones, and may vary between habitat patches (Jennions & Petrie, 1997; Cotton, Small & Pomiankowski, 2006; Miller & Svensson, 2014). On the other hand, male quality is a complex trait, varying rather rapidly across generations and within the lifetime of an individual due to interactions with the environment (genotype-by-environment interactions), with other genes (gene-by-gene interactions), and with other genotypes (genotype-by-genotype interactions) (Ingleby, Hunt & Hosken, 2010; Getty, 2014; Holman & Kokko, 2014; Wade, 2014). Additionally, it may also vary with the male's experience, condition and age (Stearns, 1992; Kokko, 1997; Verzijden et al., 2012; Harrison et al., 2013). This means that one male that performs really well in one given context will not necessarily perform equally well if the conditions change. Hence, female genetically inherited preferences for males with particular traits may not adequately reflect male quality in all possible contexts (Jennions & Petrie, 1997; Danchin et al., 2004; Cotton et al., 2006; Danchin, Giraldeau & Wagner, 2008; Miller & Svensson, 2014; Wade, 2014).
Thus, to improve their chances of choosing the best male, females need to identify the relevant ecological and social conditions under which to best raise an offspring and then assess male performance under those conditions (Wade, 2014). Gathering this amount of information would, however, significantly increase the costs of female choice (Wade, 2014). This would be particularly challenging to females in low condition (Jennions & Petrie, 1997; Cotton et al., 2006), as it would require the rejection of a great proportion of the available males and/or to spend more time and energy sampling more males (Cotton et al., 2006).
Social learning has the ability to reduce such costs, by reducing search time and increasing female accuracy in perceiving the environment and the quality of potential mates (Danchin et al., 2004; Wagner & Danchin, 2010). Moreover, similarly to the genetic mechanisms of female mate choice, social-learning processes can lead to stable shifts in female mate preferences, that can cause or increase skews in male mating success, affecting the directionality and rate of sexual selection (Wade & Pruett-Jones, 1990; Laland, 1994b; ten Cate, 2000; Verzijden et al., 2012; Santos, Matos & Varela, 2014).
Therefore, several researchers have suggested that non-genetically inherited information, particularly in the context of sexual selection, can also affect speciation (e.g. Laland, 1994b; Jablonka, Lamb & Avital, 1998; ten Cate, 2000; Danchin et al., 2004, 2011; Grant & Grant, 2009; Danchin & Wagner, 2010; Verzijden et al., 2012; Dukas, 2013; Laland et al., 2015; Lindholm, 2015). ‘Ecological feedbacks, speciation, and evolutionary dynamics not only result from gene flows, but also more generally from “information flows” among demes’ (Danchin, 2013, p. 356). In other words, developmental processes, like social learning during mate choice, can ‘create novel variants, contribute to heredity, generate adaptive fit, and thereby direct the course of evolution’ (Laland et al., 2015, p. 6).
Previously, emphasis has been given to the role of sexual imprinting in speciation (Laland, 1994a; Irwin & Price, 1999; Owens, Rowe & Thomas, 1999; ten Cate, 2000; Grant & Grant, 2009; Verzijden et al., 2012; Lindholm, 2015), as it is a type of preference learning that happens very early in the life of an organism and that has remarkable stability throughout life, affecting mate choice once the organism reaches sexual maturity (Lorenz, 1935; Immelman, 1975). Maternal imprinting, in particular, generates assortative mating, maintains male polymorphisms and preserves linkage disequilibrium between the female preference and the male trait, which are the necessary ingredients for sympatric speciation (Verzijden, Lachlan & Servedio, 2005; Verzijden et al., 2012). But beyond sexual imprinting, mate-choice copying is the best example of a learning process influencing mate choice, with properties of its own (see Table 1).
| Mate-choice copying | Sexual imprinting | |
|---|---|---|
| Type of learning: | ||
| At what age? | At maturity | Very early in life |
| By observing the mating interactions of other females? | Yes | No |
| Does it reveal male performance? | Yes | No |
| Criteria for information transmission: | ||
| Social learning | Yes | Yes |
| Social generalization | Yes | Yes |
| Informational cascades | Yes | No |
| Transmission across generations | Mainly oblique (i.e. between unrelated individuals) | Mainly vertical (i.e. between related individuals) |
| Durability | Yes, but largely unknown and can be reversed | Yes and cannot be reversed (at least easily) |
| Contribution to speciation | Theoretically yes (but only with some degree of spatial structuring) | Yes (but only via maternal imprinting) |
| Contribution to hybridization | Theoretically yes | Yes |
Mate-choice copying occurs much later in life than sexual imprinting, when a sexually mature individual, although potentially inexperienced, observes and copies the mating decisions of other individuals (Pruett-Jones, 1992; Dugatkin, 1996a). Over the past 20 years, mate-choice copying has consistently been shown to affect mate choice in a variety of species, by altering the genetically based expression of mating preferences (see reviews in Vakirtzis, 2011; Witte, Kniel & Kureck, 2015). The consequences are that the selection pressures on both copying females and their chosen males can change radically, affecting the direction and strength of sexual selection. This is why it has been suggested that mate-choice copying may cause speciation if its effects persist long enough in the behaviour of copier individuals (e.g. Gibson & Höglund, 1992; Brooks, 1998; Witte & Noltemeier, 2002; Danchin et al., 2004; Leadbeater, 2009; Mery et al., 2009; Fowler-Finn et al., 2015) (Table 1).
What is poorly understood, however, are the exact conditions, and their likelihood, under which mate-choice copying can effectively cause speciation. In contrast with the impact of maternal sexual imprinting, speciation by mate-choice copying seems less likely, because it tends to generate positive frequency dependence for the most common male phenotype, which makes it difficult to maintain polymorphisms, and also because linkage disequilibrium cannot be established between the copied preference by the female and the male trait (Verzijden et al., 2005, 2012). We hypothesize that more restricted assumptions, such as fine-grained habitat structuring (micro-allopatry), with some degree of ecological barriers (particularly involving habitat selection), and genotype-by-environment interactions are necessary for mate-choice copying to be able to create and stabilize behavioural isolation. Herein, we provide a detailed analysis of those particular circumstances under which mate-choice copying can disrupt and canalize female mating preferences for certain male phenotypes, creating and potentiating divergence and speciation (Figs 1 and 2).
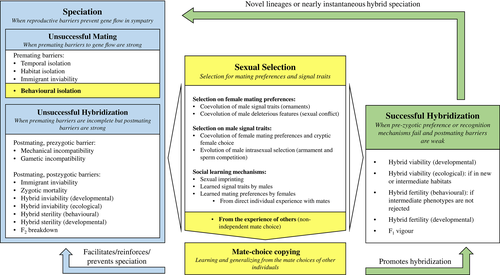
Additionally, and similarly to sexual imprinting that sometimes may also initiate hybridization events – when animals misimprint on heterospecifics' traits, making them recognize heterospecifics as quality mating partners (Irwin & Price, 1999; ten Cate & Vos, 1999; ten Cate, 2000; Grant & Grant, 2008, 2009) – we propose the hypothesis that mate-choice copying may likewise favour hybridization, by increasing female acceptance of heterospecific male phenotypes. This can happen when heterospecific demonstrator females are seen as good models to copy (Hill & Ryan, 2006), as well as when conspecific model females mate with heterospecific males (Schlupp, Marler & Ryan, 1994; Heubel et al., 2008). We suggest that – just as in sexual imprinting – copying ‘mistakes’ can be caused by incomplete species' recognition systems and could lead to successful hybridization, but also to reproductive interference. This potential role of mate-choice copying in promoting hybridization has been overlooked so far, although the conditions under which it might occur are much more likely, or less restricted, than those favouring speciation, which could be indicative of its importance. We thus propose for the first time that mate-choice copying may cause hybridization and that it can contribute, if hybridization is successful, to either the establishment and/or maintenance of hybrid zones, or to new reproductive isolation. If, on the other hand, hybridization is unsuccessful, with fitness costs to both the copying female and the preferred male – as both would be spending time and energy with unsuccessful matings and unviable offspring – mate-choice copying can lead to reproductive interference, increasing species extinction risk. As a result, there might be selection against mate-choice copying, or selection to reinforce species recognition, thereby, preserving or reinforcing reproductive barriers (Figs 1 and 2). In the following sections, we discuss scenarios (Fig. 2) and mechanisms (Figs 3-7) by which mate-choice copying can weaken or strengthen gene flow within and between populations, with consequences for both speciation and hybridization.
II. MATE-CHOICE COPYING, A NON-GENETIC MECHANISM OF SEXUAL SELECTION
(1) What is mate-choice copying?
Mate-choice copying is a non-independent mechanism of (mostly) female mate choice (Fig. 1). It is a type of social learning and occurs when the mating preference of an observer female for a particular male increases or decreases, depending on whether that male mated previously or was rejected by another female (Wade & Pruett-Jones, 1990; Dugatkin, 1992, 1996a; Pruett-Jones, 1992). This behaviour shows that mating preferences are not fixed for life, but can change via observing other females' choices. In other words, it represents a shift or a novelty in an individual's mate-choice behaviour (Lindholm, 2015).
Mate-choice copying was originally proposed in the context of lekking birds' mating systems, where females visit male displaying arenas multiple times, observe the mating decisions of several previous females, and where only a small proportion of males cumulate all mating success (Bradbury, 1981; Bradbury & Gibson, 1983; Losey et al., 1986; Wade & Pruett-Jones, 1990; Gibson, Bradbury & Vehrencamp, 1991; Gibson & Höglund, 1992). It was first tested by Dugatkin (1992) and Dugatkin & Godin (1992) using guppies Poecilia reticulata as models, and later by Galef & White (1998) using Japanese quails Coturnix japonica. Subsequent field and laboratory studies found evidence for copying in sailfin mollies Poecilia latipinna (e.g. Schlupp et al., 1994; Witte & Ryan, 2002), ocellated wrasses Symphodus ocellatus (Alonzo, 2008), zebra finches Taeniopygia guttata (e.g. Swaddle et al., 2005), black grouse Tetrao tetrix (Höglund et al., 1995), house mice Mus musculus (Kavaliers et al., 2006) and humans (e.g. Waynforth, 2007; Yorzinski & Platt, 2010). These are only a few of the many examples that can be found in the literature (reviewed in Galef & White, 2000; Westneat et al., 2000; Valone & Templeton, 2002; Vakirtzis, 2011; Witte et al., 2015), including the first evidence of mate-choice copying in an invertebrate species, Drosophila melanogaster (Mery et al., 2009), and more recently in Schizocosa wolf spiders (Fowler-Finn et al., 2015). Finding mate-choice copying also in invertebrates has considerable importance because it indicates that copying could be widespread in nature, representing a general rule, instead of being an exclusive ability of more complex cognitive systems, such as those of vertebrates (Leadbeater, 2009).
Interestingly, the first studies on mate-choice copying (Bradbury, 1981; Bradbury & Gibson, 1983; Losey et al., 1986; Wade & Pruett-Jones, 1990; Gibson et al., 1991; Dugatkin, 1992; Dugatkin & Godin, 1992; Gibson & Höglund, 1992; Pruett-Jones, 1992; Galef & White, 1998) are contemporary with the first compelling studies supporting the genetic-based hypotheses of female mate choice (Lande, 1981; Andersson, 1982, 1994; Kirkpatrick, 1982; Majerus, 1986; Majerus et al., 1986; Møller, 1994; Mead & Arnold, 2004). Despite this, the implications of mate-choice copying for the evolutionary mechanisms of sexual selection have not yet been given much attention.
(2) Why should females rely on mate-choice copying?
Mate-choice copying is thought to be a strategy used by females to assess the quality of potential mates more effectively (Wade & Pruett-Jones, 1990; Gibson & Höglund, 1992; Pruett-Jones, 1992; Brooks, 1998; Nordell & Valone, 1998; Valone & Templeton, 2002; Danchin et al., 2004; Dugatkin, 2005; Wagner & Danchin, 2010). This is because mate-choice copying is based on the inadvertent social information provided by model females about the mating performance of their mates - whether they accepted or rejected them for copulations (Danchin et al., 2004; Wagner & Danchin, 2010). In other words, the information obtained (acceptance or rejection) does not involve active signalling by the males and, consequently, cannot be dishonestly manipulated by them, which increases its reliability (Danchin et al., 2004).
Moreover, because male genotypes may not always match up correctly with the environmental context in which they are found – male quality is a complex trait (Ingleby et al., 2010; Getty, 2014; Holman & Kokko, 2014; Wade, 2014)-female uncertainty about male quality may be quite high (Getty, 2014; Wade, 2014). Genotype-by-environment interactions, in particular, augment phenotypic variability within and across habitat patches (Ingleby et al., 2010; Holman & Kokko, 2014; Wade, 2014) and can compromise the honesty of sexual signals (Greenfield & Rodriguez, 2004; Getty, 2014; Miller & Svensson, 2014; Wade, 2014). Therefore, copying other females' choices may be one important component of a more flexible mating behaviour.
A second, not mutually exclusive, hypothesis is that mate-choice copying could reduce the time and energy invested into the independent assessment of several potential mates (Wade & Pruett-Jones, 1990; Gibson & Höglund, 1992; Pruett-Jones, 1992; Briggs, Godin & Dugatkin, 1996; Schlupp & Ryan, 1996; Dugatkin & Godin, 1998). By doing so, it may reduce mate-searching time, as well as the costs of direct interactions with males, such as those from sexual harassment, sexually transmitted diseases, parasite exposure and vulnerability to predation (Pomiankowski, 1987; Reynolds & Gross, 1990; Andersson, 1994; Dugatkin & Höglund, 1995; Godin & Briggs, 1996; Dugatkin, 2005).
(3) How did mate-choice copying evolve?
Game-theoretic (Pruett-Jones, 1992; Dugatkin & Höglund, 1995; Dugatkin, 2005; Brennan, Flaxman & Alonzo, 2008) and stochastic (Losey et al., 1986) models, studying the evolution of mate-choice copying have provided evidence for both hypotheses. They found that populations of pure choosers can always be invaded by individuals carrying a copying gene (and vice versa) and that this can only be achieved if there are costs associated with direct mate assessment (Pruett-Jones, 1992; Dugatkin & Höglund, 1995;Dugatkin, 2005; Brennan et al., 2008), if mate-quality assessment is difficult (Dugatkin, 2005; Brennan et al., 2008) and if assessment is skewed towards selecting the best quality mates (Losey et al., 1986). Increasing such effects also increases the frequency of the copying strategy in the population, so that the number of copiers can even exceed the number of choosers.
This reasoning assumes that chooser females have enough personal knowledge about male variability in quality so that they will make the right decision. If, however, they lack that knowledge and mate randomly, they could mislead the mating choices of copying females. Even though, the overall outcome for copying females would be no different as if they had also chosen randomly, so copying behaviour does not increase the females' probability of making wrong choices (Nordell & Valone, 1998). Furthermore, mathematical models have shown that mate-choice copying is even more likely to evolve when choosers have incomplete information about male quality and, consequently, make assessment errors (Sirot, 2001; Dugatkin, 2005; Brennan et al., 2008; see also Rendell et al., 2010). This output is generated because chooser females pay both the cost of choice and the cost of assessment errors, while copier females pay only the cost of copying a wrong choice, which gives copying females a fitness advantage (Dugatkin, 2005; Brennan et al., 2008).
Nonetheless, when mate-choice copying is an optional strategy – when females are non-pure copiers, because they have both the copying gene and the ability for direct mate quality assessment – it is thought to be prevalent only in females with poor ability to discriminate (Nordell & Valone, 1998). Such females are generally younger and inexperienced or are females that had previous unsuccessful breeding attempts (Stöhr, 1998; Danchin et al., 2008). Accordingly, only older/experienced and high-quality model females are generally used as reliable models from which to copy (Dugatkin & Godin, 1993; Amlacher & Dugatkin, 2005; Hill & Ryan, 2006; Vukomanovic & Rodd, 2007; Waynforth, 2007; Yorzinski & Platt, 2010; and see Vakirtzis, 2011, for a review). On the other hand, copying should increase in frequency, in all types of females, if the discrimination task becomes increasingly more difficult or costly (Nordell & Valone, 1998). Empirical evidence shows that this can happen when male trait variability is low but still meaningful (Dugatkin, 1996b; Witte & Ryan, 1998), or quite large (Mery et al., 2009; see also Smolla et al., 2016). This might seem contradictory, but does not have to be: in the former case, naïve females adopt the copying strategy because they probably do not know what phenotypic trait distinguishes both males (the meaningful differences are not noticeable to them; Ryan, Akre & Kirkpatrick, 2007); while, in the latter case, male phenotypes are so different or unknown to the females that it should be difficult to assess their relative quality (the meaningful differences are larger than the differences necessary for discrimination; Ryan et al., 2007). Copying might be the safest strategy in this latter case, because naive females will at least be able to minimize the difference of their reproductive success with that of more experienced females (the bet-hedging hypothesis; Losey et al., 1986; Sirot, 2001).
Finally, with population-genetic models, mate-choice copying has also been proposed to have evolved by indirect selection (Kirkpatrick & Dugatkin, 1994; Servedio & Kirkpatrick, 1996; Santos et al., 2017). In this case, copying is not optional – there are both pure choosers and pure copiers in the population. It is modelled assuming assessment costs for choosers and mild learning costs for copiers. The copying allele spreads in the population from very low frequencies until above 50% by hitchhiking and, at the same time, facilitating the spread of a new and fitter male-trait allele. This is relevant, because through this process mate-choice copying can help the invasion of fitter male traits, that otherwise would not be able to invade (Santos et al., 2014). However, this process is unlikely if chooser females are monomorphic in their mate preferences. Genetic variation for innate preferences has to persist in the population, since at least a proportion of chooser females needs to show a preference for the new males. Only then will mate-choice copying be able to coevolve with the male trait (Santos et al., 2017).
Taken together, mate-choice copying theory predicts that the use of social information may be a cost-effective and reliable approach to mate choice, or a strategy with only indirect benefits. Theoretical models have provided evidence for each of these mechanisms. However, from an empirical point of view, only the role of mate-choice copying in facilitating male-quality assessment and discrimination has yet received compelling support (reviewed in Vakirtzis, 2011 and Witte et al., 2015).
(4) Why does mate-choice copying matter to species evolution?
Mate-choice copying can have evolutionary consequences when it gives rise to stable informational cascades and if it is followed by the social generalization of the learned mating preferences. The concept of informational cascades was proposed by Bikhchandani, Hirshleifer & Welch (1992, 1998) to explain localized conformity and fragility of mass behaviour in humans, but may also apply to mate-choice copying (Gibson & Höglund, 1992; Giraldeau, Valone & Templeton, 2002; Kendal et al., 2005; Rieucau & Giraldeau, 2011). The model posits that any behaviour can spread rapidly through a population with a single individual as the starting point if observers copy the relevant behaviour. Informational cascades could either propagate accurate or erroneous mating decisions (Bikhchandani et al., 1992, 1998). This is possible in the context of mate-choice copying because as it is defined, it is based not on the courtship signalling of males towards the model females, but on the outcome of those interactions, i.e. on the social cues that are inadvertently produced by model females during their mating decisions (Danchin et al., 2004; Giraldeau et al., 2002). And when the key information is the behavioural decision of the demonstrator individual (accepting or rejecting mating with a certain male) and not the actual information on which the demonstrator based her decision (the male courtship behaviour), erroneous information can be transmitted (Bikhchandani et al., 1992, 1998; Gibson & Höglund, 1992; Dugatkin, 1996a; Giraldeau et al., 2002; Kendal et al., 2005; Rieucau & Giraldeau, 2011). There is evidence that animals engage in informational cascades in the context of food choice (e.g. Aplin et al., 2015; and see reviews in Giraldeau et al., 2002; Kendal et al., 2005; Rieucau & Giraldeau, 2011). However, no informational cascade on mate choice copying has yet been formally tested, although theoretical work assumes the informational cascade process when copier individuals become demonstrators themselves (e.g.Kirkpatrick & Dugatkin, 1994; Laland, 1994b; Agrawal, 2001; Santos et al., 2014).
Social generalization, on the other hand, occurs when the observer female learns to copy the choice of the male phenotype by the demonstrator female and not necessarily or exclusively the choice of the individual male (Brooks, 1998). This means that the entire population of males will be affected by such a shift or novelty in female mating behaviour. There is empirical evidence of mate-choice copying generalization for new male ornaments in four vertebrate species: Japanese quails (White & Galef, 2000), guppies (Godin, Herdman & Dugatkin, 2005), sailfin mollies (Witte & Noltemeier, 2002) and zebra finches (e.g. Drullion & Dubois, 2008), but also in fruit flies (Mery et al., 2009).
When informational cascades and social generalization of preferences are in place, mate-choice copying acquires the potential to modify the selection pressures for the preferred male traits, reducing behavioural variability between females, and changing the rate and direction of sexual selection (Kirkpatrick & Dugatkin, 1994; Laland, 1994b; Agrawal, 2001; Danchin & Wagner, 2010; Santos et al., 2014; Witte et al., 2015). For that reason, it has been hypothesized that mate-choice copying could have favoured the emergence of new species, by inducing reproductive isolation between individuals with different copying traditions (e.g. Gibson & Höglund, 1992; Witte & Noltemeier, 2002; Danchin et al., 2004; Leadbeater, 2009; Mery et al., 2009; Fowler-Finn et al., 2015). Copying has thus been seen as an additional mechanism to the diversification of species.
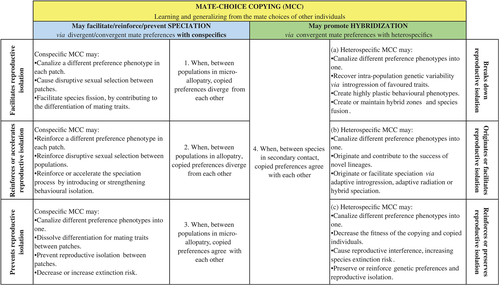
Surprisingly the hypothesis has never been fully formalized. It is presented as a very straightforward prediction of empirical findings in mate-choice copying studies. Such findings are said to have ‘implications for evolution given that socially learned mate preferences may lead to reproductive isolation, setting the stage for speciation’ (Mery et al., 2009, p. 730; see also Danchin et al., 2004; Leadbeater, 2009; Fowler-Finn et al., 2015). Such a prediction is indeed very intuitive and powerful. However, put this way, it is also too simplistic. The hypothesis needs a much deeper reflection of its assumptions and predictions. Besides, as we propose here, mate-choice copying may also reduce behavioural variability among populations and species, canalizing different preference phenotypes into one, leading conspecific and heterospecific populations to hybridize (Fig. 1). In the next sections, we provide such thorough reflection on the role of mate-choice copying in both speciation and hybridization (Fig. 2).
III. THE ROLE OF MATE-CHOICE COPYING IN SPECIATION
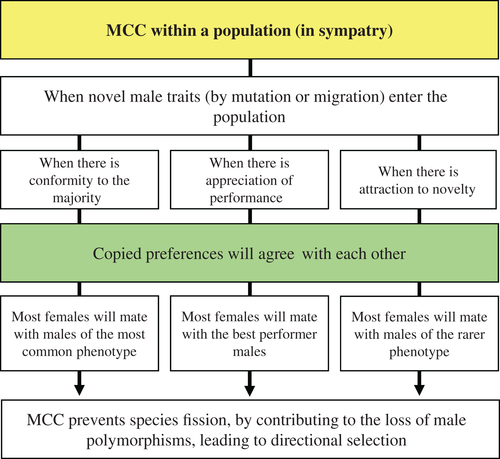
(1) When, within a population (in sympatry), copied preferences diverge from each other
The hypothesis that mate-choice copying can cause species fission, implicitly describes a scenario of sympatric speciation, where copied preferences spread through a certain proportion of the females' population that, with no more information available, would have chosen males based on their innate preferences (Leadbeater, 2009; Mery et al., 2009; Fowler-Finn et al., 2015; see also Danchin et al., 2004). For instance, it is known that, in guppies, females have a genetic preference for males with large areas of orange pigment in their colour patterns (Houde, 1988; Dugatkin, 1996b). There is, however, within- and between-population variation in the degree of female preference for orange (Houde, 1988; Houde & Endler, 1990) and also limited time and energy for females to assess every male in a population (Endler, 1983). This makes it possible that if a new male phenotype, with smaller orange areas, enters a population (by migration or mutation) a number of females may choose the new phenotype. Knowing, additionally, that there is mate-choice copying in this species (e.g., Dugatkin, 1992, 1996b; Dugatkin & Godin, 1992, 1993; Amlacher & Dugatkin, 2005; Godin & Hair, 2009), it is likely that a proportion of the females' population will rely on the choices of other females. This has been shown by Dugatkin (1996b), where observer females copied the mate choice of model females for males with smaller orange areas. This could trigger an alternative informational cascade to that of the choice of males with larger orange areas (Dugatkin, 1996b), causing a behavioural divergence within the population. But would this set the stage for reproductive isolation and ultimately sympatric speciation?
We think not, because copying females can obtain their information from any other females in the population, and most likely from unrelated model females. In fact, one of the important characteristics of non-genetic information is that it can be transmitted in all three directions: vertical, horizontal and oblique (Danchin & Wagner, 2010; Danchin et al., 2011; Danchin, 2013). Mate-choice copying is not an exception (Danchin et al., 2004). Therefore, any type of linkage disequilibrium between the learned preference (non-genetic information) and the male trait (e.g. some ornament that is genetically inherited) will be disrupted when the information inheritance is not vertical. Yet, speciation with gene flow and without linkage disequilibrium has been found theoretically unlikely (e.g. Felsenstein, 1981; Arnegard & Kondrashov, 2004; Verzijden et al., 2005), and is subject to debate (Via, 2001; Kraaijeveld et al., 2011; Butlin & The Marie Curie Speciation Network, 2012; Servedio & Boughman, 2017). Indeed, in models where different inheritance mechanisms of mate choice were tested (Verzijden et al., 2005; Servedio, Sæther & Sætre, 2009), the copying scenario (that the authors modelled as oblique sexual imprinting) did not succeed in causing disruptive divergence in sympatry. This was not the case for maternal imprinting because that information runs between related individuals – females learn from their mothers – and the linkage disequilibrium between the genomes of the females that learn and the genomes of the male traits is preserved. Verzijden & ten Cate (2007) provided an empirical example of how sexual imprinting seems to have participated in the reproductive isolation of a species pair of African cichlids in Lake Victoria.
Additionally, theoretical models have been studying the ways in which mate-choice copying can help the spread of novel male traits in a population (Kirkpatrick & Dugatkin, 1994; Laland, 1994b; Agrawal, 2001; Santos et al., 2014), potentially affecting their genetic variability and rate of divergence. However, all models agree that copying is strongly affected by a positive frequency-dependent advantage of the most common male phenotype – the ‘conformity to the majority’ rule (Bikhchandani et al., 1998; Fig. 3) – that prevents the spread of novel (by mutation or migration) male-trait alleles in a population by mate-choice copying (Kirkpatrick & Dugatkin, 1994; Laland, 1994b; Agrawal, 2001; Santos et al., 2014). Novel traits are rare in the first generations and so only a few females will be observed mating with these males, which will generate very little positive information about them. Moreover, if the novel trait provides a competitive advantage or courtship vigour to the males, or exploits a female sensory bias, it can spread rapidly and achieve fixation without the help of copying (Santos et al., 2014). This means that a mate-choice copying informational cascade favouring the most common phenotype (the phenotype with larger orange areas in the guppy example) would prevent the establishment of the information pathway for males with the new phenotype (smaller orange areas), hampering disruptive sexual selection.
Alternatively, copying could still have an effect on the spread of novel male traits if females are more strongly influenced by the observation of successful matings with novel males than by the observation of successful matings with common males. This could generate directional selection towards the novel male trait – the ‘attraction to novelty’ rule (Fig. 3) –, as was shown by Agrawal's (2001) mathematical model, but not disruptive sexual selection.
More recently, Santos et al. (2014) included in their mathematical model another important element of mate-choice copying theory: the transmission of negative information about males. When females observe model females accepting matings with certain types of males, the inadvertent social information that the females will be producing about the males' performance is positive information. However, model females also reject males and, in this case, they would be inadvertently advertising the males' lack of success (negative information). It has been demonstrated empirically that females do copy male rejection (Witte & Ueding, 2003) and Santos et al. (2014) showed that by incorporating negative information in the simulated population, the positive frequency dependence favouring the most common phenotype loses strength (because many more common males are observed being rejected than rare males) and so the novel trait, when associated with male vigour, may spread in the population by mate-choice copying and achieve fixation. In this case, females would be incorporating in their decisions the complete information about the males' mating performance – the ‘appreciation of performance’ rule (Fig. 3) – as mate-choice copying theory predicts (Pruett-Jones, 1992; Danchin et al., 2004; Santos et al., 2014), instead of relying exclusively on conformity (that only takes positive information into account), or novelty (that gives more weight to positive information from males with rare phenotypes).
Nonetheless, whatever the mate-choice copying rule applied, the outcome of mathematical simulations is the fixation of one of the alleles (the resident or the novel male trait). This way, mate-choice copying does not contribute to the evolution of divergence, in sympatry, between the two male phenotypes (of large and small orange areas in the guppy example), but to directional selection instead. This leads to the loss of male polymorphisms, in the absence of which sympatric speciation cannot occur (Verzijden et al., 2005; Servedio et al., 2009; Fig. 3).
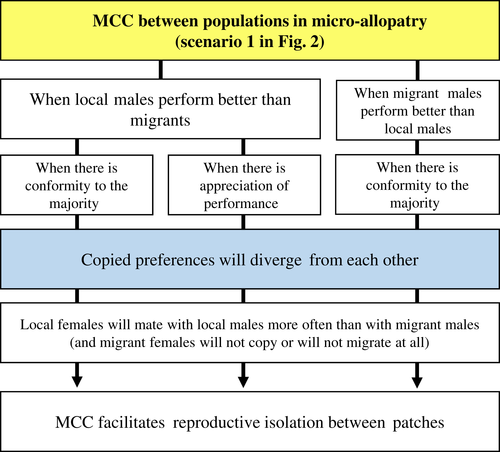
(2) When, between populations in micro-allopatry, copied preferences diverge from each other and facilitate reproductive isolation
In complex and dynamic environments, habitat conditions differ and change rapidly across space and time. It has been recognized recently that such ecological complexity affects the dynamics of sexual selection (Cornwallis & Uller, 2010; Butlin & The Marie Curie Speciation Network, 2012; Miller & Svensson, 2014; Wade, 2014), creating a mosaic of female preferences and male sexual traits (Gosden & Svensson, 2008), where genotype-by-environment interactions set the stage for local adaptation (Ingleby et al., 2010; Getty, 2014; Holman & Kokko, 2014; Wade, 2014). Mate-choice copying is more likely to have a role in speciation under such a fine-grained mosaic scenario, such as a situation of micro-allopatry, with early stages of reproductive isolation caused by ecological divergence (Streelman & Danley, 2003; Arnegard & Kondrashov, 2004; Coyne & Orr, 2004; Nosil, 2012), than under a scenario of full sympatry. This is represented in the first scenario of Fig. 2 and in Fig. 4.
In micro-allopatry, when populations are structured by habitat selection, with divergent adaptation to local conditions (micro-ecological barriers, with micro-spatial variation), mate-choice copying can have a role in further divergence through the positive-frequency-dependent effect towards the more common male – the conformity to the majority rule (Kirkpatrick & Dugatkin, 1994; Laland, 1994b; Agrawal, 2001; Santos et al., 2014). This is likely because it would preserve the phenotypes of local males – even when migrant males have better performance – facilitating reproductive isolation between patches. The appreciation of performance rule also applies here, as local females would mate more often with local males than with migrant ones, which would also facilitate directional selection towards local males. But this rule would only apply if local males perform better than migrants, which is the most likely case under local adaptation . However, for both rules to apply, only local females should be prone to copy, which is a much more restricted assumption. Migrant females should either remain faithful to migrant males (via mate preferences that they. However, for both rules to apply, only local females should be prone to copy, which is a much more restricted assumption) or do not migrate at all (via male sex-biased dispersal). Otherwise, conspecific hybridization between migrant females and local males would not be avoided, leading to genetic introgression and hence disrupting the genetic divergence between patches promoted by local females.
Therefore, mate-choice copying might not be at the origin of speciation in scenarios of sympatry, but can have a facilitating role in the subsequent process of divergence in micro-allopatry – although under restricted conditions –, by conditioning local (but not migrant) females to certain mate choices locally. By doing so, mate-choice copying will help to create different canalized female-preference phenotypes in each habitat patch and cause disruptive sexual selection for male traits between patches. This will ultimately facilitate species fission.
The recent finding that great tits Parus major use social learning to acquire novel foraging techniques and that they retain the tradition that is most common to their population, or, even more remarkably, adopt the local tradition when dispersing to neighbouring populations due to social conformity (Aplin et al., 2015), is a strong example of how copying behaviour can indeed condition the prevailing behaviour in an entire population and cause divergence of preferences between habitat patches. This leaves the suggestion that copying foraging techniques could initiate a process of local adaptation, to which mate-choice copying could subsequently add a second selective force. Examples like this are scarce in the literature, but we recognize that this is not easily tested empirically. On the other hand, theoretical models could much more easily consider the joint actions of ecological divergence and mate-choice copying, as well as of foraging and habitat copying with mate-choice copying.
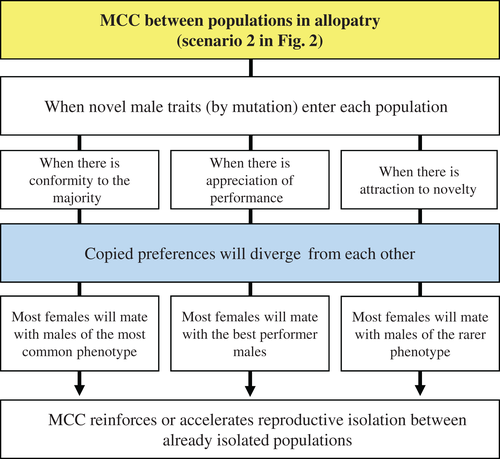
(3) When, between populations in allopatry, copied preferences diverge from each other and reinforce or accelerate reproductive isolation
This scenario describes the divergence process at a larger scale, between populations that have been evolving in allopatry or have recently become isolated. Here, the same reasoning of habitat structuring, genotype-by-environment interactions and adaptation to local conditions applies, as described in the previous section. Under those conditions, mate-choice copying could reinforce or accelerate the speciation process by introducing or strengthening the mechanism of behavioural isolation. This corresponds to the second scenario in Fig. 2. All copying rules apply here (Fig. 5): with conformity to the majority, males of the most common phenotype in each population will be favoured and copying will help the corresponding male trait alleles to reach fixation; with appreciation of performance, the best performer males and their alleles – which will depend on environmental conditions and vary between populations – will be favoured; and with attraction to novelty, it will be the males with the new phenotypes that will be preferred. In this case, the new phenotypes will independently arise in each population by mutation instead of migration.
In guppies, for example, there are populations subjected to high and low predation pressure (Endler, 1995). With high predation, males with small areas of orange pigment in their colour patterns have an advantage because they are less visible to predators (Endler, 1983) and females prefer to mate with less-orange males in those circumstances (Houde & Endler, 1990; Endler & Houde, 1995; Godin & Briggs, 1996). Mate-choice copying could help the adaptive process in populations with high predation risk, by creating an informational cascade favouring the less-orange males. This phenotypic plasticity generated by the learning process (Verzijden et al., 2012) could make a difference, because it would probably take a while until the allelic variant of female preference for males with small orange areas will invade the population. Mate-choice copying can rapidly produce that change in females' behaviour and accelerate speciation as a by-product. Moreover, mate-choice copying will allow the population to survive to predation until that allelic variant becomes more common. Besides, according to mate-choice copying theory, females should even be more prone to copy under high predation pressure (Wade & Pruett-Jones, 1990; Gibson & Höglund, 1992; Pruett-Jones, 1992; Briggs et al., 1996), which would accelerate the adaptive process even more. This is, however, less likely, since Briggs et al. (1996) found no evidence that guppy females are more prone to copy in the presence than in the absence of a predator. Still, this study indicates that guppy females do copy under predation pressure and by doing so, an informational cascade for less-orange males is possible. The adaptive advantage of such an informational cascade over the one favouring the males with larger orange areas will do the rest. Hence, again, mate-choice copying should not be seen as a cause of speciation, but as a secondary vehicle by which speciation could be achieved.
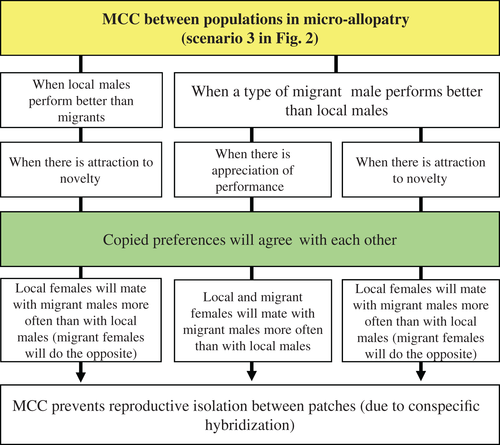
(4) When, between populations in micro-allopatry, copied preferences agree with each other and prevent reproductive isolation
This is represented in the third scenario of Fig. 2 and in Fig. 6. It describes the cases where there is no divergence of the copied preferences between populations under micro-allopatry, but convergence instead. Only two copying rules apply here. If there is attraction to novelty (Agrawal, 2001), local females will be strongly biased to mate with migrant males when they observe model females mating with them. By the same reasoning, migrant females will be biased to mate with local males. Put together, the two effects result in the constant mixing of phenotypes among habitat patches – conspecific hybridization – preventing reproductive isolation among them, as well as the loss of male polymorphisms. This rule would apply both when local males perform better or worse than migrant ones.
On the other hand, if migrants from one of the neighbouring patches perform better than local males in the remaining patches and the appreciation of performance rule applies, local and migrant females will both mate with migrant males more often than with local males, canalising selection towards a unique male phenotype. This would also prevent reproductive isolation between patches, but this time by eroding genetic variability and promoting directional selection. Notwithstanding, the loss of male polymorphisms among patches by mate-choice copying would be achieved at a slower rate than in an evolutionary model without copying This is so, because with mate-choice copying, the informational cascade for migrant males would have to compete with the informational cascade for local males. In this scenario, even if migrant males perform better than local ones, they are less abundant in each habitat patch at the beginning of the invasion process, which slows down the increase in frequency of the new male trait allele. Without copying, however, there is no informational cascade ‘protecting’ the resident allele and, given the fitness advantages of mating with migrant males, the new allele will invade each habitat patch rather rapidly. Such an outcome of a slower invasion of a new male allele in the presence of mate-choice copying was shown by simulations considering novel mutations (Santos et al., 2014), but would equally apply to the migration of a fitter allele. Therefore, according to Santos et al. (2014, p. 669), mate-choice copying seems ‘to have a more conservative role in the evolution of male traits, by reducing the strength and direction of selection’. This effect is significant because it could give a population increased or decreased chances of survival under environmental change.
Sexual selection has been suggested to increase species' extinction rates (Promislow, Montgomerie & Martin, 1992; Tanaka, 1996; Kokko & Brooks, 2003; but see Lumley et al., 2015, and Servedio & Boughman, 2017), which could explain why there is less evidence for speciation by sexual selection than by ecological divergence (Kraaijeveld et al., 2011). Several mechanisms could operate independently, namely the trade-off between male mating success and viability (Kokko & Brooks, 2003), where males with extreme traits are preferred by females and experience higher mating success but at the cost of decreased viability. The paradigmatic example is that of the Irish elk (Megaloceros giganteus), which is thought to have become extinct due to its gigantic antlers. The energetic requirements for antler growth were probably incompatible with a decline in environmental conditions due to climate change (e.g. Moen, Pastor & Cohen, 1999). Using a mathematical model, Kokko & Brooks (2003) arrived at similar predictions: in variable environments, extreme ornaments drive a population to extinction if the extreme male-trait allele reaches fixation before the environmental change happens. Mate-choice copying, by delaying the loss of male polymorphisms, would allow a population to cope better with environmental changes because the population would include males with alternative phenotypes for a larger number of generations. If, on the other hand, the male trait increases viability, mate-choice copying, by delaying fixation of the favourable trait, would also delay the population's short-term response to environmental change (evolutionary rescue: Carlson, Cunningham & Westley, 2014), putting the population at risk. A new mathematical model, similar to that of Kokko & Brooks (2003), simulating trade-offs between male mating success and viability, could easily incorporate mate-choice copying to test its impact on species extinction risk when new male traits either increase or decrease viability. This effect is compatible with the role of learning in phenotypic plasticity (Verzijden et al., 2012), as plasticity gives a species increased resilience to environmental change (Canale & Henry, 2010) and behavioural plasticity could, in fact, have an impact on the speed of evolution (Price, Qvarnstrom & Irwin, 2003; Duckworth, 2009).
IV. THE ROLE OF MATE-CHOICE COPYING IN HYBRIDIZATION
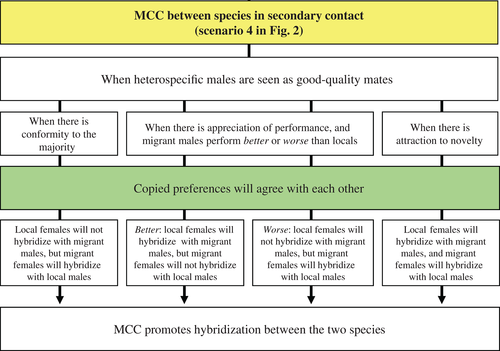
Additionally to the traditional view that mate-choice copying can favour the emergence of new species, we propose a new hypothesis that mate-choice copying may also have the potential to direct sexual selection towards interspecific hybridization if individuals from distinct species copy each other's mate choice. In other words, hybridization may be facilitated when copied preferences agree with each other between species in secondary contact (scenario 4 in Fig. 2).
Interspecific hybridization is common in nature, occurring in at least 10% of animal species (Coyne & Orr, 2004; Seehausen, 2004; Mallet, 2007; Grant & Grant, 2009). It occurs when the mechanisms of species recognition are incomplete (Burdfield-Steel & Shuker, 2011), meaning that species see each other as good-quality mates – heterospecific mating signals are interpreted as good-quality ones (Mendelson & Shaw, 2012). This may eventually produce hybrids that could be successful or unsuccessful depending on the strength of postzygotic barriers (Coyne & Orr, 2004; Fig. 1). Under these conditions of incomplete recognition systems, mate-choice copying may promote hybridization even further, as it can lead females from different species to learn from each other, that is, to interpret the mating decisions of heterospecific females as good-quality ones. This will increase the attractiveness of heterospecific males and induce ‘erroneous’ informational cascades (Giraldeau et al., 2002), speeding up the spread of social information that mating with heterospecifics is at least as good as mating with conspecifics. This is compatible with the mate-choice copying theory, because it is when females lack the ability to discriminate between males – in this case, when females lack a complete recognition system – that mate-choice copying is most likely to be utilized by choosing females (Nordell & Valone, 1998; Mery et al., 2009).
The, the mechanisms by which mate-choice copying can cause hybridization are similar to those described for speciation (Fig. 7) but are dependent upon the species' recognition abilities and the circumstances under which they came into secondary contact. If secondary contact is caused by migration, and the conformity to the majority rule applies, mate-choice copying will facilitate hybridization of migrant females with local males and inhibit hybridization of local females with migrant males, due to positive frequency-dependence favouring the local males. On the other hand, when the copying rule favours the use of both positive and negative information about males' performance – the appreciation of performance rule – migrating males may be considered more attractive than locals if they perform better, or less attractive if they perform worse. In the first case, both local and migrant females will mate with migrant males, since migrant females will reinforce their preference for conspecific males; in the second case, migrant females will hybridize with local males and the local females will reinforce their preference for conspecific males. Finally, if the copying rule favours the new male phenotype – the attraction to novelty rule – both local and migrant females will hybridize. This same reasoning can be applied when secondary contact is caused by overlap of the species ranges. In this case, the number of encounters with heterospecifics could be considerably higher, along with mate-choice copying effects.
Hybridization is one of the phenomena that contributes strongly to biodiversity changes (Seehausen, 2004; Mallet, 2007; Nolte & Tautz, 2010; Abbott et al., 2013). The outcomes of hybridization are diverse and will be no different from when it is caused or facilitated by mate-choice copying: it can lead to species fusion and adaptive introgression, by creating or maintaining hybrid zones (scenario 4a in Fig. 2); it can contribute to the success of novel lineages by hybrid speciation (scenario 4b in Fig. 2); or if hybridization costs are involved, it can cause reproductive interference and increase species extinction risk, which then could reinforce or preserve already existent reproductive isolation (scenario 4c in Fig. 2). In the next sections, we outline each of these scenarios.
(1) When, between species in secondary contact, copied preferences break down reproductive isolation
When hybridization occurs and there is a certain degree of hybrid viability, either developmental or ecological (Fig. 1), mate-choice copying, as a promoter of hybridization, will canalize different preference phenotypes into one, contributing to the creation or maintenance of hybrid zones (Abbott et al., 2013). This could eventually lead to species fusion (Grant & Grant, 2008), recovering, via adaptive introgression, intra-population genetic variability and producing highly plastic behavioural phenotypes that will be better in coping with environmental changes (Mallet, 2007; Salazar et al., 2010; Butlin & The Marie Curie Speciation Network, 2012; Abbott et al., 2013; Seehausen, 2013; scenario 4a in Fig. 2).
A well-known example of a hybrid zone is that between the two subspecies of house mice: Mus musculus musculus and Mus musculus domesticus. The subspecies are found across Europe and Northern Asia, and their ranges overlap in Central Europe, with the formation of a hybrid zone that spans from Denmark to Bulgaria (Boursot et al., 1993). It has been shown that M. m. musculus females generally prefer males from their own subspecies and that M. m. domesticus females do not discriminate between them (Zinck & Lima, 2013). Hence, the hybrid zone could be the result of M. m. domesticus females' inability to recognise their own subspecies. Additionally, if they rely on learning to decide with whom to mate, it creates opportunities for mate-choice copying. It has been shown, indeed, that the laboratory CF-1 albino strain of house mice has the ability to mate-choice copy (Kavaliers et al., 2006). This study is a case of conspecific copying but suggests an important role of this type of learning in the mating behaviour of the house mouse. The question is how often the two subspecies could hybridize by mate-choice copying. It will depend on how often they actually meet in the contact zone, on the copying rule and on the relative abundance of each subspecies. It is possible that M. m. domesticus is more abundant in certain areas of the contact zone and M. m. musculus in others. If M. m. domesticus females conform to the majority, they will then copy the choices of the most abundant population and, hence, more opportunities for hybridization will exist if the M. m. musculus population is more abundant. If they are attracted to novelty, they will copy the choices of the less-abundant population and, hence, more opportunities for hybridization will exist if M. m. musculus is less abundant. Finally, if one type of male performs better than the other and if M. m. domesticus females rely on performance, they will choose the best performers in each location, regardless of each subspecies' relative abundance. All copying rules described in Fig. 7 could, therefore, operate. Heterospecific mate-choice copying experiments between the two subspecies would be necessary to test these hypotheses.
After hybridization events, the introgression of alleles from one of the species involved into the gene pool of the other, and vice versa, becomes possible if hybrids mate with the parental species. This process is adaptive if the introgressed alleles are favoured by selection, which has indeed been demonstrated for the house mouse (Staubach et al., 2012). Adaptive introgression has also been thoroughly studied in sympatric species of Darwin's ground finches, namely in Geospiza fortis and G. scandens (e.g. Grant & Grant, 2008, 2009). They live on the island of Daphne Major in the Galapagos archipelago. Hybridization, though apparently rare, has been present across these species' evolutionary history, contributing to persistent genetic introgression. It is the result of sexual imprinting of one species on the song of the other. Since females mate according to song type, misimprinted females mate with the wrong species, producing hybrids. Hybrids themselves also mate according to song type, thus mating with the paternal species. The resulting introgression increases the phenotypic and genotypic variation of the backcrossed populations that improves their ability to explore environmental conditions that were inaccessible to the parental species. This way, the backcrossed populations can respond more rapidly to the challenge of environmental change than if the two parental species were fully genetically isolated, setting the stage for adaptive radiation (Seehausen, 2004; Grant & Grant, 2008, 2009). The question is whether mate-choice copying, besides sexual imprinting, can also facilitate hybridization between two species under similar circumstances. If this is the case, and given that mate-choice copying causes informational cascades, the resulting hybridization events would be more common than those from imprinting mistakes. This would also increase the rate of adaptive introgression between the species involved. Hybridization events typically have very low impact on the genetic architecture of a species (Abbott et al., 2013). But if hybridization occurs with a certain frequency among closely related species, the probability that one of those events will contribute to adaptive introgression is higher (Abbott et al., 2013). Mate-choice copying might operate exactly here, by increasing the number of hybridization events and by consequence the opportunities for adaptive introgression.
When hybridization is adaptive, it may also be facultative. This is the case for spadefoot toads, Spea bombifrons and Spea multiplicata, which actively hybridize when the habitat conditions are favourable to hybrid offspring (Pfenning, 2007). This behaviour is asymmetric, i.e. hybridization is more common in, and more advantageous to, S. bombifrons females than S. multiplicata females. Metamorphosis of S. bombifrons tadpoles is slower and consequently they risk not achieving adulthood if developing in shallow ponds that dry quickly. By hybridizing with S. multiplicata males, S. bombifrons females produce hybrid tadpoles that develop faster than non-hybrids, which compensates for their lower fertility. Such facultative context-dependent female mate-choice behaviour apparently does not have an underlying genetic mechanism, or there is still no evidence that such exists (Reyer, 2008). An alternative hypothesis is that of a learning mechanism, with facultative mate choice being either dependent on trial-and-error learning, or on mate-choice copying. If copying is involved, facultative mate choice could, therefore, be the result of inexperienced females copying the mate choices of older females that by their previous breeding experience have better knowledge of their environment and thus can more reliably predict breeding success. This is exactly why mate-choice copying is thought to be adaptive (Nordell & Valone, 1998; Danchin et al., 2004; Vakirtzis, 2011). A mate-choice copying experiment might not be difficult to conduct in these species and would allow testing this hypothesis.
(2) When, between species in secondary contact, copied preferences originate or facilitate reproductive isolation
When hybridization is successful (Fig. 1), besides breaking down reproductive isolation in the short term, in the long term it can also act as an additional source of differentiation between populations, originating new reproductive isolation (Mallet, 2007; Nolte & Tautz, 2010; Salazar et al., 2010; Butlin & The Marie Curie Speciation Network, 2012; Abbott et al., 2013; Seehausen, 2013). In fact, the accumulation of genetic incompatibilities between populations of the same species that would lead to reproductive isolation, occurs generally too slowly to be able to explain all speciation events by ecological divergence (Nosil, 2012). Hybridization between related species can act as an additional mechanism of divergence – an alternative to mutation – and may be more common than previously appreciated (Grant & Grant, 2008, 2009; Fitzpatrick et al., 2009; Whitney, Randell & Rieseberg, 2010; Kunte et al., 2011). This could be achieved as a result of adaptive introgression, adaptive radiation, or even hybrid speciation (Seehausen, 2004, 2013; Abbott et al., 2013). The role of mate-choice copying in originating or facilitating reproductive isolation is described in scenario 4b of Fig. 2. Examples of hybridization by mate-choice copying that could result in adaptive introgression and adaptive radiation were given in Section IV.1. Regarding hybrid speciation, there is the interesting case of the Amazon molly species complex, which is the only example in the literature of mate-choice copying involving different species.
The Amazon molly (Poecilia formosa) is a gynogenetic species and a case of hybrid speciation. It is an all-female fish that reproduces clonally. However, to initiate embryogenesis, the species relies on sperm from males of several heterospecific species, including its parental species, the sailfin molly (P. latipinna) and the Atlantic molly (P. mexicana). Heterospecific matings are therefore obligatory for the gynogenetic females to persist in nature (Schlupp, 2005). Interestingly, Schlupp et al. (1994) found that mate-choice copying occurs between Amazon and sailfin mollies, with sailfin males becoming more attractive to their conspecific females after having mated with the Amazons. The same was later confirmed for Atlantic mollies (Heubel et al., 2008). In other words, heterospecific Amazon females are seen by sailfin and Atlantic females as reliable models to copy. Although this study is not a case of true heterospecific mate-choice copying (sailfin females did not learn to prefer Amazon males, and could not, because there are no Amazon males), it provides evidence for the participation of mate-choice copying in complex relationships between species sharing the same ecology – throughout its geographic range, Amazons always live in sympatry with at least one of their parental species (Schlupp, Parzefall & Schartl, 2002). Mate-choice copying could, indeed, be contributing to the maintenance of this and other complex gynogenetic breeding systems. It also throws light on the donor males' behavioural paradox, since males were thought to derive no benefits from mating with heterospecific females (Schlupp et al., 1994; Heubel et al., 2008).
Additionally, this example also raises the question of whether mate-choice copying could facilitate or even be at the origin of such hybrid speciation events. This could be tested by studying the incidence of mate-choice copying in both the parental and hybrid species of known cases of hybridization, like the Amazon molly complex, but also others such as Squalius alburnoides (Cyprinidae fish) (Cunha et al., 2011), Heliconius butterflies (Mavarez et al., 2006), African cichlid fishes (Seehausen, 2004; Selz et al., 2014) and Darwin's finches (Grant & Grant, 2008, 2009). The single hybridization event giving rise to the Amazon molly occurred probably 100000 years ago (Schlupp, 2005; Stöck et al., 2010). Researchers have been trying to recreate the hybrid in the laboratory but with no success to date. Therefore, it will be extremely hard – not to say impossible – to determine the role of mate-choice copying in the origin of the Amazon molly. Nonetheless, heterospecific mate-choice copying experiments between the Amazon molly parental species have never been done. In these species, mate-choice copying is stronger in a conspecific context (Hill & Ryan, 2006; Heubel et al., 2008), but it would be important to know how much of this dynamic can change in a heterospecific context. Such experiments would be extremely helpful to ascertain whether mate-choice copying increases the frequency with which Atlantic molly females (the maternal species) mate with sailfin molly males (the paternal species) and, hence, how many opportunities mate-choice copying may have provided in the past for the hybrid formation of the Amazon molly. For Heliconius butterflies, hybrid phenotypes were successfully obtained in the laboratory (Mavarez et al., 2006; Salazar et al., 2010), meaning that in this case mate-choice copying experiments could even attempt to measure not only mating preference but also the number of hybrid offspring produced with and without copying.
(3) When, between species in secondary contact, copied preferences reinforce or preserve reproductive isolation
If hybridization is unsuccessful – when premating barriers are incomplete but postmating barriers are strong (Fig. 1) – the outcome will be very different from the above scenarios (scenario 4c in Fig. 2). Hybrids will be less vigorous and could even be inviable or sterile (Coyne & Orr, 2004). Therefore, the third possible consequence of mate-choice copying as a promoter of heterospecific matings is a significant reduction in the fitness of the copying females and/or of their selected males. Heterospecific mate-choice copying will become decreasingly adaptive in this scenario, at least to one of the species involved, which in the short term can even increase the risk of local extinction (Todesco et al., 2016) and be interpreted as a type of reproductive interference.
Reproductive interference occurs when two species interfere with each other during mating, either by participating in heterospecific matings or by hampering the other species' conspecific matings (Gröning & Hochkirch, 2008; Burdfield-Steel & Shuker, 2011). This could have fitness consequences to at least one of the species, leading to its competitive exclusion (Kishi, Nishida & Tsubaki, 2009). Reproductive interference is caused by incomplete species recognition and can occur in at least seven different ways: signal jamming, heterospecific rivalry, misdirected courtship, erroneous female choice, heterospecific mating attempts, heterospecific matings and actual hybridization through the production of hybrid offspring (Gröning & Hochkirch, 2008). Mate-choice copying, by increasing the attractiveness of heterospecific males, will be probably enhancing every one of these mechanisms. Copying could thus be substantially interfering in the way species communicate, potentially handicapping one or both species' competitive abilities.
A case of misdirected courtship and heterospecific mating attempts is that between introduced Trinidadian guppies and the resident, endangered, Skiffia bilineata species, another viviparous fish native to Central Mexico (Valero, Garcia & Magurran, 2008). S. bilineata females, which are larger than guppy females but morphologically similar, probably represent a supernormal stimulus to male guppies, who are attracted to large females (Herdman, Kelly & Godin, 2004). Since these species cannot effectively hybridize as they are phylogenetically too distant – guppies belong to the Poeciliidae family and S. bilineata to the Goodeidae family – male guppies do not appear to derive benefits from courting heterospecific females. But given that female guppies mate-choice copy, it is not impossible that male guppies gain the advantage of becoming more attractive to conspecific females by courting and mating with heterospecific females, in much the same way as male sailfin mollies become more attractive to conspecific females by courting Amazon mollies (Schlupp et al., 1994; Heubel et al., 2008). This would not promote actual hybridization, since the two species do not produce viable hybrids, and guppy females, instead of S. bilineata females, would be the copiers. Nonetheless, it suggests how mate-choice copying by guppy females can interfere with the reproductive system of the other species. Besides, mate-choice copying by S. bilineata females could also exist and is worth testing for. Such experiments could be done rather easily and if mate-choice copying is demonstrated, it could be enhancing reproductive interference effects for S. bilineata, which has already gone extinct from 50% of its native distribution (De La Vega-Salazar, Avila-Luna & Macías-Garcia, 2003).
In the long term, it is predictable that reproductive interference via mate-choice copying will reinforce the process of species recognition, facilitating further speciation. Reinforcement will occur either because, in the copying species, it favours females that do not copy – selection against mate-choice copying –, or because, in the copying or copied species, it leads populations to local extinction. Alternatively, the behaviour of mate-choice copying may be preserved if selection pressures act directly on the recognition system itself. This could still reinforce reproductive barriers by preventing heterospecific mate-choice copying while preserving the females' ability for conspecific mate-choice copying.
This poses the question of how species recognition, or behavioural isolation in general, evolves. If females share information with each other, behavioural isolation should also involve the ability to identify as poor-quality information the cues and signals coming from heterospecific females. The mate-choice copying literature provides evidence that females evaluate whether other females are good models to copy or not (Dugatkin & Godin, 1993; Amlacher & Dugatkin, 2005; Hill & Ryan, 2006; Vukomanovic & Rodd, 2007; Waynforth, 2007; Yorzinski & Platt, 2010). Then, it is possible that species that do not perform heterospecific mate-choice copying are species where female–female recognition has successfully evolved as an additional reproductive barrier to gene flow.
In all three hybridization scenarios (4a to 4c in Fig. 2), the fate of the F1s and F2s is also very important. As we have seen, hybrid fitness will determine whether hybridization is participating in reinforcing barriers to gene flow (if hybrids pay costs), or whether hybridization is generating new species, or recovering genetic variability (if there is some degree of hybrid viability) (Fig. 1). But other questions remain. Will hybrids be more or less prone to copy? In which direction will they copy more often, towards the maternal or the paternal species? The patterns of mate-choice behaviour of the hybrid generations will, therefore, be crucial to the role of mate-choice copying on long-term genetic introgression or adaptive radiation and hybrid speciation patterns that will follow, as well as to the ecological competences of the new lineages.
V. CONCLUSIONS
(1) We have described and reviewed mate-choice copying behaviour by females, and have detailed why it is considered an important mechanism of female mate choice. Furthermore, we outline how it can subsequently affect the course of sexual selection, by strengthening or weakening barriers to reproductive isolation. Such a conceptual framework was lacking in the literature, and here we present a comprehensive theoretical basis for the role of mate-choice copying in both speciation and hybridization.
(2) Previous studies have suggested the participation of mate-choice copying in the speciation process, but no specific evolutionary scenario has been proposed. Here, by merging mate-choice copying theory with speciation theory, we envisioned the ways by which this behaviour can affect speciation. By predicting in which scenarios mate-choice copying is more or less prone to facilitate or reinforce reproductive isolation, we can now study its occurrence and prevalence in a number of new species and ecological conditions. We particularly highlight the importance of environmental complexity and genotype-by-environment interactions, leading to fine-grained spatial variation and local adaptation, which are fundamental conditions for mate-choice copying to emerge as a driving force for speciation.
(3) Mate-choice copying may not only facilitate reproductive isolation setting the stage for speciation, but at the same time, and with less-restricted assumptions, it may also have the potential to direct sexual selection towards hybridization. Interestingly, it is also by promoting or facilitating hybridization that mate-choice copying could be increasing species' opportunities for adaptive introgression and radiation, hybrid speciation and reinforcement, which are hybridization outcomes that have been increasingly recognized to have potentially major roles in speciation events.
(4) These new hypotheses challenge not only the view of the role of social learning in species evolution but also shed light on the behavioural mechanisms that could be at the origin of speciation and hybridization, opening a new avenue of research for both theoretical and experimental studies.
VI. ACKNOWLEDGEMENTS
We are grateful to two anonymous reviewers for their constructive suggestions and comments on a previous version of this manuscript. This study was financed by Portuguese national funds, through Fundação para a Ciência e a Tecnologia (FCT), within the cE3c unit funding UID/BIA/00329/2013 and through a post-doctoral research grant to S.A.M.V. (SFRH/BPD/66042/2009), and by the University of Oklahoma.




